Abstract
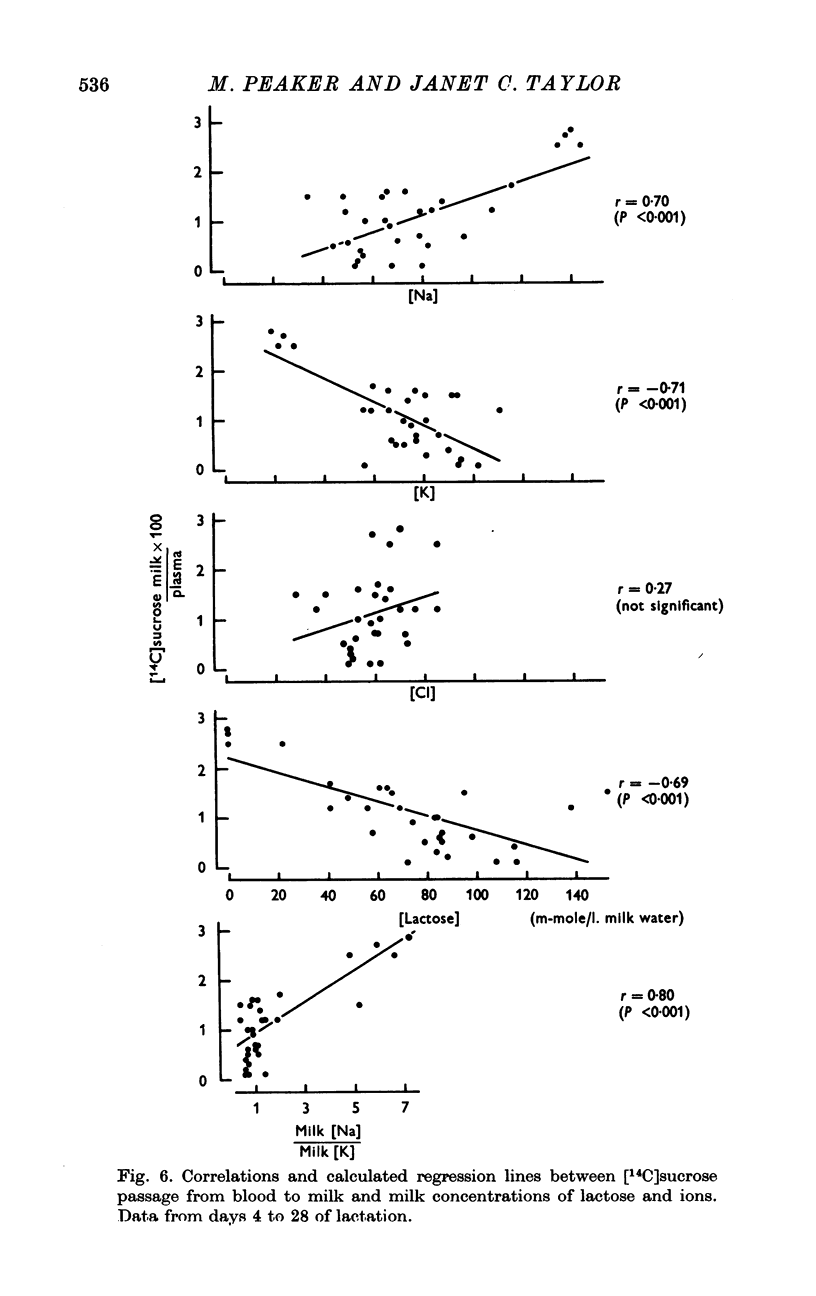
Changes in the yield and composition of milk and in the permeability of the mammary epithelium to labelled disaccharides and ions have been studied during lactation in rabbits of the Dutch breed. 2. Milk yield increased to reach a peak on day 20 of lactation and then declined, but by 30-32 days the yield was still relatively high. Milk [protein] and [fat] increased in late lactation; [Na] and [Cl] decreased from early to established lactation (11-14 days) and then increased, whereas milk [K] and [lactose] showed an inverse pattern to that displayed by[Na] and [Cl]. 3. At all stages of lactation [Na] and [Cl] were both inversely related to milk [lactose] while [K] showed a positive correlation. 4. Labelled lactose and sucrose were found to cross the mammary epithelium at all stages but in increased amounts during late lactation. Sucrose entry from blood into milk was positively correlated with milk [Na], and inversely correlated with [K] and [lactose]. 5. The entry of (24)Na and (36)Cl into milk from blood paralleled the changes in milk [Na] and [Cl]. 6. Intracellular ionic composition determined in vitro was similar for [Na] and [K] in both established (11-14 days) and late (25-28 days) lactation, but [Cl] was higher in late lactation. 7. Intracellular potentials recorded in vivo were -31 mV (mean) and -36 mV in established and late lactation respectively. Transepithelial p.d. was close to zero at both stages. 8. It is suggested that ions and lactose and other small molecules can cross the mammary epithelium by a paracellular, as well as by a transcellular route, throughout lactation and that the paracellular pathway is increased in late lactation. 9. The site of the proposed paracellular pathway and the implications of such a pathway's presence on ion transport are discussed, and a scheme is suggested to account for the ionic composition of milk in this species.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CROSS B. A., HARRIS G. W. The role of the neurohypophysis in the milk-ejection reflex. J Endocrinol. 1952 Apr;8(2):148–161. doi: 10.1677/joe.0.0080148. [DOI] [PubMed] [Google Scholar]
