Abstract
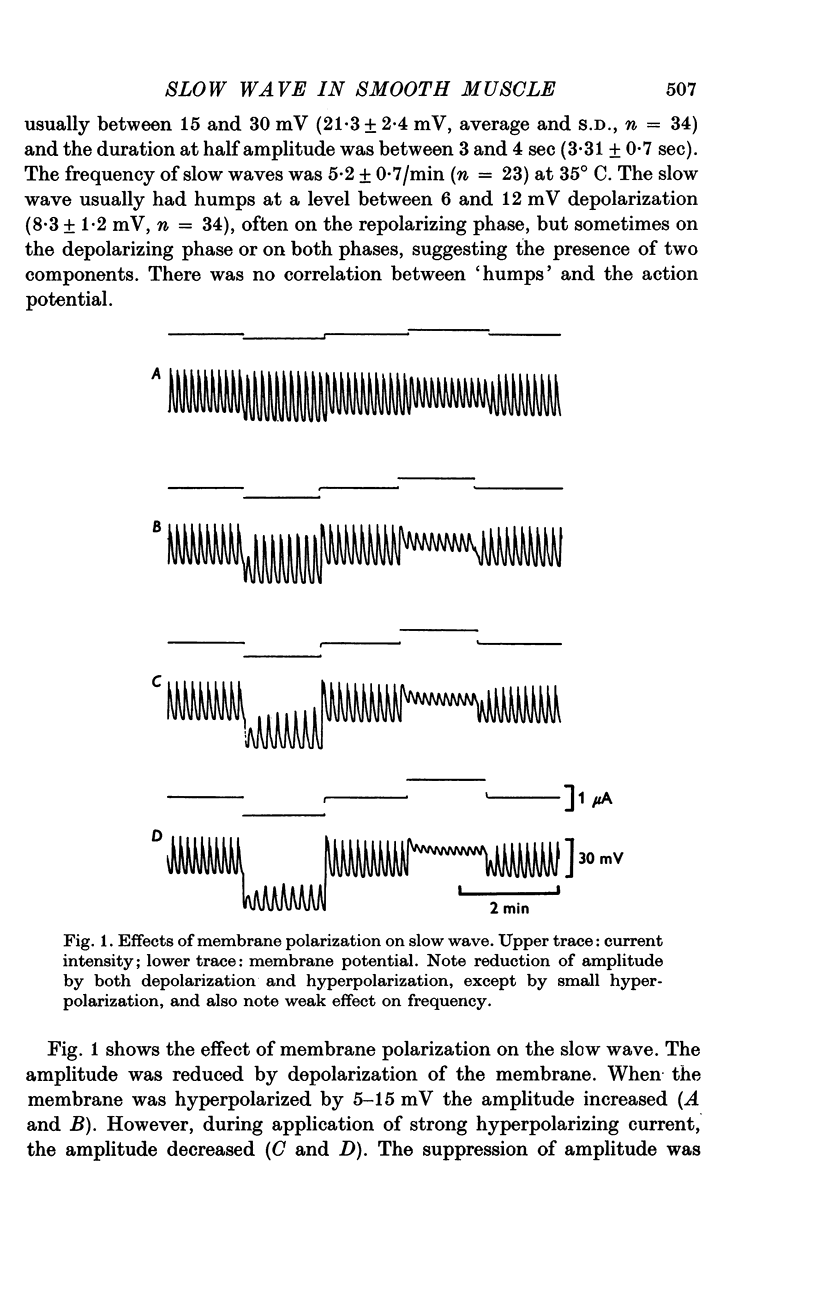
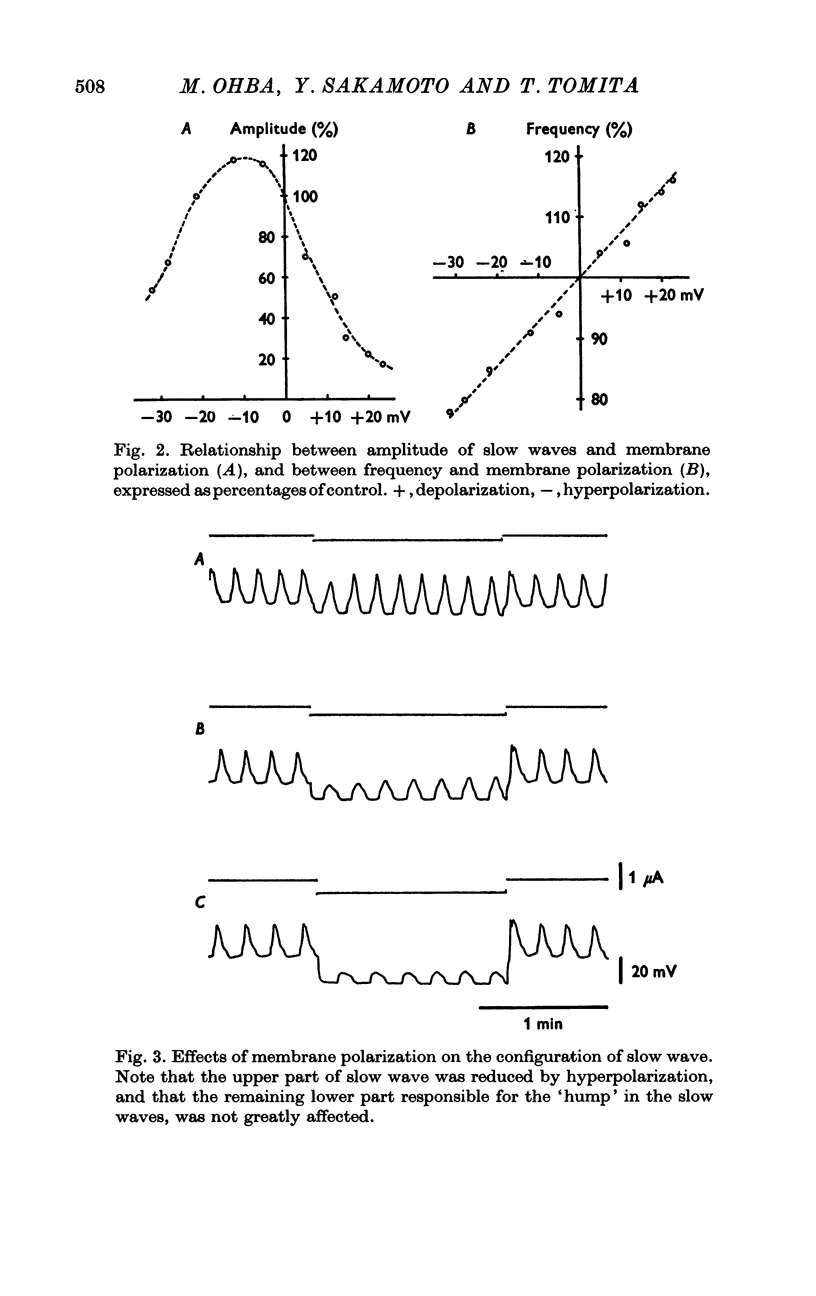
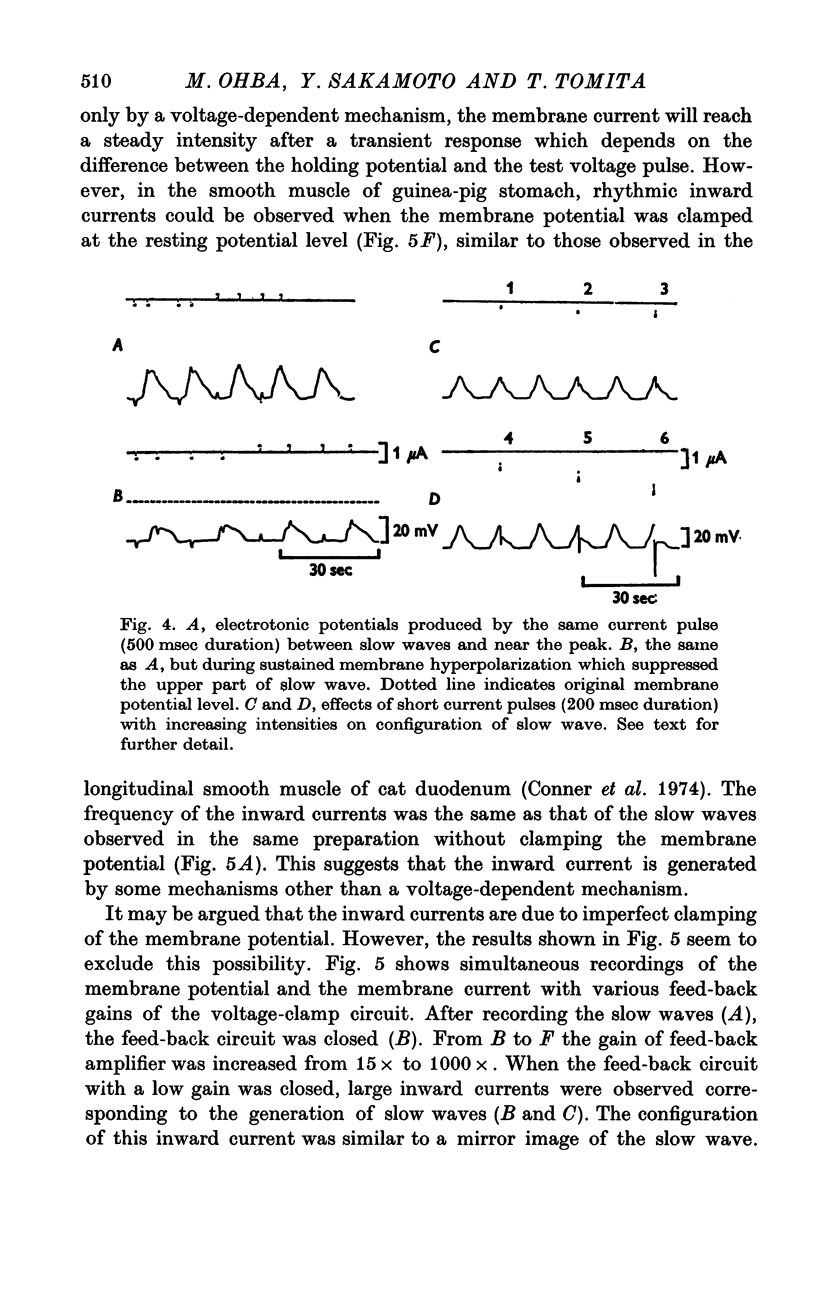
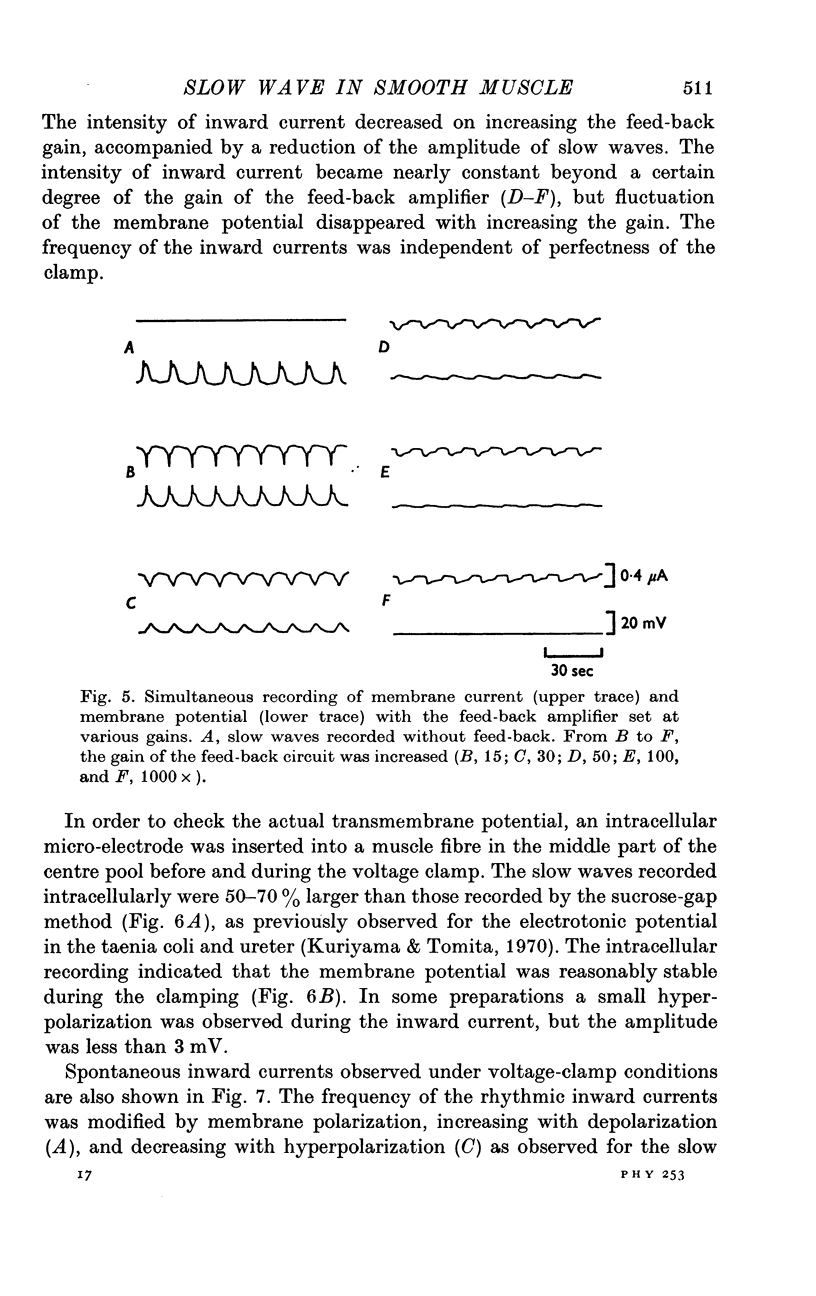
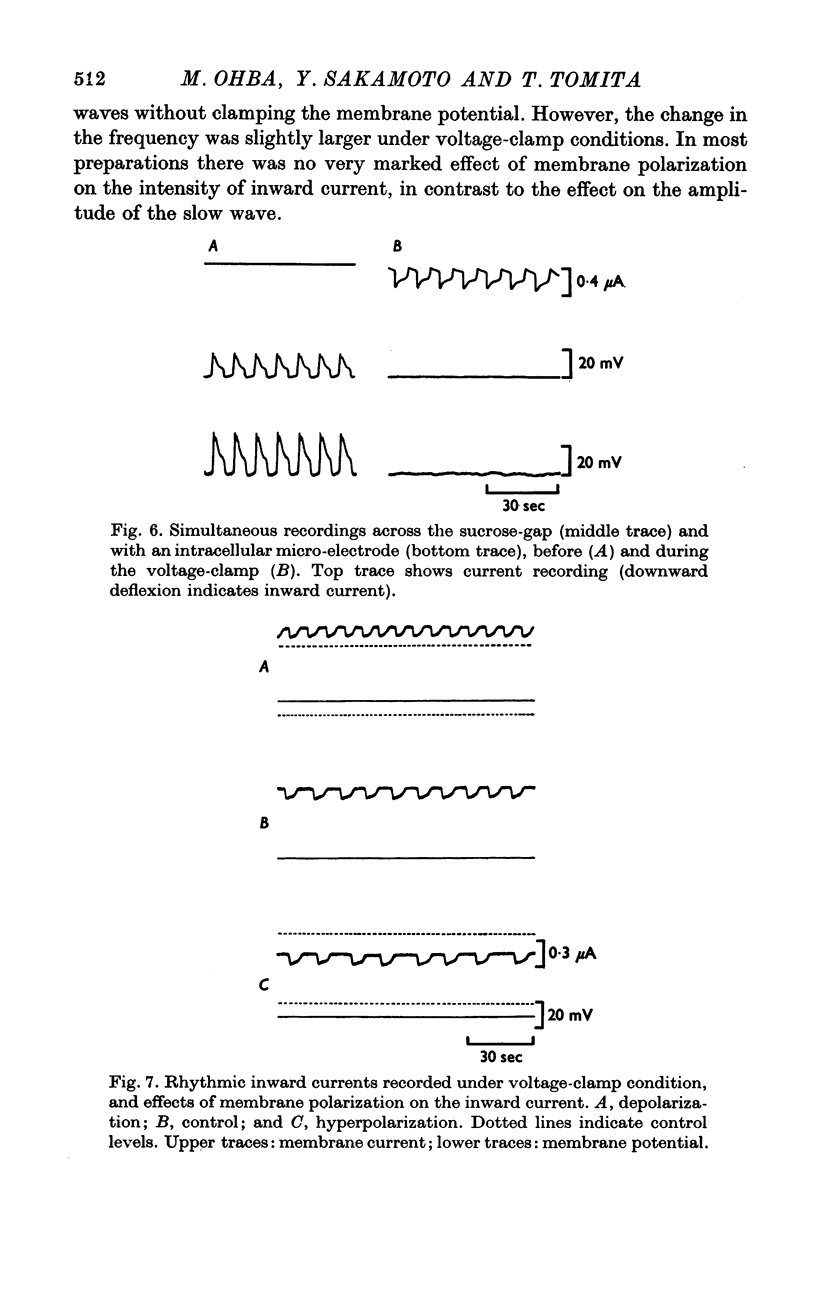
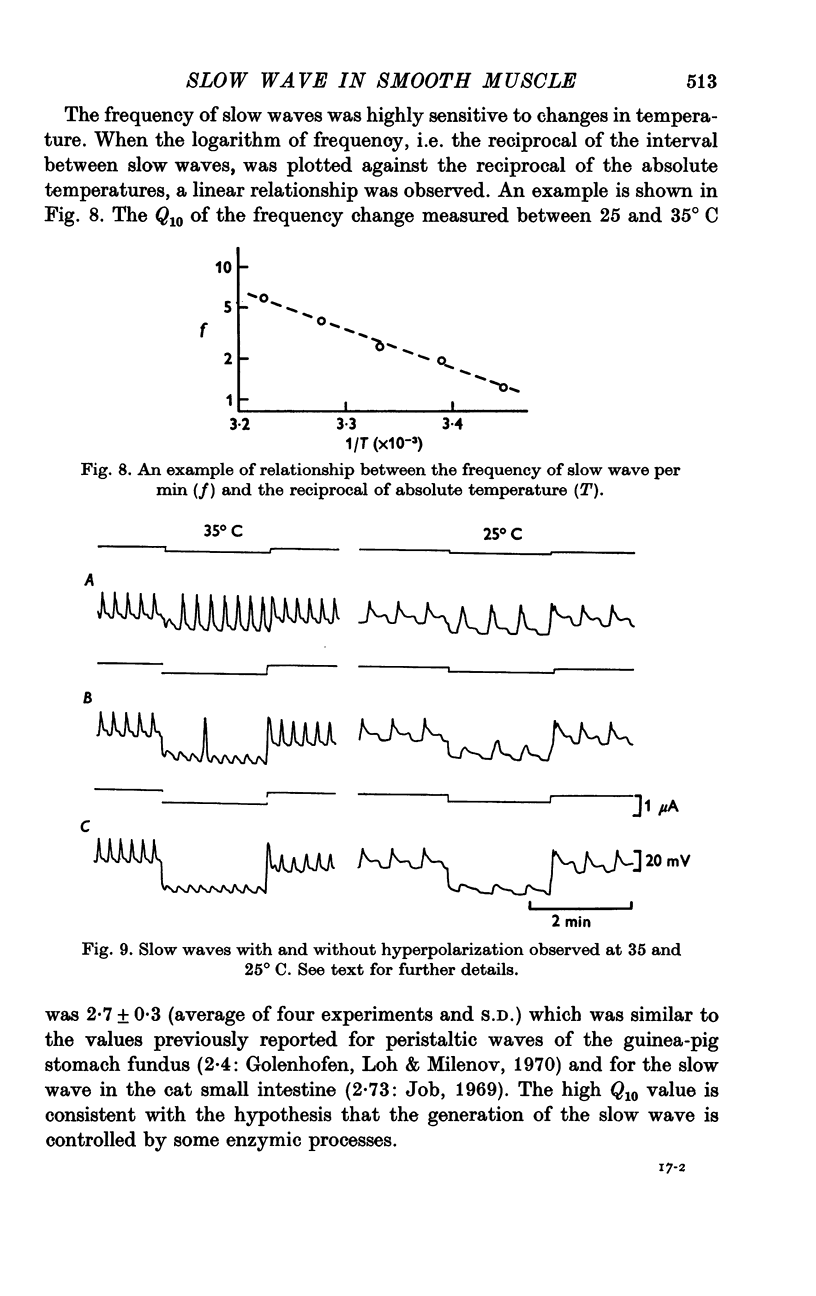
The slow wave in the circular muscle of guinea-pig stomach was investigated with the double sucrose-gap method. 2. The amplitude of the slow wave was reduced by depolarization, and it was increased by a small hyperpolarization (5-10 mV). With hyperpolarization greater than 15 mV the amplitude decreased, and the slow wave became reduced, and less dependent on polarization. This residual was not abolished by strong hyperpolarizing current pulses. 