Abstract
Periplasmic nitrate reductase (NapABC enzyme) has been characterized from a variety of proteobacteria, especially Paracoccus pantotrophus. Whole-genome sequencing of Escherichia coli revealed the structural genes napFDAGHBC, which encode NapABC enzyme and associated electron transfer components. E. coli also expresses two membrane-bound proton-translocating nitrate reductases, encoded by the narGHJI and narZYWV operons. We measured reduced viologen-dependent nitrate reductase activity in a series of strains with combinations of nar and nap null alleles. The napF operon-encoded nitrate reductase activity was not sensitive to azide, as shown previously for the P. pantotrophus NapA enzyme. A strain carrying null alleles of narG and narZ grew exponentially on glycerol with nitrate as the respiratory oxidant (anaerobic respiration), whereas a strain also carrying a null allele of napA did not. By contrast, the presence of napA+ had no influence on the more rapid growth of narG+ strains. These results indicate that periplasmic nitrate reductase, like fumarate reductase, can function in anaerobic respiration but does not constitute a site for generating proton motive force. The time course of Φ(napF-lacZ) expression during growth in batch culture displayed a complex pattern in response to the dynamic nitrate/nitrite ratio. Our results are consistent with the observation that Φ(napF-lacZ) is expressed preferentially at relatively low nitrate concentrations in continuous cultures (H. Wang, C.-P. Tseng, and R. P. Gunsalus, J. Bacteriol. 181:5303-5308, 1999). This finding and other considerations support the hypothesis that NapABC enzyme may function in E. coli when low nitrate concentrations limit the bioenergetic efficiency of nitrate respiration via NarGHI enzyme.
Nitrate (NO3−), which is relatively abundant in many environments, has three functions in bacterial physiology. Nitrate assimilation provides a source of ammonium for biosynthesis (reviewed in reference 29), nitrate respiration generates proton motive force for energy (reviewed in references 4, 21, and 65), and nitrate dissimilation oxidizes excess reducing equivalents (reviewed in reference 36).
Membrane-bound nitrate reductase (NarGHI enzyme; nitrate reductase A) employs a redox loop to couple quinol oxidation with proton translocation, thereby generating proton motive force for anaerobic respiration. The Escherichia coli and Paracoccus denitrificans enzymes have been the focus of most biochemical studies (reviewed in references 4, 21, and 65). This enzyme contains Mo-molybdopterin guanine dinucleotide, five iron-sulfur clusters, and diheme cytochrome b556. NarGHI enzyme activity is inhibited by azide (N3−). Enzyme synthesis is maximally induced during anaerobic growth in the presence of nitrate.
Periplasmic nitrate reductase (NapABC enzyme; nitrate reductase P) also oxidizes quinol, but it is thought that this enzyme is not a coupling site for proton translocation (reviewed in reference 4). Therefore, this enzyme is responsible for nitrate dissimilation. However, periplasmic nitrate reductase can participate indirectly in respiration, by functioning in an electron transport chain with a proton-translocating enzyme, such as NADH dehydrogenase I (NuoA-N enzyme) (reviewed in references 36 and 45). The Paracoccus pantotrophus (Thiosphaera pantotropha) enzyme has been the focus of most biochemical studies (reviewed in references 4 and 45). This enzyme contains Mo-molybdopterin guanine dinucleotide, one iron-sulfur cluster, diheme cytochrome c552 (NapB), and a tetraheme cytochrome c (NapC). NapABC enzyme activity is not sensitive to micromolar concentrations of azide.
Periplasmic nitrate reductase of Paracoccus spp. is synthesized during aerobic growth irrespective of the added nitrate and is responsible for initiating aerobic denitrification (3). Aerobic nitrate reduction at least in part disposes of excess reductant, because enzyme synthesis is enhanced by growth on highly reduced carbon substrates, such as butyrate (reviewed in reference 45). An analogous role in redox balancing during anaerobic photosynthesis has been suggested for Rhodobacter spp. (reviewed in reference 45). Structural genes for the NapABC enzyme and accessory proteins have been characterized for a range of gram-negative bacterial species (reviewed in reference 38).
Systematic sequencing of the E. coli K-12 genome revealed a napFDAGHBC-ccmABCDEFGH cluster at centisome 49.5 (7, 25; GenBank accession number U00008). The functions of the conserved NapD protein and the iron-sulfur proteins NapFGH have not been established (reviewed in reference 38). The ccmABCDEFGH genes are required for cytochrome c maturation (reviewed in references 22 and 61).
The E. coli nap-ccm locus was identified independently in a search for anonymous anaerobically expressed genes (11, 12). Comparison of available sequence data (12; GenBank accession number U00008) revealed that the sequence downstream of the aeg-46.5 control region corresponds to the 5" end of the napF gene. Expression from the napF operon promoter requires anaerobiosis, acting through the Fnr protein, and is stimulated by nitrate and nitrite, acting through the Nar regulatory system (11, 12, 16, 18). Subsequent work with E. coli has focused on verifying the synthesis and activity of periplasmic nitrate reductase (24, 60; this study), elucidating the transcriptional organization and regulation of the nap-ccm complex operon (12, 16, 18, 24, 59, 68), studying the contribution of periplasmic nitrate reductase to anaerobic metabolism (39, 40, 68; this study), and probing the functions of the ccm gene products in cytochrome c maturation (reviewed in references 22 and 61).