- Cowie A. T. Variations in the yield and composition of the milk during lactation in the rabbit and the galactopoietic effect of prolactin. J Endocrinol. 1969 Jul;44(3):437–450. doi: 10.1677/joe.0.0440437. [DOI] [PubMed] [Google Scholar]
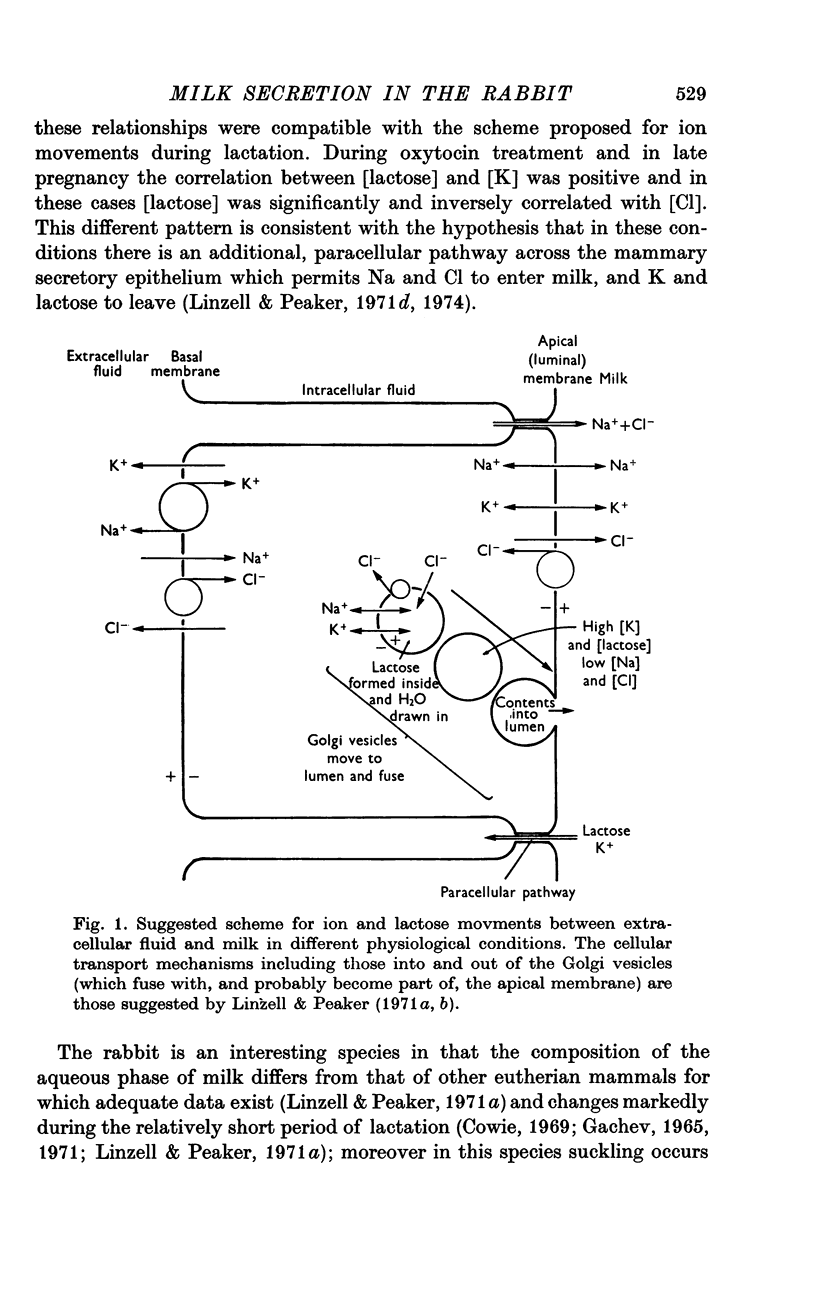
- Fleet I. R., Linzell J. L., Peaker M. The use of an autoanalyzer for the rapid analysis of milk constituents affected by subclinical mastitis. Br Vet J. 1972 Jun;128(6):297–300. doi: 10.1016/s0007-1935(17)36934-8. [DOI] [PubMed] [Google Scholar]
- Frömter E., Diamond J. Route of passive ion permeation in epithelia. Nat New Biol. 1972 Jan 5;235(53):9–13. doi: 10.1038/newbio235009a0. [DOI] [PubMed] [Google Scholar]
- Hagemeijer F., Rorive G., Schoffeniels E. The ionic composition of rat aortic smooth muscle fibres. Arch Int Physiol Biochim. 1965 Jun;73(3):453–475. doi: 10.3109/13813456509081857. [DOI] [PubMed] [Google Scholar]
- JENNESS R., REGEHR E. A., SLOAN R. E. COMPARATIVE BIOCHEMICAL STUDIES OF MILK. II. DIALYZABLE CARBOHYDRATES. Comp Biochem Physiol. 1964 Dec;13:339–352. doi: 10.1016/0010-406x(64)90028-3. [DOI] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. Changes in colostrum composition and in the permeability of the mammary epithelium at about the time of parturition in the goat. J Physiol. 1974 Nov;243(1):129–151. doi: 10.1113/jphysiol.1974.sp010746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. Intracellular concentrations of sodium, potassium and chloride in the lactating mammary gland and their relation to the secretory mechanism. J Physiol. 1971 Aug;216(3):683–700. doi: 10.1113/jphysiol.1971.sp009547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. Mechanism of milk secretion. Physiol Rev. 1971 Jul;51(3):564–597. doi: 10.1152/physrev.1971.51.3.564. [DOI] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M., Taylor J. C. The effects of prolactin and oxytocin on milk secretion and on the permeability of the mammary epithelium in the rabbit. J Physiol. 1975 Dec;253(2):547–563. doi: 10.1113/jphysiol.1975.sp011206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. The distribution and movements of carbon dioxide, carbonic acid and bicarbonate between blood and milk in the goat. J Physiol. 