3. The frequency of the slow waves was not much affected by membrane polarization. The change was only 15-20% by depolarization or hyperpolarization of 12mV. 4. Rythmic inward currents could be recorded under voltage-clamp conditions. The frequency of the inward currents was the same as that of the slow wave. The intensity of inward current was little affected by membrane polarization. 5. Lowering the temperature reduced the frequency of the slow wave. The rates of rise and fall of the component which remained during strong hyperpolarization were similarly decreased by lowering the temperature. The Q10 of the frequency was about 2-7. 6. It is suggested that the slow wave consists of two different components. One is generated by a potential independent process, and triggers the second component which is potential dependent. The first component may be controlled by some metabolic process.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. C., Jr Voltage-clamp studies on uterine smooth muscle. J Gen Physiol. 1969 Aug;54(2):145–165. doi: 10.1085/jgp.54.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoff A. Digestion: motility. Annu Rev Physiol. 1972;34:261–290. doi: 10.1146/annurev.ph.34.030172.001401. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Prosser C. L., Weems W. A. A study of pace-maker activity in intestinal smooth muscle. J Physiol. 1974 Aug;240(3):671–701. doi: 10.1113/jphysiol.1974.sp010629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golenhofen K., Lammel E. Selective suppression of some components of spontaneous activity in various types of smooth muscle by iproveratril (Verapamil). Pflugers Arch. 1972;331(3):233–243. [PubMed] [Google Scholar]
- Golenhofen K., von Loh D., Milenov K. Elektrophysiologische Untersuchungen zur Spontanaktivität isolierter Muskelpräparate aus verschiedenen Abschnitten des Meerschweinchen-Magens. Pflugers Arch. 1970;315(4):336–356. doi: 10.1007/BF00593460. [DOI] [PubMed] [Google Scholar]