Studies with several denitrifying bacteria, including P. pantotrophus, “Pseudomonas” sp. strain G-179, and Rhodobacter sphaeroides f. sp. denitrificans, have demonstrated that periplasmic nitrate reductase can support anaerobic respiration (1, 2, 30). By contrast, studies with E. coli have indicated that periplasmic nitrate reductase functions poorly in anaerobic respiration (39, 40). Furthermore, although all organisms have the napDABC genes, only denitrifiers carry the napE gene, whereas E. coli and related ammonifying bacteria carry the napGH genes (reviewed in reference 38). This raises the possibility that these accessory genes (or some other aspect of cell physiology) determine whether periplasmic nitrate reductase functions in respiration (in denitrifiers) or simply in dissimilation (in E. coli).
Although E. coli nitrate respiration has been studied for seven decades (reviewed in reference 53), the E. coli NapABC enzyme was first recognized only after whole-genome sequencing. We therefore wished to search for NapA enzyme activity in E. coli and to characterize its role in enterobacterial physiology. In this paper we describe an azide-insensitive nitrate reductase activity whose expression requires an intact nap locus, in addition to the azide-sensitive activity whose expression requires the narGHJI operon. These observations complement the results of independent studies performed in the laboratory of Jeff Cole (24, 59, 60). We also found that periplasmic nitrate reductase supports anaerobic respiration by E. coli. Finally, we describe experiments designed to examine the kinetics of napF operon expression during growth in batch culture, the results of which complement those of continuous culture studies performed in the laboratory of Rob Gunsalus (68).
(Some of the work presented here was submitted by Marianne T. Shih in 1996 as an undergraduate thesis to the Cornell University Division of Biological Sciences Honors Program.)
MATERIALS AND METHODS
Strains and plasmids.
The strains and plasmids used are listed in Table 1 and depicted in Fig. 1. Genetic crosses were performed by P1 kc-mediated generalized transduction (34). Standard methods were used for restriction endonuclease digestion, ligation, transformation, and PCR amplification of DNA (32).
TABLE 1.
Strains and plasmids
| Strain or plasmid | Genotype | Reference or source |
|---|---|---|
| Escherichia coli K-12 strains | ||
| BW18812 | Δ(lacI-codB)X74 ΔphoA532 ΔuidA Δphn | 33 |
| LCB2048 | thi-1 thr-1 leu-6 lacY1 supE44 rpsL175 Δ(narGH)::Km Δ(narUZ)::Ω-Sp | 6 |
| POI1734 | Mu dI1734 ara::(Mu cts)3 Δ(lac-proAB)X111 rpsL | 9 |
| RK4353 | araD139 Δ(argF-lac)U169 flhD5301 gyrA129 non-9 rpsL150 ptsF25 relA1 deoC1 | 55 |
| RK5268 | Like RK4353 but narG205::Tn10 | 55 |
| VJS632 | Prototroph | 56 |
| VJS676 | Like VJS632 but Δ(argF-lac)U169 | 56 |
| VJS691 | Like VJS632 but Δ(argF-lac)U169 Δ(trpEA)2 | 56 |
| VJS2197 | Like VJS676 but λΦ(narG-lacZ) | 43 |
| VJS2430 | Like VJS676 but λΦ(narG-lacZ) ΔnarX242 zch-2084::Ω-Cm | 43 |
| VJS3040 | Like VJS676 but λΦ(narG-lacZ) narQ251::Tn10d(Tc) | 43 |
| VJS3861 | Like VJS676 but λΦ(nrfA-lacZ) | This study |
| VJS4734 | Like VJS676 but λΦ(napF-lacZ) [Δ-275] | 16 |
| VJS4799 | Like VJS676 but λΦ(napF-lacZ) [Δ-275] narL215::Tn10 | 16 |
| VJS5432 | Like VJS691 but Δ(narUZ)::Ω-Sp | This study |
| VJS5433 | Like VJS691 but Δ(narUZ)::Ω-Sp ΔnapF1::Km | This study |
| VJS5435 | Like VJS691 but narG205::Tn10 ΔnapF1::Km | This study |
| VJS5436 | Like VJS691 but narG205::Tn10 Δ(narUZ)::Ω-Sp | This study |
| VJS5437 | Like VJS691 but narG205::Tn10 Δ(narUZ)::Ω-Sp ΔnapF1::Km | This study |
| VJS5917 | Like VJS6737 but Δ(narUZ)::Ω-Sp | This study |
| VJS5918 | Like VJS6737 but Δ(narUZ)::Ω-Sp ΔnapA5 | This study |
| VJS5919 | Like VJS6737 but Δ(narUZ)::Ω-Sp narG205::Tn10 | This study |
| VJS5920 | Like VJS6737 but Δ(narUZ)::Ω-Sp narG205::Tn10 ΔnapA5 | This study |
| VJS6737 | Like BW18812 but rpsL150 | This study |
| Plasmids | ||
| pHG165 | Apr, pUC8 polylinker, medium-copy-number cloning vector | 52 |
| pHP45::Ω-Cm | Apr Cmr, source of Ω-Cm interposon | 20 |
| pKAS46 | Apr KmrrpsL+, allelic-exchange vector | 51 |
| pKT53 | Tcr, source of nrfA control region | 64 |
| pRS415 | Apr, lacZ operon fusion vector | 50 |
| pUC4K | Apr Kmr, source of Kmr (aph) cassette | 66 |
| pVJS1510 | AprnapFDA", 2.9-kb PstI-BglII insert in pHG165a | This study |
| pVJS1521 | Apr Kmr, ΔnapF1::Km in pVJS1510 | This study |
| pVJS1725 | Apr Cmr Kmr, napA4::Ω-Cm in pVJS1729 | This study |
| pVJS1728 | Apr Kmr, ΔnapA5 in pVJS1729 | This study |
| pVJS1729 | Apr Kmrnap"AGHBC-ccmA", 5.5-kb NotI-BamHI insert in pKAS46a | This study |
See Fig. 1.
