Abstract
Escherichia coli cells from strain fpr, deficient in the soxRS-induced ferredoxin (flavodoxin)-NADP(H) reductase (FPR), display abnormal sensitivity to the bactericidal effects of the superoxide-generating reagent methyl viologen (MV). Neither bacteriostatic effects nor inactivation of oxidant-sensitive hydrolyases could be detected in fpr cells exposed to MV. FPR inactivation did not affect the MV-driven soxRS response, whereas FPR overexpression led to enhanced stimulation of the regulon, with concomitant oxidation of the NADPH pool. Accumulation of a site-directed FPR mutant that uses NAD(H) instead of NADP(H) had no effect on soxRS induction and failed to protect fpr cells from MV toxicity, suggesting that FPR contributes to NADP(H) homeostasis in stressed bacteria.
The soxRS regulon positively modulates the expression of at least 17 genes in Escherichia coli, mediating an oxidative stress response that protects the bacterial cells against the superoxide anion radical (O2−·), nitric oxide (NO), and redox cycling agents such as the herbicide methyl viologen (MV) (10, 36, 37). The sensor of the regulon is the SoxR protein, a dimeric transcription factor that contains [2Fe-2S] centers and whose only known target is the soxS promoter (31, 41). When E. coli cells are subjected to a challenge with O2−· , the iron sulfur clusters of SoxR may undergo univalent oxidation to yield the transcriptionally active form of the protein (7, 15). Both oxidized and reduced SoxR are able to interact with the soxS promoter, but only binding of the oxidized dimer enhances the synthesis of SoxS, a transcriptional activator of the AraC/XylS family (37). Increased SoxS levels then activate the various regulon genes via σ70 RNA polymerase (10, 37). The soxRS regulon appears to be specifically tailored to respond to O2−· (or NO) and is not induced by other sources of oxidative stress such as heat shock or ionizing radiation (10, 37).
E. coli cells exposed to a source of O2−· may undergo bacteriostatic or bactericidal effects. Bacteriostasis is related to superoxide-mediated inactivation of catalytic [4Fe-4S] clusters in hydrolyases, with the tricarboxylic acid cycle enzyme aconitase being a most sensitive target (9). Inhibition of these enzymes causes a decline in growth rates without affecting cell viability, since oxidized hydrolyases can be reactivated by a reductive system whose components are yet to be identified (12). Bactericidal effects, on the contrary, usually reflect DNA oxidation and cleavage by superoxide derivatives such as the hydroxyl (·OH) and ferryl (FeO2+) free radicals (17, 18). The balance between bacteriostasis and lethality depends on the intensity of the stress imposed, the culture conditions, and the stock of antioxidants present in a given E. coli strain, among other factors (16, 19, 30). To cope with the various hazards of O2−· toxicity, members of the soxRS regulon need to operate at different (and complementary) levels of the global cell response to the oxidative challenge. Protective functions include direct O2−· scavenging by the Mn-containing superoxide dismutase (SOD), replacement of oxidant-sensitive hydrolyases by resistant isoforms, DNA repair activities, diminished uptake, and increased elimination of xenobiotics, etc. (10, 25, 36). As part of the global response, E. coli cells also induce the synthesis of several NADP(H)-dependent dehydrogenases and oxidoreductases, including the flavoprotein ferredoxin (flavodoxin)-NADP(H) reductase (FPR) (EC 1.18.1.2) (27). These ubiquitous FAD-containing enzymes catalyze the reversible electron transfer between a single molecule of NADP(H) and two molecules of obligatory one-electron carriers such as ferredoxin or flavodoxin (1). They can also mediate the so-called “diaphorase” reaction, namely, the irreversible oxidation of NADPH by a wide variety of adventitious electron acceptors, such as viologens, quinones, substituted phenols, complexed transition metals and tetrazolium salts, among others (1, 27). The steady-state levels of FPR increased 20-fold on exposure of E. coli cells to the O2−· propagator MV (27, 38), and FPR-deficient strains (fpr) proved to be abnormally sensitive to oxidants (3, 22). Expression of this reductase from a multicopy plasmid provided increased MV tolerance to E. coli cells displaying wild-type levels of FPR synthesis and induction, indicating that the antioxidant effect was dose dependent even beyond physiological levels of the flavoenzyme (3, 21). The nature and mechanism of this defensive action, however, remain elusive, although a number of hypotheses have been advanced.
In their seminal work, Liochev et al. (27) proposed that E. coli FPR might be involved in the reduction of SoxR once the oxidative condition has subsided, so that the function of the reductase would be to provide for self-regulation of the entire soxRS system. Alternatively, FPR could participate in the reductive healing of O2−· -damaged hydrolyases (10, 24). A function of this type would be in agreement with recent observations showing that aconitase activities but not aconitase protein levels were severely depressed in a conditional yeast mutant lacking the adrenodoxin reductase homologue, a mitochondrial flavoenzyme with FPR activity (23). Still other proposals posed a role for FPR in the modulation of the NADP(H)+ homeostasis or in the reduction of an abundant cellular scavenger (22). The various invoked mechanisms are based on the promiscuity exhibited by FPR at its acceptor side, but empirical evidence for any of these contentions is scant. To gain further insight into the protective role of this reductase, we probed the effects of FPR inactivation, FPR overexpression, and FPR mutation on growth, survival, soxRS induction, and NADP(H) levels in MV-treated E. coli cells.