1975 Jan;244(3):771–782. doi: 10.1113/jphysiol.1975.sp010825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. The effects of oxytocin and milk removal on milk secretion in the goat. J Physiol. 1971 Aug;216(3):717–734. doi: 10.1113/jphysiol.1971.sp009549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. The permeability of mammary ducts. J Physiol. 1971 Aug;216(3):701–716. doi: 10.1113/jphysiol.1971.sp009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaker M. Intracellular concentrations of sodium, potassium and chloride in the salt-gland of the domestic goose and their relation to the secretory mechanism. J Physiol. 1971 Mar;213(2):399–410. doi: 10.1113/jphysiol.1971.sp009389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaker M., Linzell J. L. The effects of oestrus and exogenous oestrogens on milk secretion in the goat. J Endocrinol. 1974 May;61(2):231–240. doi: 10.1677/joe.0.0610231. [DOI] [PubMed] [Google Scholar]
- Peaker M., Phillips J. G., Wright A. The effect of prolactin on the secretory activity of the nasal salt-gland of the domestic duck (Anas platyrhynchos). J Endocrinol. 1970 May;47(1):123–127. doi: 10.1677/joe.0.0470123. [DOI] [PubMed] [Google Scholar]
- Pitelka D. R., Hamamoto S. T., Duafala J. G., Nemanic M. K. Cell contacts in the mouse mammary gland. I. Normal gland in postnatal development and the secretory cycle. J Cell Biol. 1973 Mar;56(3):797–818. doi: 10.1083/jcb.56.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G. Electrical potential differences and electromotive forces in epithelial tissues. J Gen Physiol. 1972 Jun;59(6):794–798. doi: 10.1085/jgp.59.6.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UDY D. C. A rapid method for estimating total protein in milk. Nature. 1956 Aug 11;178(4528):314–315. doi: 10.1038/178314a0. [DOI] [PubMed] [Google Scholar]
- Zarrow M. X., Denenberg V. H., Anderson C. O. Rabbit: frequency of suckling in the pup. Science. 1965 Dec 31;150(3705):1835–1836. doi: 10.1126/science.150.3705.1835. [DOI] [PubMed] [Google Scholar]