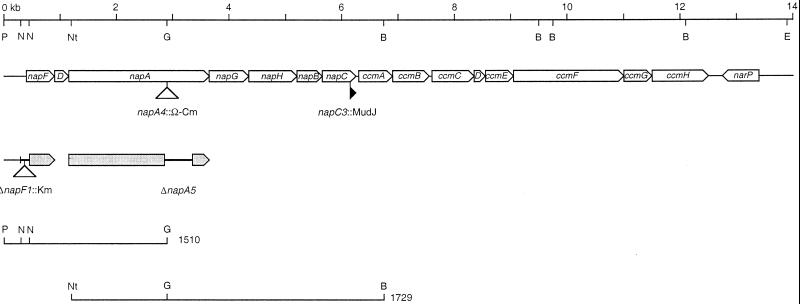
FIG. 1.
nap-ccm cluster. The seven nap genes, eight ccm genes, and the convergently transcribed narP gene are depicted to scale as open arrows. Insertions of MudJ and Ω-Cm are indicated by solid and open triangles, respectively. The deletion-substitution ΔnapF1::Km and the deletion ΔnapA5 are indicated by thick lines on gray arrows. The restriction endonuclease sites indicated are BamHI (B), BglII (G), EcoRI (E), NotI (Nt), NsiI (N), and PstI (P) sites. Only two of seven NsiI sites used to construct the ΔnapF1::Km allele are shown. The inserts for pVJS subclones listed in Table 1 are shown.
Culture media and conditions.
Defined, complex, and indicator media for genetic manipulations were used as described previously (32). The antibiotic concentrations were as follows: ampicillin, 200 μg/ml; chloramphenicol, 25 μg/ml; kanamycin, 75 μg/ml; spectinomycin, 100 μg/ml; streptomycin, 200 μg/ml; and tetracycline, 20 μg/ml.
The defined medium used to grow cultures for enzyme assays was buffered with 3-(N-morpholino)propanesulfonic acid (MOPS) as previously described (56). The initial pH of this medium is adjusted to 8.0 in order to ameliorate nitrite toxicity (62). Because the pKa" of MOPS is 7.2, the buffering capacity of this medium continually increases as acidic fermentation products accumulate; at the time of harvest, cultures typically had a pH of about 7.5.
The respiratory oxidants NaNO3, NaNO2, sodium fumarate, and trimethylamine N-oxide were added at concentrations of 40 mM, 5 mM, 50 mM, and 2%, respectively, as indicated below. The carbon sources glucose, glucitol, gluconic acid, mannose, and mannitol were each added at a concentration of 40 mM as indicated below. The carbon source glycerol was added at a concentration of 50 mM along with 0.03% acid-hydrolyzed casein (28). The complex medium used to grow cultures for nitrate reductase assays consisted of one part of MOPS-glucose medium mixed with one part of TY broth (0.8% tryptone, 0.5% yeast extract, 0.5% NaCl) and supplemented with 5 mM NaNO2.
Cultures were grown at 37°C. Culture densities were monitored with a Klett-Summerson photoelectric colorimeter (Klett Manufacturing Co., New York, N.Y.) equipped with a no. 66 (red) filter. Anaerobic cultures used for enzyme assays were grown in screw-cap tubes as described previously (56).
Enzyme assays.
β-Galactosidase activities were determined at room temperature (approximately 21°C) by monitoring the hydrolysis of o-nitrophenyl-β-d-galactoside in CHCl3-sodium dodecyl sulfate-permeabilized cells. Specific activities are expressed below in arbitrary units (34). For differential rate plots, the activities are expressed in amounts of orthonitrophenol per milliliter per minute and are not normalized for cell mass (optical density at 600 nm). All cultures were assayed in duplicate, and the values reported below are averages based on at least two independent experiments.
Differential rates of β-galactosidase synthesis (35) were determined for anaerobic cultures essentially as described previously (58). Cultures (25 ml) were continuously bubbled with N2-CO2 (95:5) to maintain anaerobiosis. Samples (1.5 ml) were withdrawn and mixed with 1.5 ml of a solution containing 50 mg of chloramphenicol per ml (to inhibit further protein synthesis) and 1.5 μl of 50 mM NaN3 (to inhibit further nitrate reduction). Samples were stored on ice before the assay was performed. The concentration of nitrite in each sample was estimated following reaction with sulfanilic acid (31).
Nitrate reductase activities were determined at room temperature by monitoring the production of nitrite in intact cells (56). Cells were suspended in 0.32 M potassium phosphate (pH 7.1) and stored on ice. Samples (0.8 ml) were mixed with 0.1 ml of a solution containing 0.5 mg of methyl viologen or benzyl viologen per ml. Reactions were started by adding 0.1 ml of a solution containing 4 mg of Na2S2O4 per ml, 4 mg of NaHCO3 per ml, and 0.5 M NaNO3. Reactions were terminated by vigorous vortex mixing (to oxidize the viologen), and 1 ml of a sulfanilic acid solution and 1 ml of an N-1-naphthylethylenediamine solution were added. Specific activities are expressed below in arbitrary units analogous to Miller units (56). All cultures were assayed in duplicate, and the values reported below are averages based on at least two independent experiments.
Construction of nap and ccm insertions and deletions.
We isolated a series of subclones (Fig. 1) derived from phage 372 (19D1) of the Kohara λ library (27). To construct Φ(nap-lacZ) and Φ(ccm-lacZ) operon (transcriptional) fusions, each subclone was subjected to insertional mutagenesis with the bacteriophage transposon MudJ (MudI 1734), which forms lacZ operon (transcriptional) fusions (9). Several insertions were characterized by restriction mapping and DNA sequence analysis to determine the exact insertion point. Five insertions, spanning the nap-ccm region (Fig. 1), were then transplanted into the chromosome by allelic exchange in a recBC sbcBC strain (69). The resulting nap::MudJ and ccm::MudJ insertions were crossed into strain VJS691 and then tested for genetic linkage to a closely linked narP::Tn10 insertion to confirm their chromosomal location.
