Abstract
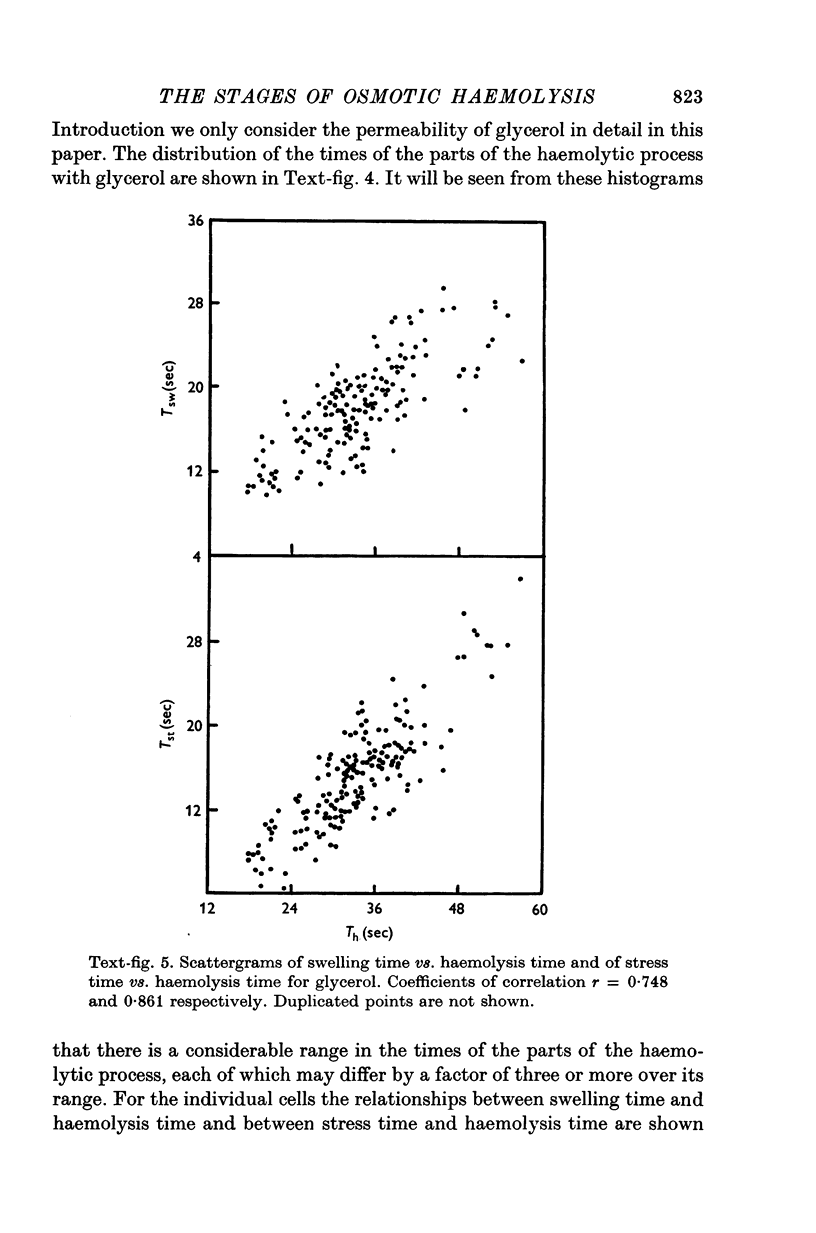
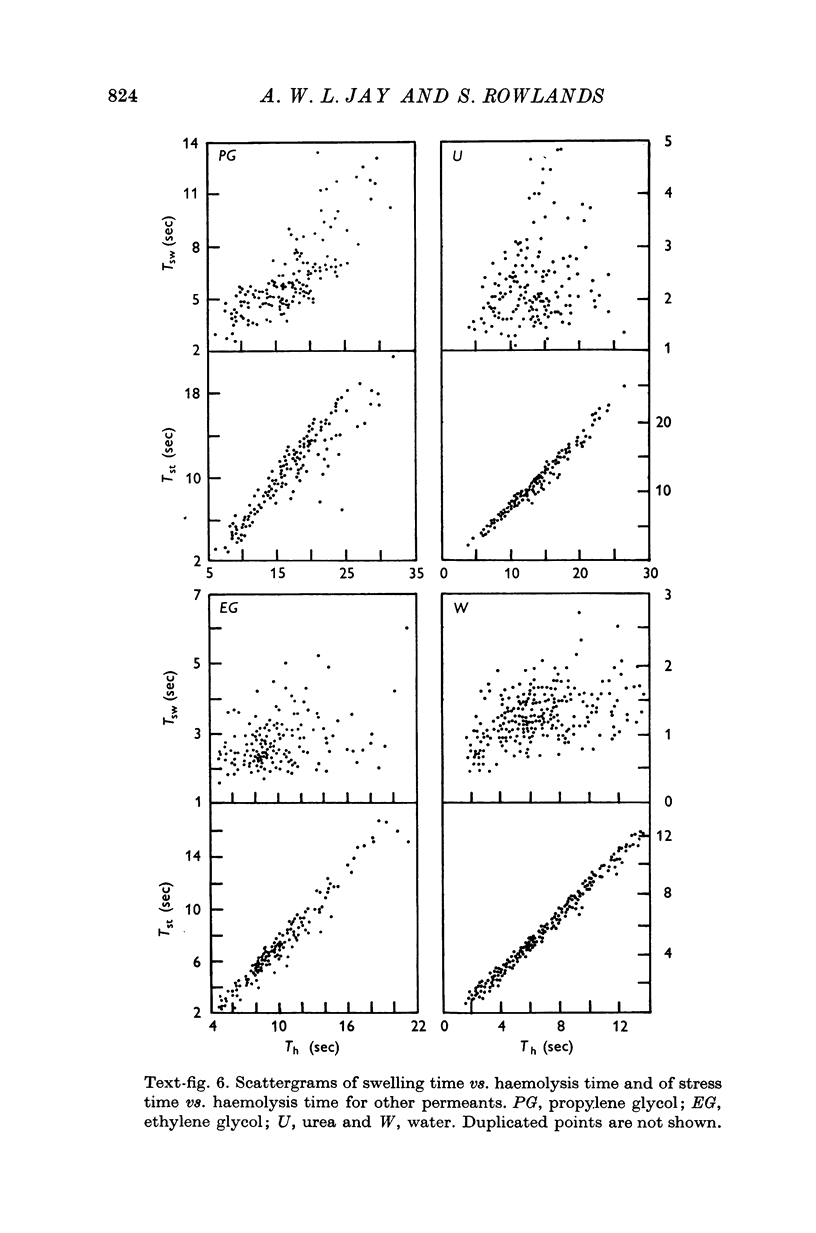
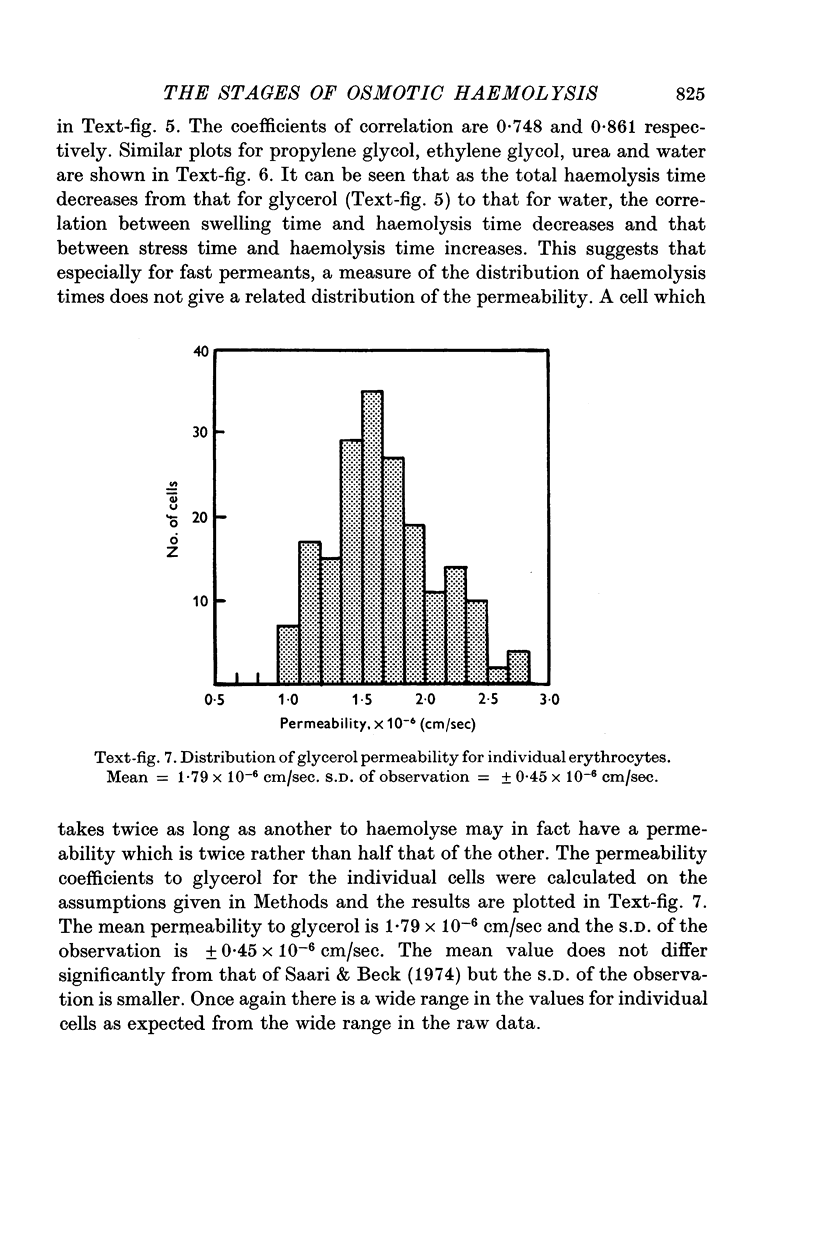
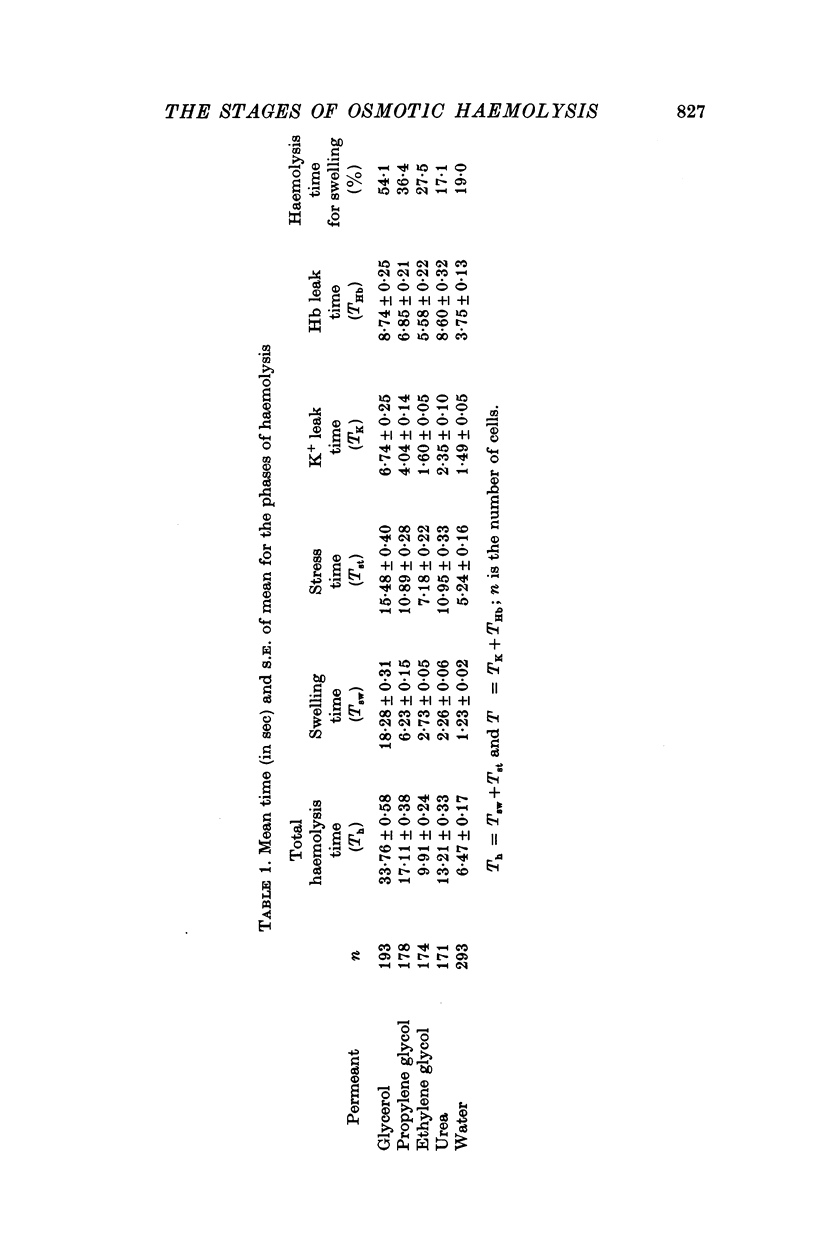
The haemolysis of individual human erythrocytes has been observed using an inverted microscope and cine-camera. 2. With each permeant (glycerol, propylene glycol, ethylene glycol, urea and water) haemolysis is a multistage process. The stages are swelling, popping, reduction in volume possibly accompanied by ion leakage and, finally, haemoglobin leakage. 3. The classical haemolysis time (Th) is made up of a swelling time (Tsw) and a stress time (Tst). Tst is not negligible and with the faster permeants it may occupy more than 75% of the haemolysis time. 4. The stress time can also be divided into two parts: a K+ leak time (TK) during which the cell shrinks and a time (THb) during which haemoglobin is leaving the cell. THb occupies a substantial part of Th, from 25 to 65%, and is relatively longer in fast haemolysis. 5. There is a wide spread in the permeability coefficient to glycerol in a population of erythrocytes. The distribution is compatible with a Gaussian distribution. The mean permeability is 1-79 X 10(-6) cm/sec and the S.D. is +/0 0-45 X 10(-6) cm/sec. 6. The correlation between haemolysis time and swelling time for individual erythrocytes is poor, especially for fast haemolysis. Consequently, a measure of the distribution of haemolysis time does not give a related distribution of the swelling time or of the calculated permeability for individual erythrocytes.