All strains used in this work were derivatives of E. coli K-12, and their relevant features are summarized in Table 1. The fpr mutation of strain C-6007 (3) was transferred into strains QC772 (sodA) and GC4468 (parental) by P1 transduction (28). Transductants were screened for kanamycin resistance (Kanr), and the properties of the presumptive fpr-inactivated clones were assessed by PCR analysis (3) and FPR inmunoreaction (38). Recombinant plasmids employed in the present study are also described in Table 1. Briefly, pEC105 contains the entire sequence of the E. coli fpr gene (3), with the initial ATG fused in-frame to codon 13 of the lacZ gene in pSU18. Plasmid pDR105 harbors a full-length cDNA encoding the mature, processed region of pea FPR (5), linked in-frame to the first 16 triplets of the β-galactosidase gene in pSU19. A site-directed mutant version of this fused gene, containing a Tyr-to-Ser replacement at position 308 (35), was also prepared in pSU19, yielding recombinant vector pY308S. Chimeric genes were placed under the control of the lacZ promoter in the three plasmids. Finally, pTN1530 contains a soxS"::lacZ operon fusion expressing β-galactosidase activity under the control of the soxS promoter (31). Transformation of competent E. coli cells with the various plasmids, isolation of genomic and plasmidic DNA, and miscellaneous recombinant DNA techniques were carried out according to established procedures (2). Unless otherwise stated, E. coli cells were grown at 37°C in Luria-Bertani (LB) broth (2). Antibiotics (100 μg of ampicillin, 100 μg of kanamycin, and/or 20 μg of chloramphenicol ml−1) were added when required (Table 1).
TABLE 1.
E. coli strains and plasmids used in this study
| Strain or plasmid | Genotype or relevant characteristicsa | Source and/or reference |
|---|---|---|
| Strains | ||
| GC4468 | F− Δlac U169 rpsL | 4 |
| QC772 | GC4468 sodA49 Camr | 4 |
| C-6007 | Cla fpr Kanr Rifr | 3 |
| RR6A | GC4468 fpr Kanr | This study |
| RR64 | QC772 fpr Kanr Camr | This study |
| B247 | MC4100 (λJW2) (lysogen) λJW2:φ (soxS"::lacZ") Ampr Kanr | 29, 41 |
| Plasmids | ||
| pEC105 | pSU18 carrying the E. coli fpr gene, Camr | This study |
| pDR105 | pSU19 carrying the pea fpr gene, Camr | D. Rial and E. A. Ceccarelli, unpublished data |
| pY308S | pSU19 carrying the Y308S mutant version of the pea fpr gene, Camr | 32; This study |
| pTN1530 | pNK1415 ΔsoxR soxS"::lacZ Ampr | 31 |
Ampr, ampicillin resistance; Rifr, rifampin resistance; Camr, chloramphenicol resistance.
To study the induction of the soxRS regulon by MV, saturated cultures of E. coli were diluted (100-fold) into fresh LB broth supplemented with the corresponding antibiotics and grown for 2 to 4 h at 37°C to reach an optical density at 600 nm (OD600) of 0.3 to 0.4. Aliquots (50 ml each) of these cultures were placed in 250-ml flasks, MV was added to a final concentration of 100 μM, and the samples were shaken vigorously (300 rpm) at 37°C for the times indicated in Fig. 2 and Table 2. For experiments on the rate of soxRS deactivation, cells induced for 1 h were collected by centrifugation, washed twice with LB broth to remove the viologen, resuspended in 50 ml of the same medium, and incubated at 37°C with vigorous shaking. Aliquots were withdrawn at the times indicated and assayed for β-galactosidase activity by the procedures of Miller (28). The OD600 was used as a measure of cell density. To evaluate MV toxicity, appropriate dilutions of cells grown to stationary phase were spread onto LB agar plates containing various concentrations of the herbicide. Colonies were enumerated after 16 and 40 h of incubation at 37°C. When required, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to the media to induce expression of genes placed under control of the lacZ promoter (Table 1).
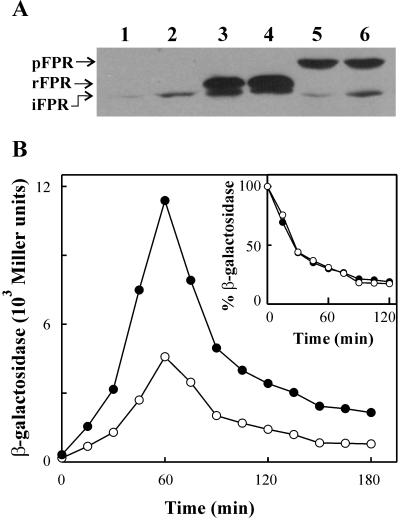
FIG. 2.