The ΔnapF1::Km allele was constructed by cloning a Kmr cassette, liberated by PstI digestion of plasmid pUC4K, between the NsiI sites at position −32 (relative to the transcription initiation site) (12) and at napF codon 5. A clone was chosen in which the cassette was oriented with its promoter directed away from the napF operon. This insertion was transplanted into the chromosome and tested for linkage as described above for the MudJ insertions.
The napA4::Ω-Cm allele was constructed by cloning the Ω-Cm interposon, liberated by BamHI digestion of plasmid pHP45::Ω-Cm, into the BglII site at napA codon 578 (7, 25, 60). (The napA gene encompasses 828 codons.) This insertion was transplanted into the chromosome by integration-excision allelic exchange (51), with selection for Cmr Smr segregants (plasmid-free segregants were selected as Smr constructs after loss of the dominant rpsL+ Sms allele). We then used generalized transduction to construct napA4::Ω-Cm nap::MudJ and napA4::Ω-Cm ccm::MudJ double mutants. The resulting strains were subjected to transduction-mediated three-point crosses, employing flanking Tn10 (Tcr) insertions, to confirm the genetic map order of the MudJ (Kmr) and Ω-Cm elements in each strain (data not shown).
The ΔnapA5 deletion was constructed by loop deletion mutagenesis (70) by using the following mutagenic oligonucleotide complementary to an internal segment of the napA gene coding strand: 5"-ACTGCCGGTGTGCCAGTGCTCGAGATCCGATTTCGCTTCGCC (the underlined XhoI site corresponds to the complement of the Glu-723 and Leu-559 codons). Thus, the deletion removed 163 codons (codons 560 through 722), including the BglII site at codon 578, while simultaneously introducing a new XhoI site.
The ΔnapA5 allele was transplanted into the chromosome (51) of a Φ(napA-uidA) derivative of strain VJS6737, in which a uidA cassette (33) was inserted into the BglII site at napF codon 578 (V. Stewart and J. Shi, unpublished data). Sms segregants were isolated on agar containing the UidA indicator X-Gluc (5-bromo-4-chloro-3-indolyl-β-d-glucuronide) to facilitate identification of UidA− strains in which the ΔnapA5 allele had replaced the resident napA::uidA allele. The presence of the deletion was confirmed by whole-colony PCR analysis (70), in which primers flanking the site of the deletion were used and the presence of the introduced XhoI site was examined.
Construction of a Φ(nrfA-lacZ) gene (translational) fusion.
The nrfA operon control region from position −209 to position 131 (relative to the transcription initiation site) was subcloned as an EcoRI-HindIII fragment (64) into a polylinker plasmid to place a BamHI site adjacent to the HindIII site. The resulting EcoRI-BamHI fragment was then cloned into plasmid pRS414 (50) to form a plasmid-borne Φ(nrfA-lacZ) gene (translational) fusion. The fusion construct was crossed into bacteriophage λRS45 (50), and monocopy lysogens were identified by a whole-colony PCR test (41). Similarly constructed λΦ(narG-lacZ) gene and λΦ(napF-lacZ) operon fusions have been described previously (16, 42).
RESULTS AND DISCUSSION
E. coli periplasmic nitrate reductase.
E. coli contains three sets of nitrate reductase genes: the narGHJI operon, encoding the membrane-bound NarGHI enzyme; the homologous narZYWV operon, encoding the membrane-bound NarZYV enzyme; and the napFDAGHBC operon, encoding the periplasmic NapABC enzyme. In order to distinguish the different activities, we used generalized transduction to construct a series of isogenic strains with all combinations of nar and nap polar null lesions. The defined null alleles narG205::Tn10 (55) and Δ(narUZ)::Ω-Sp (6) were available, so we fabricated ΔnapF1::Km, in which the napF operon promoter control region and the 5" end of the napF gene were replaced with a Kmr cassette (see Materials and Methods).
Strains were grown anaerobically with nitrite, which in batch culture induces high-level napF operon expression (24, 43, 68). Reduced viologen-linked nitrate reductase activity was measured in whole cells as described in Materials and Methods. The most reproducible results were obtained in assays performed with unbroken freshly cultured cells, suggesting that NapA activity may be relatively unstable (NarG activity is quite stable). Instability was also noted by Thomas et al. (60).
Results are shown in Table 2. The NarZYV enzyme contributed little to the overall nitrate reductase activity (compare the activities of strains VJS5435 and VJS5437), as expected since low levels of this enzyme are synthesized during exponential growth (10). The NarG+ NapA+ strain VJS5432 exhibited significant endogenous activity, which increased four- to fivefold when reduced viologen was provided as an artificial electron donor. The NarG+ NapA− strain VJS5433 exhibited approximately 80% of the NarG+ NapA+ viologen-stimulated activity, whereas the NarG− NapA+ strain VJS5436 exhibited 15 to 30% of the NarG+ NapA+ viologen-stimulated activity and very little endogenous (viologen-independent) activity.
TABLE 2.
Nitrate reductase activities of NarG−, NapA−, and NarZ− strains
| Strain | Phenotype
|
Nitrate reductase sp act with the following electron donorsa:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| None, without N3− | Methyl viologen
|
Benzyl viologen
|
||||||||
| NarG | NapA | NarZ | Without N3− | With N3− | Without N3− | With N3− | ||||
| VJS5432 | + | + | − | 86 | 330 | 190 | 480 | 250 | ||
| VJS5433 | + | − | − | 96 | 260 | 27 | 400 | 25 | ||
| VJS5436 | − | + | − | 4 | 97 | 130 | 73 | 52 | ||
| VJS5435 | − | − | + | <1 | 2 | 2 | <1 | <1 | ||
| VJS5437 | − | − | − | <1 | <1 | <1 | <1 | <1 | ||
Specific activities were determined as described in Materials and Methods and are expressed in arbitrary units. Assays were performed with different dithionite-reduced electron donors. Azide (50 μM) was added to some preparations.