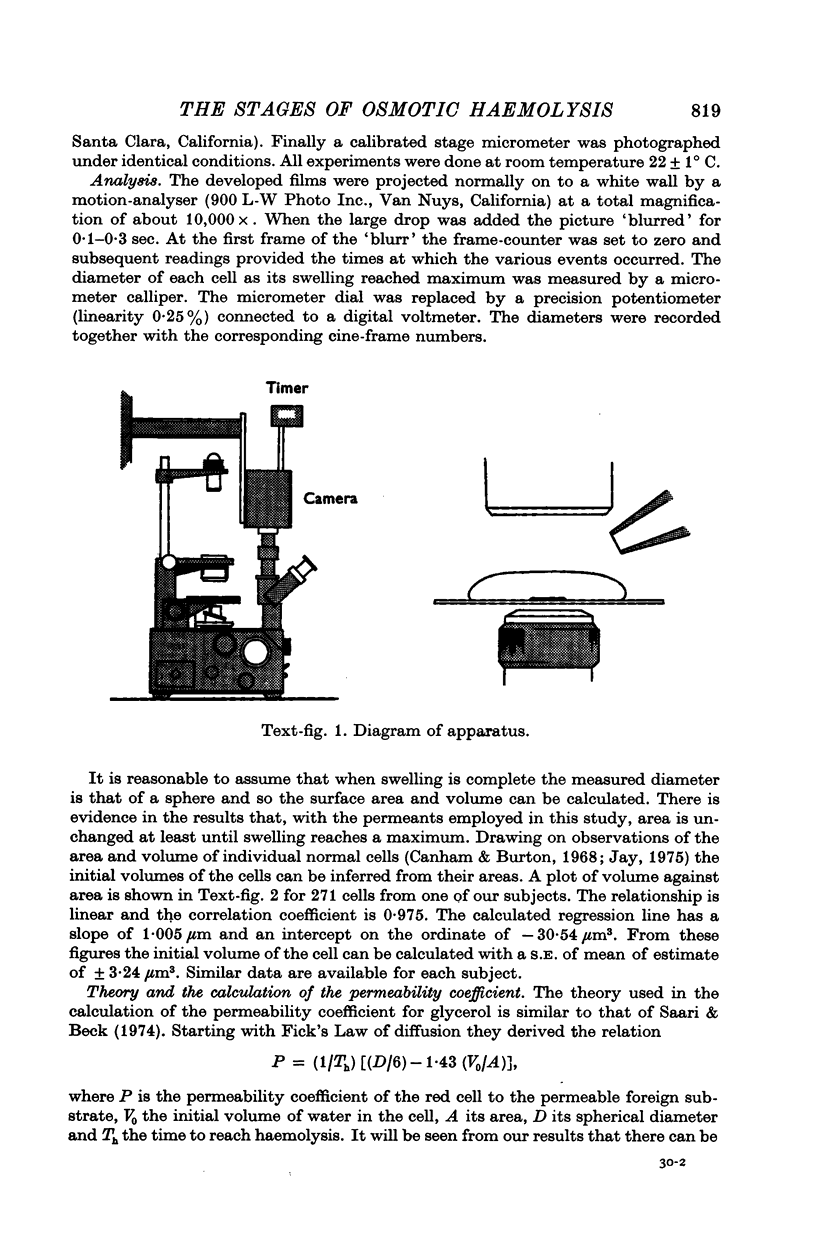
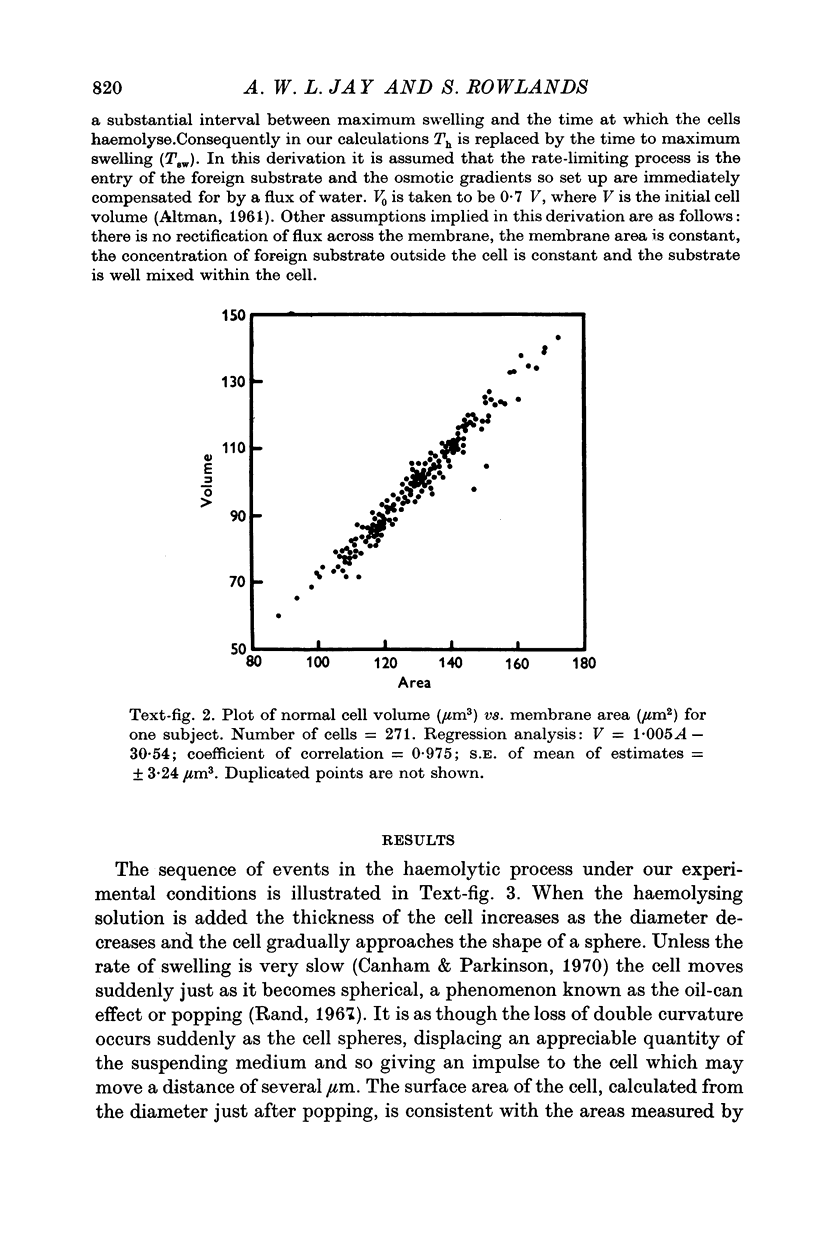
Full text
PDF

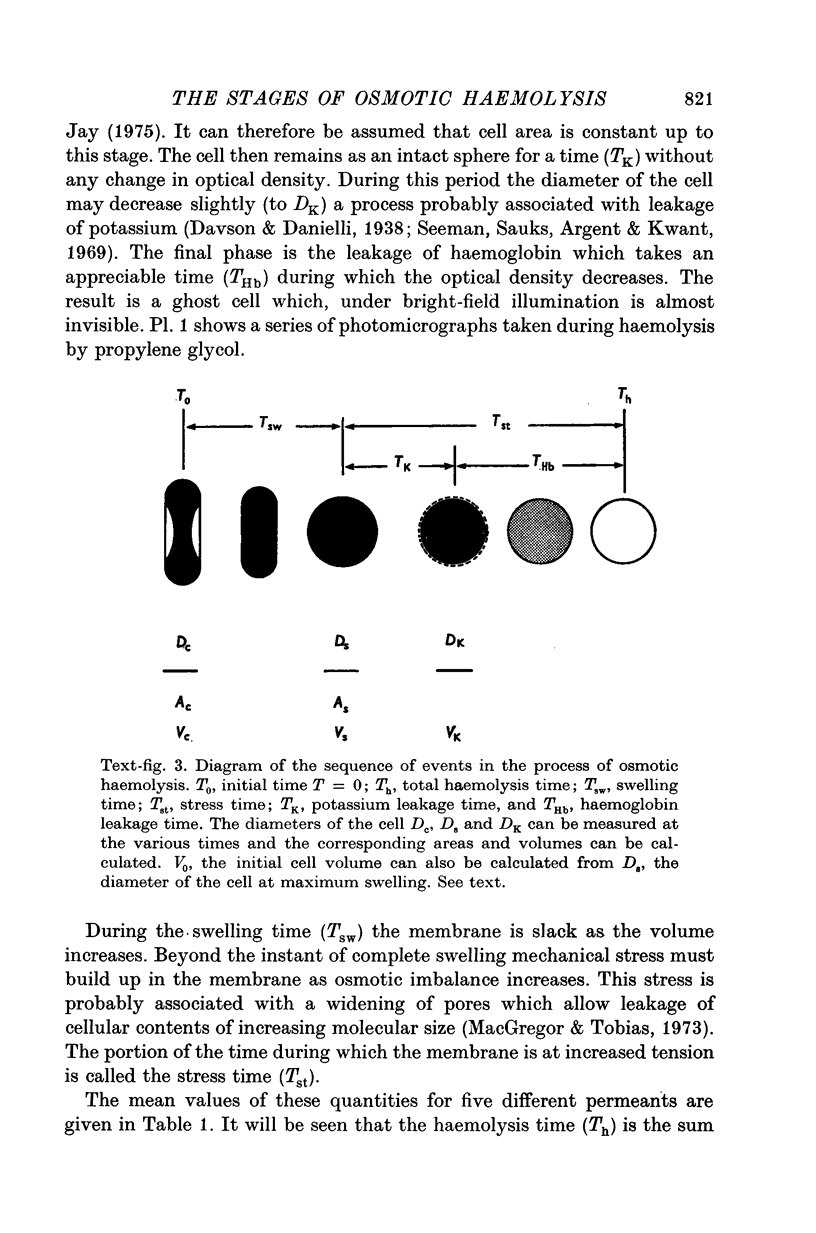
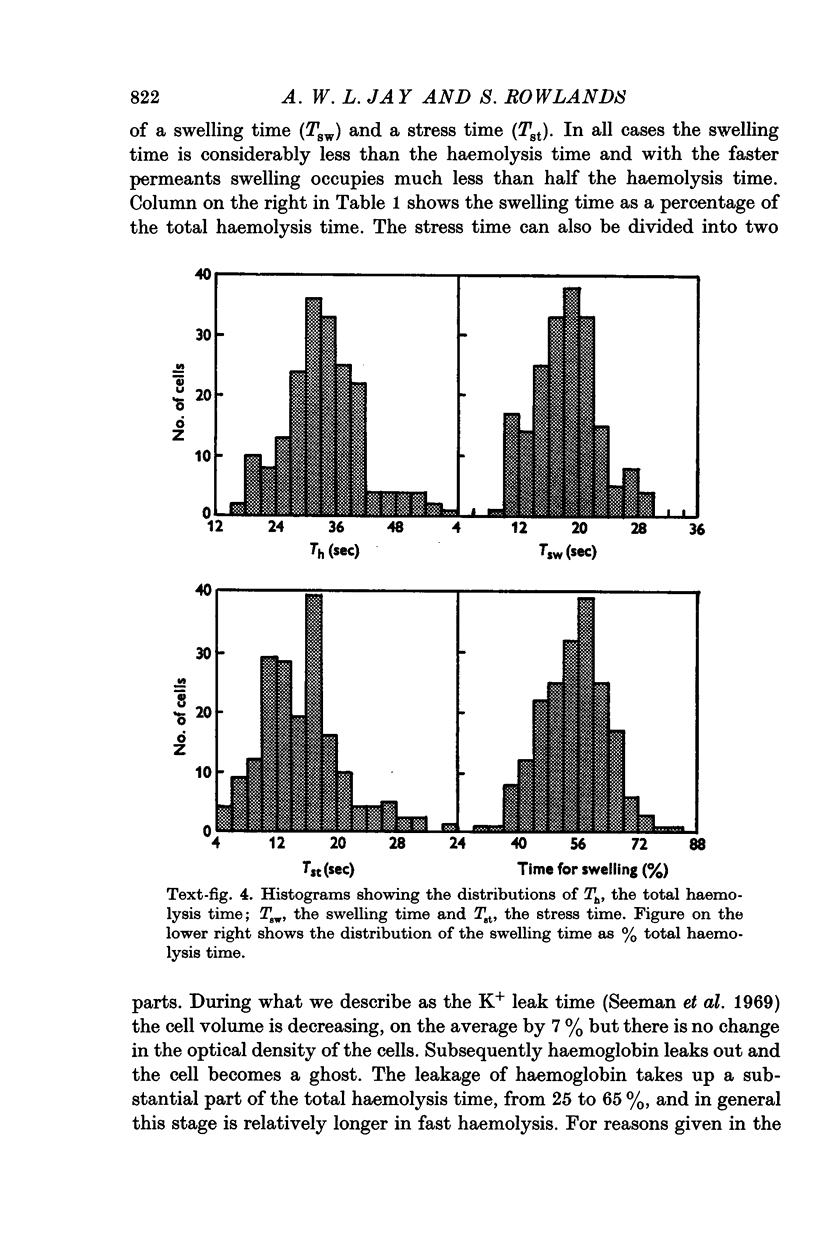




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruckdorfer K. R., Demel R. A., De Gier J., van Deenen L. L. The effect of partial replacements of membrane cholesterol by other steroids on the osmotic fragility and glycerol permeability of erythrocytes. Biochim Biophys Acta. 1969 Jul 15;183(2):334–345. doi: 10.1016/0005-2736(69)90089-3. [DOI] [PubMed] [Google Scholar]