Expression of FPR stimulates the soxRS response in E. coli. (A) Accumulation of indigenous and recombinant FPR in E. coli. Cells from strain B247, transformed with either pSU19 (lanes 1 and 2), pEC105 (lanes 3 and 4), or pDR105 (lanes 5 and 6), were cultured in LB broth containing 0.5 mM IPTG. The samples corresponding to lanes 2, 4, and 6 were further induced by exposure to 100 μM MV for 1 h. Cleared supernatants corresponding to 15 μg of protein were resolved by SDS-PAGE and blotted onto nitrocellulose membranes. Filters were probed with a succession of antisera raised against both E. coli and pea FPR. The electrophoretic mobilities of the various FPR variants are indicated on the left. The slower migration of the recombinant E. coli FPR (rFPR), relative to the indigenous form (iFPR), is caused by the extra residues present at the amino terminus of the plasmid-borne reductase. pFPR, recombinant pea FPR. (B) Effect of FPR overproduction on MV-induced soxRS activation in wild-type E. coli cells. Strain B247, containing a chromosome copy of a soxS"::lacZ operon fusion (41), was transformed with the FPR-producing plasmid pDR105 (•), or with the supporting plasmid pSU19 (○). Cells were cultured in LB medium supplemented with 0.5 mM IPTG, 100 μM MV, and the corresponding antibiotics (Table 1). After 1 h of treatment with MV and IPTG, cells were collected by centrifugation, washed, and assayed for β-galactosidase as described in the text. (Inset) Relative decrease of soxS"::lacZ expression in each strain was calculated as the fraction of the β-galactosidase activity induced by MV treatment for 1 h.
TABLE 2.
Effect of FPR expression on NADP(H) levels and soxRS induction in various E. coli strainsa
| Strain | Plasmid | Plasmid-borne activity | Mean FPR-dependent activity (U/mg) ± SE with:
|
Mean NADPH/NADP+ ratiob ± SE with:
|
soxRS induction in 0.1 mM MVc | ||
|---|---|---|---|---|---|---|---|
| No MV | 0.1 mM MV | No MV | 0.1 mM MV | ||||
| B247 | pSU18 | None | 4.8 ± 0.4 | 6.9 ± 0.5 | 4.4 ± 0.6 | 1.6 ± 0.4 | 1 |
| B247 | pEC105 | E. coli FPR | 20.2 ± 1.4 | 23.8 ± 1.0 | 1.3 ± 0.3 | 0.7 ± 0.1 | 2.7 |
| B247 | pDR105 | Pea FPR | 27.1 ± 0.4 | 30.5 ± 3.1 | 2.1 ± 0.3 | 1.1 ± 0.1 | 2.8 |
| B247 | pY308S | NADH-dependent pea FPR | 5.0 ± 0.8, 19.0 ± 1.6d | 6.3 ± 0.7, 20.3 ± 2.1d | 6.0 ± 0.6 | 2.0 ± 0.2 | 0.8 |
| GC4468 | pTN1530 | SoxS-driven β-galactosidase | 6.5 ± 0.2 | 8.0 ± 0.7 | 4.6 ± 1.4 | 1.9 ± 0.3 | 1 |
| RR6A (fpr) | pTN1530 | SoxS-driven β-galactosidase | 5.2 ± 0.8 | 4.2 ± 1.0 | 6.1 ± 1.5 | 3.2 ± 0.3 | 1.1 |
E. coli cells were grown in LB broth to an OD600 of ≈0.4, incubated for an extra hour in the same medium with or without MV and assayed for FPR- dependent cytochrome c reductase activity, β-galactosidase activity, and NADP(H) contents by the procedures described in the text. Values are the means of four experiments ± the SE.
Total NADP(H) levels ranged from 6.0 to 12.9 nmol per 109 cells, with <15% variation between isogenic strains.
The β-galactosidase activities of the derivative strains B247/pEC105, B247/pDR105, B247/pY308S, and RR6A were referred to those of the corresponding controls, B247/pSU18 and GC4468, respectively, which were taken as unity.
FPR activity was estimated by using 5 mM NADH as an electron donor.
The presence of bacterial or plant-derived FPR in cleared supernatants from E. coli cell lysates was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting (22, 38). Ferredoxin-dependent cytochrome c reductase and ferricyanide-dependent diaphorase activities of FPR were determined as described before (32). Aconitase activities were assayed by the method of Flint et al. (9). The NADP(H) levels were estimated in the various strains by a redox cycling assay (40), after alkaline extraction of the pyridine nucleotides. Portions (200 μl) of cells grown to an OD600 of ∼0.5, treated or not with 100 μM MV (1 h at 37°C), were mixed with an equal volume of 0.5 M KOH. For the estimation of total NADP(H) amounts, the mixture was immediately neutralized by the addition of 100 μl of a solution containing 0.5 M triethanolamine, 0.4 M KH2PO4, and 0.1 M K2HPO4. To determine the NADPH contents, alkaline mixtures were incubated for 60 min at 60°C prior to neutralization in order to selectively destroy the NADP+. Proteins were then extracted twice with 500 μl of chloroform, and pyridine nucleotides were assayed in the upper aqueous phase. Each determination was made in triplicate.
Elimination of FPR increases the susceptibility of E. coli cells to the bactericidal effect of MV.
If FPR were involved in the reductive activation of oxidized hydrolyases, bacteriostatic effects should become apparent when FPR-deficient bacteria are challenged with low doses of MV. Figure 1A shows the behavior of E. coli cells from fpr and sodA strains, and from a sodA fpr double mutant, when exposed to MV in solid media. Bacteria carrying the sodA mutation lack the Mn-containing SOD (4, 16). They showed a clear growth inhibition when exposed to MV, reflecting the abnormal accumulation of O2−· in the cytosol. At 16 h after challenge, only a few colonies were visible in the MV plates, but at 40 h the numbers of developing colonies were similar to those of the untreated controls (Fig. 1A), although of a smaller size (data not shown). These results indicate that the effect of MV was reversible in this strain and of a static nature.