Previously characterized NapA enzymes are not sensitive to azide, in contrast to NarG enzyme (reviewed in references 4 and 21). Our results (Table 2) revealed that activity of the NarG+ NapA− strain VJS5433 was inhibited 8- to 10-fold by azide, whereas activity of the NarG− NapA+ strain was not sensitive to azide. These results established that an azide-insensitive nitrate reductase activity is present in E. coli and that this activity requires an intact napF operon.
Grove et al. employed activity staining of native gels to detect nitrate reductase activity in the periplasmic fraction of a narG null strain, and this activity was not present in extracts of a nap null strain (23). These authors also presented evidence that napB and napC encode Nap-associated cytochromes c. Subsequently, Thomas et al. determined the amino-terminal sequences of the NapA and NapB proteins, confirming the structural gene assignments (60). These results, together with those presented above, demonstrate that E. coli synthesizes a periplasmic azide-insensitive NapABC enzyme that is homologous to enzymes characterized in other bacteria (reviewed in reference 4).
Potter et al. detected only very low levels of NapA activity when reduced methyl viologen was used as the electron donor in broken cells, yet they detected significant levels of activity when formate or glycerol was used as the electron donor in whole cells (40). In our viologen-dependent assay, which detected substantial levels of NapA activity (Table 2), we used freshly harvested whole cells.
Function of E. coli periplasmic nitrate reductase.
We sought culture conditions under which the presence of the NapABC enzyme would result in a clear phenotype. Therefore, we constructed an in-frame deletion in the napA gene in order to eliminate Nap enzyme activity while avoiding polarity on ccm gene expression (since respiratory nitrite reductase requires the Ccm+ function for activity). The ΔnapA5 deletion (Fig. 1) was constructed and transplanted into the chromosome as described in Materials and Methods.
To determine whether the ΔnapA5 deletion was polar on downstream gene expression, we monitored β-galactosidase expression in strains carrying the downstream napC3::MudJ insertion (Fig. 1). Strains carrying napC3::MudJ in combination with napA+, napA4::Ω-Cm, or ΔnapA5 were cultured anaerobically in MOPS-glucose medium with and without nitrite, and β-galactosidase activities were measured. The napA+ strain exhibited approximately 20-fold induction of Φ(napC-lacZ) expression by nitrite. A similar level of nitrite-induced Φ(napC-lacZ) expression was observed in the ΔnapA5 strain. By contrast, the napA::Ω-Cm insertion significantly reduced Φ(napC-lacZ) expression due to strong transcriptional polarity. We concluded that the ΔnapA5 allele exerts no significant polarity on downstream nap-ccm gene expression.
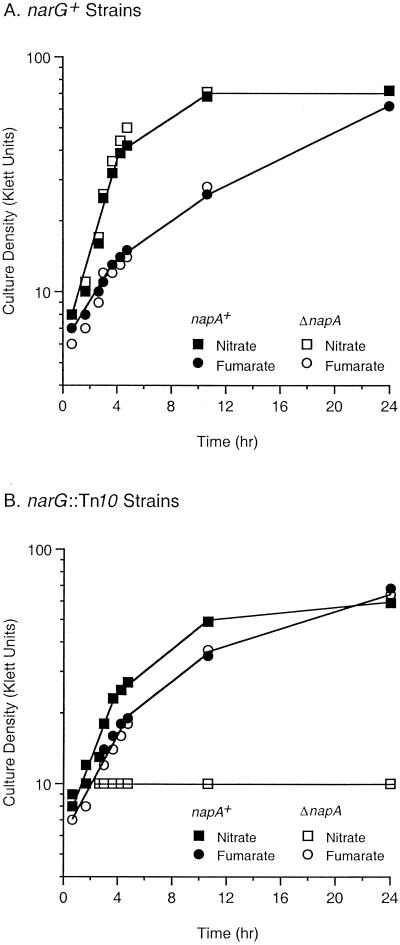
Isogenic strains carrying narG+ or narG205::Tn10 in combination with napA+ or ΔnapA5 were batch cultured anaerobically in MOPS-glycerol-casein medium supplemented with nitrate or fumarate as described in Materials and Methods. (Anaerobic growth with glycerol requires respiration with a terminal oxidant, which in this case was either nitrate or fumarate.) The narG+ strain grew on nitrate with an early-exponential-phase doubling time of about 85 min irrespective of the presence of napA+ (Fig. 2A). By contrast, the narG::Tn10 napA+ strain grew on nitrate (early-exponential-phase doubling time, about 150 min) only slightly faster than on fumarate (early-exponential-phase doubling time, about 165 min) (Fig. 2B). Strikingly, the narG::Tn10 ΔnapA double mutant did not grow with nitrate as the electron acceptor. All four strains had equivalent growth rates on fumarate.
FIG. 2.
tk;2Anaerobic respiration: growth curves for anaerobic cultures in MOPS-glycerol-acid-hydrolyzed casein medium supplemented with nitrate or fumarate as the respiratory oxidant. (A) Data for the narG+ strains VJS5917 (napA+) and VJS5918 (ΔnapA5). (B) Data for the narG:: Tn10 strains VJS5919 (napA+) and VJS5920 (ΔnapA5). All strains carry Δ(narUZ)::Ω-Sp.