- Job D. D. Ionic basis of intestinal electrical activity. Am J Physiol. 1969 Nov;217(5):1534–1541. doi: 10.1152/ajplegacy.1969.217.5.1534. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Osa T., Tasaki H. Electrophysiological studies of the antrum muscle fibers of the guinea pig stomach. J Gen Physiol. 1970 Jan;55(1):48–62. doi: 10.1085/jgp.55.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Tomita T. The action potential in the smooth muscle of the guinea pig taenia coli and ureter studied by the double sucrose-gap method. J Gen Physiol. 1970 Feb;55(2):147–162. doi: 10.1085/jgp.55.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magaribuchi T., Obu T., Sakamoto Y., Yamamoto Y. Some electrical properties of the slow potential changes recorded from the guinea pig stomach in relation to drug actions. Jpn J Physiol. 1972 Jun;22(3):333–352. doi: 10.2170/jjphysiol.22.333. [DOI] [PubMed] [Google Scholar]
- Mills R. G., Taylor G. S. Studies of intestinal slow wave activity with a double sucrose gap apparatus. Life Sci I. 1971 Mar 15;10(6):347–353. doi: 10.1016/0024-3205(71)90134-2. [DOI] [PubMed] [Google Scholar]
- Prosser C. L. Smooth muscle. Annu Rev Physiol. 1974;36:503–535. doi: 10.1146/annurev.ph.36.030174.002443. [DOI] [PubMed] [Google Scholar]
- Tomita T., Watanabe H. Factors controlling myogenic activity in smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):73–85. doi: 10.1098/rstb.1973.0010. [DOI] [PubMed] [Google Scholar]