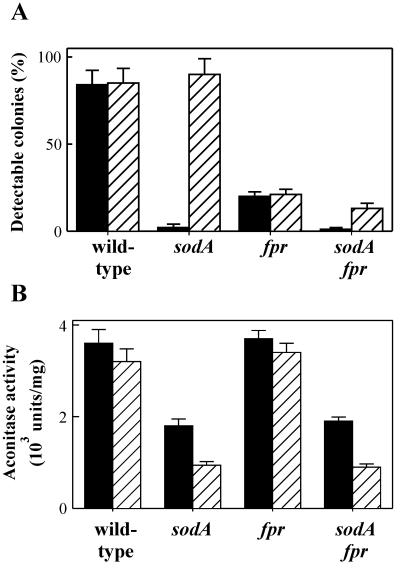
FIG. 1.
Cell survival and aconitase levels in fpr and sodA single and double E. coli mutant bacteria exposed to MV. (A) Growth of E. coli mutants in the presence of MV. Cells from strains GC4468 (wild type), RR6A (fpr), QC772 (sodA), and RR64 (sodA fpr) were grown (16 h at 37°C) in LB broth with antibiotics (Table 1), and appropriate dilutions were then spread onto LB agar plates containing 100 μM MV. Colonies were counted after 16 h (closed bars) and 40 h (hatched bars) of incubation at 37°C, and percentages refer to the colony numbers obtained in plates lacking the herbicide. Results from four different experiments were averaged, and the standard error (SE) values are indicated in the middle of the bar. (B) Effect of MV on aconitase activities of E. coli mutants. Aerobic cultures of the various E. coli strains, initiated with 5% (vol/vol) inocula from overnight-grown cells, were incubated for an additional 4 to 6 h at 37°C in glucose minimal medium (12) either in the absence (solid bars) or in the presence (hatched bars) of 3 μM MV. Extracts were prepared from the collected bacteria essentially as described by Gardner and Fridovich (11). Aconitase activities were measured in the soluble fractions and refer to total protein contents, as determined by a dye-binding assay (39). One activity unit is defined as the amount of enzyme capable of catalyzing the formation of 1 nmol of aconitate per min under the conditions of the reaction. Bars represent the averages of three experiments ± the SE.
Survival of the fpr mutants also declined as the MV concentration was raised, but the numbers of colonies counted at 16 or 40 h postchallenge were essentially the same (Fig. 1A), indicating that MV caused irreversible damage to these bacteria. Moreover, the average sizes of colonies surviving a 40-h treatment with 100 μM MV were not significantly different from those obtained in the absence of the herbicide (data not shown), suggesting that the growth rates of the spared cells were not affected by the stress imposed. Both the bactericidal and the bacteriostatic effects of MV were apparent in fpr sodA double mutants (Fig. 1A).
MV-mediated damage to aconitase, a typical [4Fe-4S]-containing hydrolyase belonging to the tricarboxylic acid cycle, could be demonstrated in sodA mutants, whereas introduction of FPR deficiency had no further effect on aconitase inactivation (Fig. 1B). Similar results were obtained when we assayed other sensitive hydrolyases, such as the 6-phosphogluconate dehydratase of the Entner-Doudoroff pathway or the dihydroxy acid dehydratase of branched amino acid synthesis (data not shown). Accordingly, fpr mutants failed to show added auxotrophies related to respiratory substrates (i.e., glycerol), gluconate, or amino acids (data not shown). The collected results indicate that E. coli FPR is either not involved in reactivation of oxidized hydrolyases or, if it does play any role, is not limiting under physiological or stressed conditions.
Effect of ferredoxin-NADP(H) reductase levels on soxR activation.
We then evaluated whether FPR levels could affect the activation state of the soxRS regulon. Bacterial or plant-type FPR versions were overexpressed in wild-type E. coli cells containing a single chromosomal copy of a soxS"::lacZ operon fusion (41). Transformed bacteria could accumulate large amounts of recombinant FPR from either origin when supplemented with the gratuitous lacZ inducer IPTG. Under the conditions employed here, plasmid-borne FPR expression levels were 5- to 10-fold greater than those observed upon maximal induction of the indigenous fpr gene in MV-treated cells (Fig. 2A) and were accompanied by a concomitant increase in FPR-dependent activities (Table 2).
The extent of SoxR activation in the presence of 100 μM MV (as monitored by the β-galactosidase activity assay) was significantly higher in cells expressing a recombinant FPR relative to those transformed with the supporting vector (Fig. 2B). Even in the absence of the herbicide, FPR expression led to a major increase of SoxS-dependent activities, from 165 ± 20 to 321 ± 48 β-galactosidase units. Similar results were obtained irrespective of whether the pea (Fig. 2B) or the E. coli (Table 2) reductase isoforms were used. As expected, MV removal resulted in a rapid decline of the activated state of SoxR (Fig. 2B). Although cells overexpressing FPR displayed consistently higher β-galactosidase activities compared to those transformed with pSU supporting plasmids, the kinetics of soxRS deactivation were identical in the two types of bacteria (Fig. 2B, inset).