These results demonstrate that E. coli can use periplasmic nitrate reductase and fumarate reductase equally well in a respiratory chain involving glycerol 3-phosphate oxidation. Fumarate reductase itself does not constitute a coupling site for generating proton motive force from quinol oxidation (63; reviewed in references 21 and 65). Therefore, the observed growth phenotypes support the conclusion that periplasmic nitrate reductase is not a coupling site (4, 36). These observations do not eliminate the formal possibility that periplasmic nitrate reductase simply provides nitrite as the substrate for periplasmic nitrite reductase (NrfABCD enzyme), which is known to support anaerobic respiration (reviewed in references 5 and 21). However, expression of the nrfABCDEFG operon is strongly inhibited by nitrate (64) (see Fig. 6). Therefore, the most economical hypothesis is that E. coli periplasmic nitrate reductase functions directly to support anaerobic respiration.
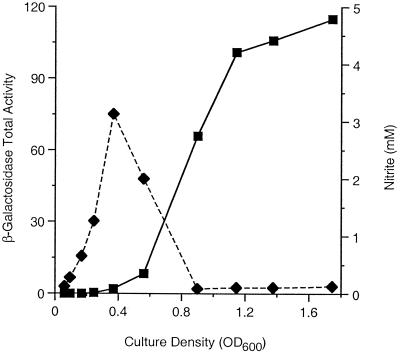
FIG. 6.
Differential rate of Φ(nrfA-lacZ) expression. A culture of the λΦ(nrfA-lacZ) strain VJS3861 was grown anaerobically in MOPS-glucose medium supplemented with 5 mM nitrate. Samples were withdrawn and assayed for total β-galactosidase activity (▪) and nitrite concentration (⧫). OD600, optical density at 600 nm.
Studies with a P. pantotrophus double mutant carrying a null allele of narH in addition to an operator-constitutive mutation in the napE operon control region (49) revealed that periplasmic nitrate reductase can support anaerobic respiration on nitrate with about one-third the wild-type doubling time (2). By contrast, however, an E. coli narG narZ double-null nap+ strain grew only arithmetically in defined glycerol-casein medium supplemented with 20 mM nitrate (39). Subsequent work indicated that this growth defect was partially suppressed by introduction of a narL null allele (40). Strains derived from both the MC4100 (RK4353) and C600 (LCB320) lineages exhibited this phenotype. The authors of those studies concluded that NarL antagonism of napF operon expression (16) caused the growth defect on nitrate.
By contrast, we observed robust exponential growth with strains derived from both the BD792 (BW18812) and RK4353 lineages (Fig. 2 and data not shown). We used λΦ(narG-lacZ) specialized transducing phage to confirm that the strains which we used harbored narL+ alleles (54). We note that Φ(napF-lacZ) expression during batch culture of narL+ strains is induced about 10-fold by nitrate, compared to the approximately 20-fold induction by nitrite (16, 68). Thus, the inhibitory effect of NarL and nitrate is relatively minor compared to the overall induction of Φ(napF-lacZ) expression.
Carbon source oxidation state does not regulate napF operon expression.
In P. pantotrophus, P. denitrificans, and Rhodobacter capsulatus, growth on reduced carbon sources results in increased NapABC synthesis compared to the synthesis observed when the organisms are grown on oxidized carbon sources (46, 47, 49). This reflects the enzyme's role in these organisms in maintaining redox balance (nitrate dissimilation) even in aerated cultures. We therefore batch cultured the nar+ nap+ λΦ(napF-lacZ) strain VJS4734 anaerobically in MOPS-nitrite medium supplemented with carbon sources having different oxidation states. However, the relative levels of Φ(napF-lacZ) expression in mid-exponential-phase cultures differed less than twofold, and the small variations observed did not reflect the relative oxidation states of the carbon sources (data not shown). Expression was slightly higher in the culture grown with glucitol (more reduced) than in the culture grown with glucose or glucuronic acid (more oxidized). Conversely, expression was slightly higher in the culture grown with mannose (more oxidized) than in the culture grown with mannitol (more reduced). Indeed, the patterns were more reminiscent of catabolite repression, as glucose and mannitol are more effective elicitors of catabolite repression than glucitol and mannose are (37). Thus, at least for the carbon sources tested, we observed no effect of oxidation state on Φ(napF-lacZ) expression.
Differential regulation by nitrate and nitrite: kinetics of Φ(napF-lacZ) induction.
Our previous conclusions regarding expression of the napF, narG, and other Nar-regulated operons were based on measurements obtained for mid-exponential-phase batch cultures started with either 40 mM nitrate or 5 mM nitrite. Because growing cultures quickly consume nitrate, we use an excess to ensure that saturating (millimolar) concentrations were present at the time of harvest. The concentration of nitrite employed is sufficient to observe regulated gene expression without inhibiting growth rates and yields (see also Materials and Methods). Thus, these cultures allow us to draw conclusions regarding differential responses to saturating concentrations of the structurally related signals nitrate and nitrite. They do not allow us to draw conclusions regarding responses to limiting concentrations of these compounds (68).
Cognizant of these limitations of batch culture analysis, we hypothesized that the napF promoter might be more efficiently expressed during growth with a limiting concentration of nitrate. We therefore examined the kinetics of Φ(napF-lacZ) induction in response to different nitrate concentrations. Two parallel batch cultures of the nar+ nap+ λΦ(napF-lacZ) strain VJS4734 were grown to the early exponential phase in MOPS-glucose medium. Nitrate was added to a final concentration of 1 mM to one culture and to a final concentration of 10 mM to the other. Samples were withdrawn and used to measure β-galactosidase activity and nitrite concentration (see Materials and Methods), and the results were analyzed by constructing differential rate plots (26, 35). We observed no difference in the initial rates of β-galactosidase synthesis in the two cultures (data not shown).