- Canham P. B., Burton A. C. Distribution of size and shape in populations of normal human red cells. Circ Res. 1968 Mar;22(3):405–422. doi: 10.1161/01.res.22.3.405. [DOI] [PubMed] [Google Scholar]
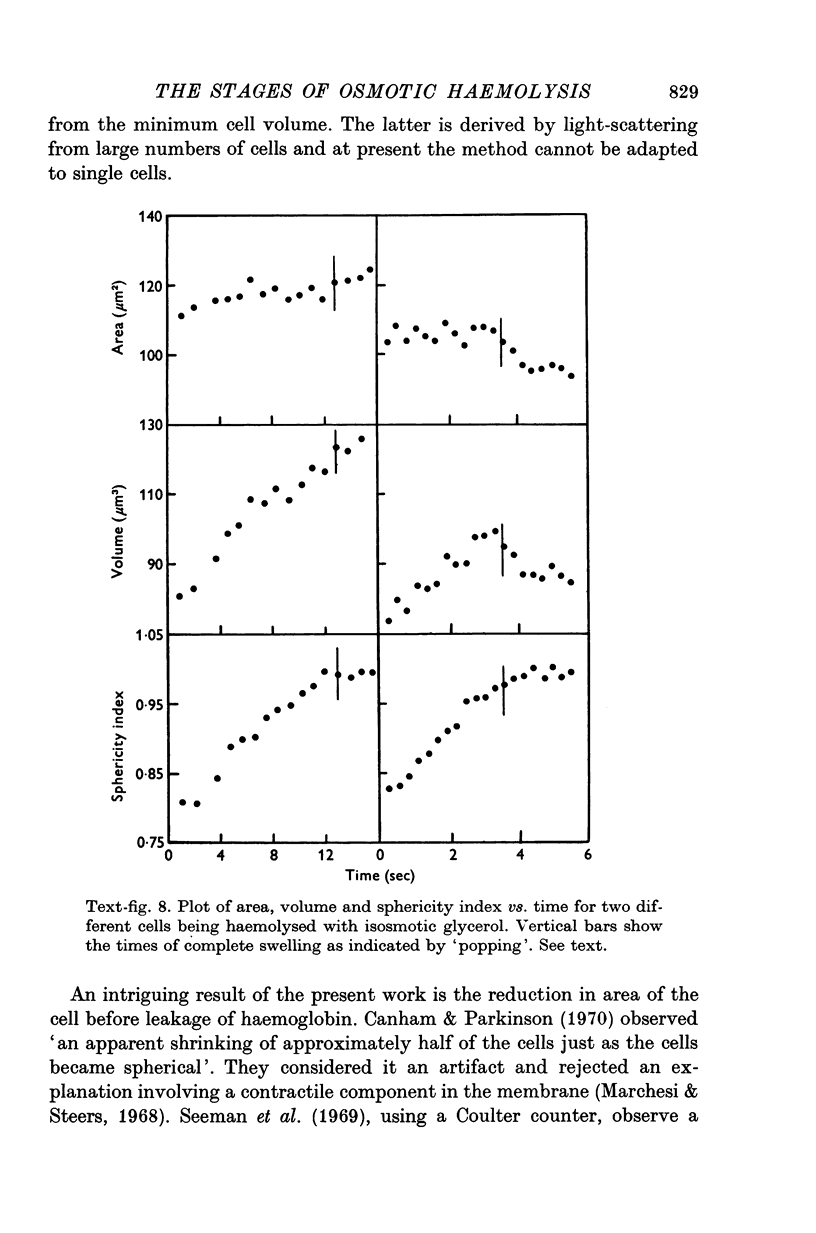
- Canham P. B., Parkinson D. R. The area and volume of single human erythrocytes during gradual osmotic swelling to hemolysis. Can J Physiol Pharmacol. 1970 Jun;48(6):369–376. doi: 10.1139/y70-059. [DOI] [PubMed] [Google Scholar]
- Davson H., Danielli J. F. Studies on the permeability of erythrocytes: Factors in cation permeability. Biochem J. 1938 Jun;32(6):991–1001. doi: 