The effect of decreasing FPR concentrations below physiological levels was also investigated by using the fpr mutant strain RR6A (Table 1) and a soxS"::lacZ operon fusion encoded by a multicopy plasmid (31). Neither the extent nor the time course of the soxRS response elicited by MV was significantly affected by FPR inactivation in the mutant cells compared to FPR-proficient bacteria (data not shown). Similar results had been obtained by P. Gaudu and D. Touati (unpublished data), who studied the MV-dependent induction of a sodA"::lacZ operon fusion in FPR-deficient cells. Moreover, Demple and coworkers also failed to observe changes in soxRS activation in both fpr and fnr E. coli strains compared to the corresponding parentals (E. Hidalgo, B. Gonzalez Flecha, and B. Demple, unpublished data).
FPR modulates NADP(H) levels during the soxRS response of E. coli.
The results summarized in the previous section indicate that FPR overproduction stimulates the induction of the soxRS system. It is unlikely, however, that this effect could involve direct participation of the reductase in SoxR oxidation. An alternative possibility is that FPR could modulate the soxRS response through indirect mechanisms, namely, by regulating the accumulation of electron donors in the bacterial cytosol. The inhibitory effect of NADPH (but not of NADH) on SoxR activation has been recognized (13). To evaluate this possibility, we probed the effect of a site-directed mutant version of pea FPR that displays a shift in substrate preference from NADP(H) to NAD(H) (Fig. 3A [see also reference 35]). The expression of this mutant FPR in wild-type E. coli cells did not affect the magnitude (Table 2) or the time course (data not shown) of soxRS induction by the herbicide. Moreover, the NAD(H)-dependent flavoenzyme provided no additional protection to fpr mutant bacteria exposed to MV toxicity (Fig. 3B).
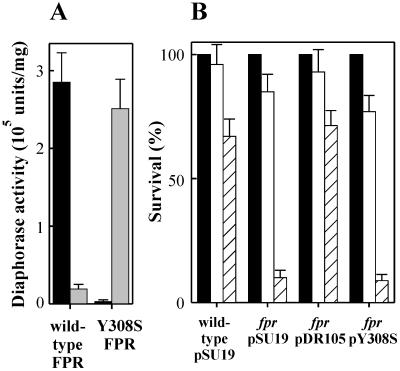
FIG.3.
A site-directed NAD(H)-dependent ferredoxin-NADP(H) reductase mutant does not complement the fpr deficiency in E. coli. (A) NADP(H)-dependent (solid bars) and NAD(H)-dependent (shaded bars) diaphorase activities of purified wild-type and Y308S FPR, assayed with potassium ferricyanide as an electron acceptor (32). The Y308S FPR mutant, in which the carboxyl-terminal tyrosine residue was replaced by a serine, was designed and purified as reported elsewhere (32, 35). (B) Survival of fpr mutant bacteria expressing NADP(H)- or NAD(H)-dependent FPR versions to MV toxicity. Parental cells from strain GC4468 were transformed with pSU19, whereas bacteria from the fpr mutant genotype RR6A were transformed with pSU19 or with the recombinant plasmids pDR105 or pY308S harboring the wild-type or Y308S mutant FPR genes, respectively. Plating experiments were carried out as described in the legend to Fig. 1A, with the addition of 0.5 mM IPTG and either no (solid bars), 50 μM (open bars), or 100 μM (hatched bars) MV added to the agar broth. The heights of the bars represent the averages of three experiments ± the SE.
In the absence of oxidants, 80 to 85% of the NADP(H) pool of the wild-type E. coli cells was in the reduced state (Table 2). Disruption of the fpr gene resulted in a moderate increase of the NADPH/NADP+ ratio (Table 2), reflecting a minor contribution of FPR to NADP(H) homeostasis in unstressed E. coli cells. The observed effect, albeit small, supports the notion that this flavoenzyme behaves as an NADPH consumer in the E. coli cytosol (22). Accordingly, overexpression of FPR in an otherwise wild-type background resulted in a significant decline of the cytosolic NADPH pool (Table 2). Exposure of the cells to MV resulted in a further decrease of NADPH relative to NADP+ in all strains assayed, with the FPR-producing bacteria displaying the lowest levels (Table 2). As could be anticipated, expression of the NADH-consuming Y308S FPR version had little or no effect on the redox state of the NADP(H) pool (Table 2).
Concluding remarks.
Based on the ability of FPR to mediate rapid electron transfer from NADPH to different types of acceptors, several authors have suggested that the FPR antioxidant role could be related to its participation in a putative redox pathway leading to reductive reconstitution of O2−·-damaged hydrolyases (10, 24) or to reductive deactivation of the sensor protein SoxR (27, 42, 43). Although either of the two mechanisms could explain the need for FPR recruitment during the soxRS adaptive response, our results argue against both possibilities. Indeed, growth arrest and hydrolyase inactivation did not contribute significantly to the MV sensitivity of fpr mutant bacteria (Fig. 1). This behavior contrasts with that exhibited by a conditional yeast mutant with reduced FPR activity, which developed a complex syndrome including hydrolyase inactivation and abnormal iron trafficking (23). On the other hand, FPR did interfere with the SoxR reduction pathway but not in the way predicted originally. Accumulation of recombinant reductase above MV-induced levels led to stimulation of the soxRS response (Fig. 2) rather than to the depression that could have been expected from FPR involvement in SoxR reduction and/or inactivation (27, 42).