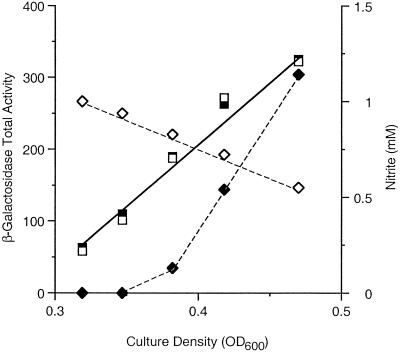
We next investigated the effects of nitrate versus the effects of nitrite. Two parallel cultures were grown to the early exponential phase. Nitrate was added to a final concentration of 10 mM to one culture, and nitrite was added to a final concentration of 1 mM to the other. Samples were withdrawn and used to measure β-galactosidase activity and nitrite concentration. The results are shown in Fig. 3. The nitrite-induced culture steadily consumed nitrite, whereas the nitrate-induced culture rapidly accumulated nitrite. Again, the initial rates of Φ(napF-lacZ) expression were the same in both cultures for about one-half a generation (Fig. 3). Therefore, the initial rates of Φ(napF-lacZ) expression were the same upon induction with 1 mM nitrate, 10 mM nitrate, and 1 mM nitrite.
FIG. 3.
Differential rates of Φ(napF-lacZ) induction. Duplicate cultures of strain VJS4734 were grown anaerobically in MOPS-glucose medium. At the early exponential phase, nitrate (solid symbols) and nitrite (open symbols) were added to final concentrations of 10 and 1 mM, respectively. Samples were withdrawn and assayed for total β-galactosidase activity (▪ and □) and nitrite concentration (⧫ and ◊). OD600, optical density at 600 nm.
Differential regulation by nitrate and nitrite: time course of Φ(napF-lacZ) expression.
Although the initial rates of enzyme synthesis were indistinguishable, we observed that the rates in the cultures supplemented with 10 mM nitrate began to fluctuate within 1 doubling time (not shown in Fig. 3). We reasoned that the decrease observed was due to NarL-mediated antagonism (16). To test this hypothesis, we monitored the differential rates of Φ(napF-lacZ) expression in both narL+ and narL::Tn10 strains grown in batch cultures started with 5 mM nitrate (Fig. 4). This experiment differed from those described above because nitrate was present from the beginning of growth. In this experiment, we were concerned with the patterns of gene expression as nitrate was depleted from the medium rather than with the initial rates of synthesis when nitrate was added.
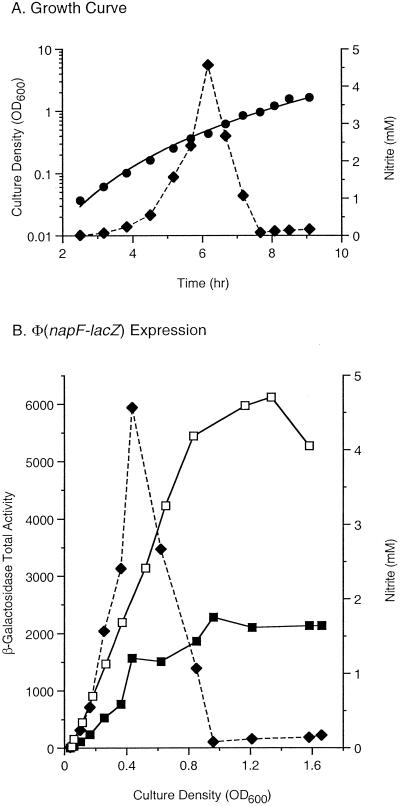
FIG. 4.
Differential rates of Φ(napF-lacZ) expression. Cultures of the λΦ(napF-lacZ) strains VJS4734 (narL+) and VJS4799 (narL::Tn10) were grown anaerobically in MOPS-glucose medium supplemented with 5 mM nitrate. (A) Growth curve (•) and nitrite concentrations (⧫) for strain VJS4734. This pattern was typical for strains used in this experiment and in the experiments whose results are shown in Fig. 5 and 6. (B) Total β-galactosidase activities in cultures of strains VJS4734 (▪) and VJS4799 (□) and, for reference, nitrite concentrations in the culture of strain VJS4734 (⧫). OD600, optical density at 600 nm.
Figure 4A shows a representative growth curve for the nar+ nap+ λΦ(napF-lacZ) strain VJS4734. At each time point, a sample was withdrawn and assayed for β-galactosidase activity and for nitrite concentration as described in Materials and Methods. Nitrite, formed by the action of nitrate reductase, accumulated nearly quantitatively and was then consumed by the action of nitrite reductase (forming ammonium). This pattern of nitrite accumulation and consumption was previously noted by DeMoss and Hsu (19). Thus, we were able to monitor the effects on gene expression of continually changing ratios of the two signal ligands.
In the narL+ strain, the rate of Φ(napF-lacZ) expression exhibited a complex pattern. The rate seemed to increase about twofold as the nitrite concentration peaked (i.e., at about the time that the nitrate in the culture medium was exhausted) (Fig. 4B and data not shown). This is consistent with the conclusion of Wang et al. (68), drawn from studies of continuous cultures, that Φ(napF-lacZ) expression is maximal at submillimolar concentrations of nitrate. As nitrite was rapidly consumed, β-galactosidase synthesis transiently stopped and then resumed. Finally, when the nitrite in the culture medium was exhausted, synthesis again stopped (Fig. 4B and data not shown).