10.1042/bj0320991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried E. L., Robertson N. A. Glycerol lysis time as a screening test for erythrocyte disorders. J Lab Clin Med. 1974 Feb;83(2):323–333. [PubMed] [Google Scholar]
- Jay A. W. Geometry of the human erythrocyte. I. Effect of albumin on cell geometry. Biophys J. 1975 Mar;15(3):205–222. doi: 10.1016/S0006-3495(75)85812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T., Steers E., Jr Selective solubilization of a protein component of the red cell membrane. Science. 1968 Jan 12;159(3811):203–204. doi: 10.1126/science.159.3811.203. [DOI] [PubMed] [Google Scholar]
- PAGANELLI C. V., SOLOMON A. K. The rate of exchange of tritiated water across the human red cell membrane. J Gen Physiol. 1957 Nov 20;41(2):259–277. doi: 10.1085/jgp.41.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand R. P. Some biophysical considerations of the red cell membrane. Fed Proc. 1967 Nov-Dec;26(6):1780–1784. [PubMed] [Google Scholar]
- SIDEL V. W., SOLOMON A. K. Entrance of water into human red cells under an osmotic pressure gradient. J Gen Physiol. 1957 Nov 20;41(2):243–257. doi: 10.1085/jgp.41.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
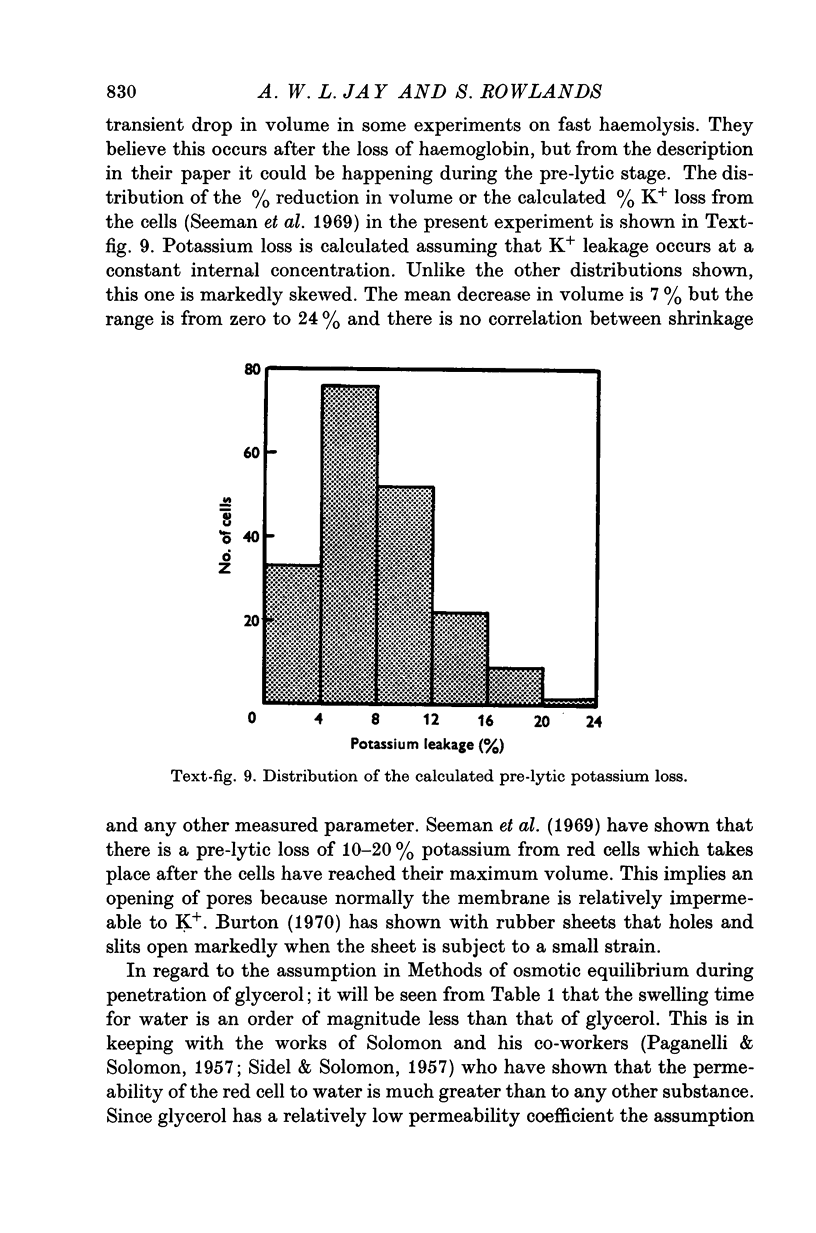
- Seeman P., Sauks T., Argent W., Kwant W. O. The effect of membrane-strain rate and of temperature on erythrocyte fragility and critical hemolytic volume. Biochim Biophys Acta. 1969;183(3):476–489. doi: 10.1016/0005-2736(69)90162-x. [DOI] [PubMed] [Google Scholar]
- Sha'afi R. I., Rich G. T., Mikulecky D. C., Solomon A. K. Determination of urea permeability in red cells by minimum method. A test of the phenomenological equations. J Gen Physiol. 1970 Apr;55(4):427–450. doi: 10.1085/jgp.55.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]