In general, FPR's protective effects were linked to its NADP(H)-dependent activities. Predictable changes in the redox state of the NAD(H) pool resulting from overproduction of an NADH-oxidizing FPR mutant had no effect on either soxRS induction or MV tolerance (Table 2, Fig. 3). The ability of the flavoenzyme to reduce its physiological substrates ferredoxin or flavodoxin also seems to be dispensable for its protective action (22). The question remains open as to how the FPR-mediated oxidation of NADPH, by using whatever electron acceptor is available, relates to its well-documented antioxidant role. One possible mechanism is proposed in Fig. 4. FPR induction at the onset of the soxRS response might be instrumental to keep NADPH at tolerable levels during the progress of the stress condition. The potential toxicity of NADPH (and NADH) derives from its ability to rapidly reduce iron and other transition metals required for the synthesis of highly toxic hydroxyl radicals through Fenton-type chemistry (Fig. 4). As early as 1988, Imlay and Linn (17) proposed that cells might deliberately deplete the pyridine nucleotide pool to impair production of Fenton-class radicals. The identity of the electron donor(s) driving iron reduction in vivo remains unclear but noteworthy NADPH concentrations may rise to 150 μM in the E. coli cytosol, compared to <20 μM NADH (33). It should be kept in mind, however, that other possible protective mechanisms cannot be ruled out by these experiments and that further research will be required to evaluate the general validity of this proposal.
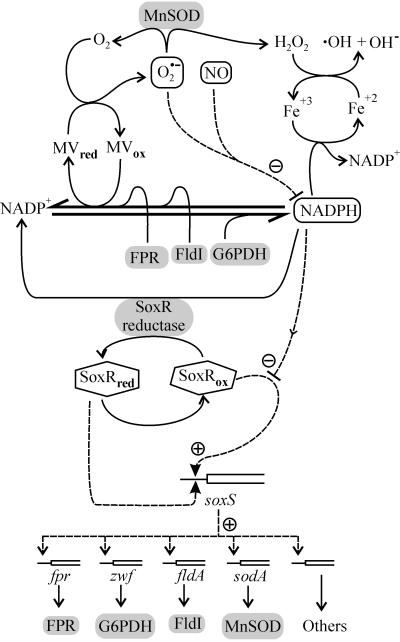
FIG. 4.
Integrated model for FPR participation in the soxRS response of E. coli. At least part of the FPR protective action is proposed to be related to its ability to oxidize the NADPH pool, since NADPH accumulation during the oxidative stress condition might favor propagation of active oxygen species such as · OH through the reduction of Fe3+ and other transition metals (17). FPR is not required for regulation of the soxRS response, although depletion of the electron source might contribute to SoxR activation (see below). Under conditions of normal aerobic growth, a reductive pathway presumably involving a SoxR reductase (20) maintains the SoxR dimer in a reduced inactive state (SoxRred). During oxidative stress, the dimer is converted into the transcriptionally active form with oxidized iron-sulfur centers (SoxRox). Both forms of SoxR bind to the soxS promoter, but only the interaction of SoxRox stimulates transcription (arrow with plus symbol). SoxS then activates expression of the other members of the regulon, including FPR, glucose-6-phosphate dehydrogenase (G6PDH), flavodoxin I (FldI), and Mn-dependent SOD. The balance between SoxRox and SoxRred will be biased toward SoxR oxidation whenever electrons are drained from the cellular pool of reduced metabolites. Factors affecting this balance comprise inactivation of key enzymes involved in NADPH synthesis (G6PDH) and the accumulation of chemicals or proteins causing the oxidation or destruction of reduced metabolites. The latter class includes oxygen-centered radicals such as superoxide or nitric oxide, redox cycling compounds such as MV, and electron acceptor proteins and enzymes such as FldI, desulfoferrodoxin, or FPR.
The response of the soxRS regulon to abnormal accumulation of NADPH-consuming FPR illustrates further the complex nature of this adaptive system, whose general features are also described schematically in Fig. 4. A common property of many soxRS inducers, including nitric oxide, redox cycling compounds, and even superoxide itself, is that they can deplete the cellular pool of reduced metabolites. This led Liochev and Fridovich (26) to postulate that SoxR might be actually sensing changes in the redox status of the cell. In vitro, both NADPH (13) and GSH (6) are able to inhibit SoxR activity. Thus, even though specific mechanisms such as SoxR nitrosylation may be operating under certain circumstances (8), many oxidants could trigger induction of the regulon simply by draining the cellular electron source. The accumulation of flavodoxin I, one of the FPR physiological substrate acceptors and a recently added member of the soxRS system, is indeed reported to cause enhanced induction of the regulon upon MV treatment of the bacterial host (43). Similar results have been obtained when E. coli cells were transformed with a plasmid expressing desulfoferrodoxin from sulfate-reducing bacteria, whose accumulation triggers the soxRS response presumably by impairing SoxR reduction (14, 34). A comparable stimulation of the regulon was also observed in zwf cells, deficient in the SoxS-dependent NADPH producer glucose-6-phosphate dehydrogenase (14, 26). The molecular events involved in that type of SoxR sensing are most probably related to faulty reduction of the sensor protein rather than to SoxR oxidation itself. The sensitivity of the soxRS response to multiple signals most certainly confers evolutionary advantages to cells thriving in changing media by allowing the system to adapt to a number of environmental and physiological challenges other than oxidative stress.
Acknowledgments
This research was supported by grant BID 802/0C-AR from the National Research Agency (ANPCyT, Argentina). A.R.K., H.O.P., and N.C. are members of the National Research Council (CONICET, Argentina); R.E.R. and J.F.P. are fellows of the same institution.
We thank several colleagues for the generous gift of plasmids and strains: D. Rial and E. A. Ceccarelli (IBR Rosario, Argentina) for pDR105, B. Demple (Harvard School of Public Health, Boston, Mass.) for pTN1530; E. Haggård-Ljungquist (Stockholm University, Sweden) for plasmid pEE1010 and strain C-6007; D. Touati (Institute Jacques Monod, Paris, France) for strains GC4468 and QC772; and P. Miller and J. Liu (Parke-Davis Pharmaceutical Research, Ann Arbor, Mich.) for strain B247. We also thank D. de Mendoza (Instituto de Biolog|$$|Aa|fia Molecular y Celular, Rosario, Argentina) and D. Touati and B. Demple for helpful suggestions and critical reading of the manuscript.
REFERENCES
- 1.Arakaki, A. K., E. A. Ceccarelli, and N. Carrillo. 1997. Plant-type ferredoxin-NADP+ reductases: a basal structural framework and a multiplicity of functions. FASEB J. 11:133-140. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular cloning. John Wiley & Sons, Inc., New York, N.Y.
- 3.Bianchi, V., E. Haggard-Ljungquist, E. Pontis, and P. Reichard. 1995. Interruption of the ferredoxin (flavodoxin) NADP+ oxidoreductase gene of Escherichia coli does not affect anaerobic growth but increases sensitivity to paraquat. J. Bacteriol. 177:4528-4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlioz, A., and D. Touati. 1986. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 5:623-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceccarelli, E. A., A. M. Viale, A. R. Krapp, and N. Carrillo. 1991. Expression, assembly, and processing of an active plant ferredoxin-NADP+ oxidoreductase and its precursor protein in Escherichia coli. J. Biol. Chem. 266:14283-14287. [PubMed] [Google Scholar]
- 6.Ding, H., and B. Demple. 1996. Glutathione-mediated destabilization in vitro of [2Fe-2S] centers in the SoxR regulatory protein. Proc. Natl. Acad. Sci. USA 93:9449-9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding, H., and B. Demple. 1997. In vivo kinetics of a redox-regulated transcriptional switch. Proc. Natl. Acad. Sci. USA 94:8445-8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding, H., and B. Demple. 2000. Direct nitric oxide signal transduction via nitrosylation of iron-sulfur centers in the SoxR transcription activator. Proc. Natl. Acad. Sci. USA 97:5146-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flint, D. H., J. F. Tuminello, and M. H. Emptage. 1993. The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J. Biol. Chem. 268:22369-22376. [PubMed] [Google Scholar]
- 10.Fridovich, I. 1997. Superoxide anion radical (O2−· ), superoxide dismutases, and related matters. J. Biol. Chem. 272:18515-18517. [DOI] [PubMed] [Google Scholar]
- 11.Gardner, P. R., and I. Fridovich. 1991. Superoxide sensitivity of the Escherichia coli 6-phosphogluconate dehydratase. J. Biol. Chem. 266:1478-1483. [PubMed] [Google Scholar]
- 12.Gardner, P. R., and I. Fridovich. 1992. Inactivation-reactivation of aconitase in Escherichia coli: a sensitive measure of superoxide radical. J. Biol. Chem. 267:8757-8763. [PubMed] [Google Scholar]
- 13.Gardner, P. R., and I. Fridovich. 1993. NADPH inhibits transcription of the Escherichia coli manganese superoxide dismutase gene (sodA) in vitro. J. Biol. Chem. 268:12958-12963. [PubMed] [Google Scholar]
- 14.Gaudu, P., S. Dubrac, and D. Touati. 2000. Activation of SoxR by overproduction of desulfoferrodoxin: multiple ways to induce the soxRS regulon. J. Bacteriol. 182:1761-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaudu, P., N. Moon, and B. Weiss. 1997. Regulation of the soxRS oxidative stress regulon. Reversible oxidation of the Fe-S centers of SoxR in vivo. J. Biol. Chem. 272:5082-5086. [DOI] [PubMed] [Google Scholar]
- 16.Hopkin, K. A., M. A. Papazian, and H. M. Steinman. 1992. Functional differences between manganese and iron superoxide dismutases in Escherichia coli K-12. J. Biol. Chem. 267:24253-24258. [PubMed] [Google Scholar]
- 17.Imlay, J. A., and S. Linn. 1988. DNA damage and oxygen radical toxicity. Science 240:1302-1309. [DOI] [PubMed] [Google Scholar]
- 18.Keyer, K., A. S. Gort, and J. A. Imlay. 1995. Superoxide and the production of oxidative DNA damage. J. Bacteriol. 177:6782-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitzler, J., and I. Fridovich. 1986. The effects of paraquat on Escherichia coli: distinction between bacteriostasis and lethality. J. Free Radic. Biol. Med. 2:245-248. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi, K., and S. Tagawa. 1999. Isolation of reductase for SoxR that governs an oxidative response regulon from Escherichia coli. FEBS Lett. 451:227-230. [DOI] [PubMed] [Google Scholar]
- 21.Krapp, A. R., and N. Carrillo. 1995. Functional complementation of the mvrA mutation of Escherichia coli by plant ferredoxin-NADP+ oxidoreductase. Arch. Biochem. Biophys. 317:215-221. [DOI] [PubMed] [Google Scholar]
- 22.Krapp, A. R., V. B. Tognetti, N. Carrillo, and A. Acevedo. 1997. The role of ferredoxin-NADP+ reductase in the concerted cell defense against oxidative damage--studies using Escherichia coli mutants and cloned plant genes. Eur. J. Biochem. 249:556-563. [DOI] [PubMed] [Google Scholar]
- 23.Li, J., S. Saxena, D. Pain, and A. Dancis. 2001. Adrenodoxin reductase homolog (Arh1p) of yeast mitochondria required for iron homeostasis. J. Biol. Chem. 276:1503-1509. [DOI] [PubMed] [Google Scholar]
- 24.Li, Z., and B. Demple. 1996. Sequence specificity for DNA binding by Escherichia coli SoxS and Rob proteins. Mol. Microbiol. 20:937-945. [DOI] [PubMed] [Google Scholar]
- 25.Liochev, S. I., L. Benov, D. Touati, and I. Fridovich. 1999. Induction of the soxRS regulon of Escherichia coli by superoxide. J. Biol. Chem. 274:9479-9481. [DOI] [PubMed] [Google Scholar]
- 26.Liochev, S. I., and I. Fridovich. 1992. Fumarase C, the stable fumarase of Escherichia coli, is controlled by the soxRS regulon. Proc. Natl. Acad. Sci. USA 89:5892-5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liochev, S. I., A. Hausladen, W. F. Beyer, Jr., and I. Fridovich. 1994. NADPH: ferredoxin oxidoreductase acts as a paraquat diaphorase and is a member of the soxRS regulon. Proc. Natl. Acad. Sci. USA 91:1328-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual for E. coli and related bacteria, p. 268-274. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 29.Miller, P. F., L. F. Gambino, M. C. Sulavik, and S. J. Gracheck. 1994. Genetic relationship between soxRS and mar loci in promoting multiple antibiotic resistance in Escherichia coli. Antimicrob. Agents Chemother. 38:1773-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minakami, H., and I. Fridovich. 1990. Relationship between growth of Escherichia coli and susceptibility to the lethal effect of paraquat. FASEB J. 4:3239-3244. [DOI] [PubMed] [Google Scholar]
- 31.Nunoshiba, T., E. Hidalgo, C. F. Amabile Cuevas, and B. Demple. 1992. Two-stage control of an oxidative stress regulon: the Escherichia coli SoxR protein triggers redox-inducible expression of the soxS regulatory gene. J. Bacteriol. 174:6054-6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orellano, E. G., N. B. Calcaterra, N. Carrillo, and E. A. Ceccarelli. 1993. Probing the role of the carboxyl-terminal region of ferredoxin-NADP+ reductase by site-directed mutagenesis and deletion analysis. J. Biol. Chem. 268:19267-19273. [PubMed] [Google Scholar]
- 33.Penfound, T., and J. W. Foster. 1996. Biosynthesis and recycling of NAD, p. 721-730. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 34.Pianzzola, M. J., M. Soubes, and D. Touati. 1996. Overproduction of the rbo gene product from Desulfovibrio species suppresses all deleterious effects of lack of superoxide dismutase in Escherichia coli. J. Bacteriol. 178:6736-6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piubelli, L., A. Aliverti, A. K. Arakaki, N. Carrillo, E. A. Ceccarelli, P. A. Karplus, and G. Zanetti. 2000. Competition between C-terminal tyrosine and nicotinamide modulates pyridine nucleotide affinity and specificity in plant ferredoxin-NADP+ reductase. J. Biol. Chem. 275:10472-10476. [DOI] [PubMed] [Google Scholar]
- 36.Pomposiello, P. J., M. H. Bennik, and B. Demple. 2001. Genome-wide transcriptional profiling of the Escherichia coli responses to superoxide stress and sodium salicylate. J. Bacteriol. 183:3890-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pomposiello, P. J., and B. Demple. 2001. Redox-operated genetic switches: the SoxR and OxyR transcription factors. Trends Biotechnol. 19:109-114. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez, R. E., A. R. Krapp, and N. Carrillo. 1998. The mvrA locus of Escherichia coli does not encode a ferredoxin-NADP+ reductase. Microbiology 144:2375-2376. [DOI] [PubMed] [Google Scholar]
- 39.Sedmak, J. J., and S. E. Grossberg. 1977. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal. Biochem. 79:544-552. [DOI] [PubMed] [Google Scholar]
- 40.Slater, T. F., and B. Sawyer. 1962. A colorimetric method for estimating the pyridine nucleotide content of small amounts of animal tissue. Nature 193:454-456. [DOI] [PubMed] [Google Scholar]
- 41.Wu, J., and B. Weiss. 1992. Two-stage induction of the soxRS (superoxide response) regulon of Escherichia coli. J. Bacteriol. 174:3915-3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yannone, S. M., and B. K. Burguess. 2001. The seven-iron FdI from Azotobacter vinelandii regulates the expression of NADPH-ferredoxin reductase via an oxidative stress response. J. Biol. Inorg. Chem. 3:253-258. [Google Scholar]
- 43.Zheng, M., B. Doan, T. D. Schneider, and G. Storz. 1999. OxyR and SoxRS regulation of fur. J. Bacteriol. 181:4639-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]