In striking contrast, the narL null strain exhibited a steady rate of Φ(napF-lacZ) expression that was not affected by changes in the nitrate/nitrite ratio; synthesis stopped only after the nitrite was exhausted (Fig. 4B and data not shown). Thus, the complex pattern of Φ(napF-lacZ) expression in the narL+ strain evidently reflected antagonism by phospho-NarL, which competes with the activator phospho-NarP for a common DNA binding site upstream of the napF operon promoter (16, 18). These experiments, together with complementary studies of continuous cultures (68), revealed that the levels of Φ(napF-lacZ) expression observed in batch cultures provide only one view of the complexity that underlies transcriptional regulation of the napF promoter.
In continuous culture, Φ(napF-lacZ) expression was maximal at a relatively low concentration of nitrate, about 0.5 mM (68). Consistent with this, the time course experiments revealed that Φ(napF-lacZ) expression seemed to increase as nitrate was removed from the medium (Fig. 4B and data not shown). By contrast, maximal Φ(narG-lacZ) expression in continuous culture requires more than 5 mM nitrate. It was hypothesized that this pattern of regulation reflects a relatively low Km for nitrate of periplasmic nitrate reductase compared to the Km for nitrate of the membrane-bound enzyme (68). Arguing against this notion, studies of Paracoccus sp. enzymes in which reduced methyl viologen was used as the electron donor revealed that the Km for nitrate of NarGHI enzyme was more than fourfold lower than the Km for nitrate of NapAB enzyme (0.28 versus 1.3 mM) (8, 14). However, we note that the Km of NarGHI enzyme was 20-fold lower in assays in which a more physiologically relevant quinol analog was used as the electron donor (14); analogous studies with NapABC-NapFGH enzyme have not been described.
We suggest an alternative hypothesis to explain why periplasmic nitrate reductase might be preferentially expressed during growth in the presence of low nitrate concentrations. For nitrate respiration, which is mediated by NarGHI enzyme, nitrate must be imported to the cytoplasm; for nitrite respiration, which is mediated by NrfABC enzyme, the resultant nitrite must be exported back to the periplasm (19, 48). In the presence of excess nitrate, the energy cost for this transport would likely be more than supplanted by an NarGHI-generated proton motive force. When nitrate is limiting, however, it may be more economical to simply reduce nitrate in the periplasm (concomitant with oxidation of reducing equivalents) and deliver it directly to the periplasmic respiratory nitrite reductase (13).
Differential regulation by nitrate and nitrite: time course of Φ(narG-lacZ) and Φ(nrfA-lacZ) expression.
Because of the complex pattern for Φ(napF-lacZ) expression described above, we examined the synthesis patterns for other Nar-regulated structural genes, including those for the membrane-bound nitrate reductase (narGHIJ operon), the nitrate-inducible formate dehydrogenase (fdnGHI operon), and the periplasmic respiratory nitrite reductase (nrfA-G operon).
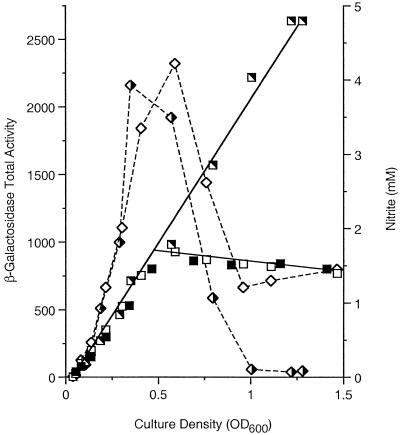
Studies performed with batch cultures have shown that induction of Φ(narG-lacZ) expression is maximal during anaerobic growth with nitrate and much weaker during growth with nitrite (43, 44, 68). Analysis of continuous cultures yielded the same results (68). Thus, we monitored the differential rates of Φ(narG-lacZ) expression in nar+, ΔnarX, and narQ::Tn10 strains grown in batch cultures started with 5 mM nitrate, as described above (Fig. 5). (The cultures of the nar+ and ΔnarX strains exhibited similar patterns of nitrite accumulation and consumption; for clarity, Fig. 5 shows only the data for the ΔnarX strain.)
FIG. 5.
Differential rates of Φ(narG-lacZ) expression. Cultures of the λΦ(narG-lacZ) strains VJS2197 (nar+) (solid symbols), VJS2430 (ΔnarX narQ+) (half-open symbols), and VJS3040 (narX+ narQ::Tn10) (open symbols) were grown anaerobically in MOPS-glucose medium supplemented with 5 mM nitrate. Samples were withdrawn and assayed for total β-galactosidase activity (squares) and nitrite concentration (diamonds). The values for nitrite concentration in the culture of strain VJS2197 are omitted for clarity. OD600, optical density at 600 nm.
Enzyme synthesis in the nar+ strain proceeded at a steady rate until the nitrite concentration peaked (i.e., until about the time that the nitrate in the culture medium was exhausted), and then synthesis stopped. The pattern of Φ(fdnG-lacZ) expression in a nar+ strain was virtually identical (data not shown), as expected (43). Expression of Φ(narG-lacZ) in the narQ null strain was likewise indistinguishable. By contrast, Φ(narG-lacZ) expression in the narX null strain continued unabated (Fig. 5). This is consistent with the postulated function of the NarX protein as a negative regulator (cophosphatase) of phospho-NarL function (reviewed in references 15 and 57) and suggests that this activity has a physiological role (namely, shutoff of target gene expression upon nitrate depletion). Thus, previous conclusions resulting from studies of mid-exponential-phase batch cultures are concordant with the results of these time course experiments.
We note in passing that the culture of the narQ null strain did not consume nitrite when it was present at a concentration below about 1 mM (Fig. 5), implying that at least one gene required for high-affinity nitrite uptake or reduction requires narQ+ for expression. We have not pursued this observation.
Studies with both batch and continuous cultures show that Φ(nrfA-lacZ) expression and Φ(narG-lacZ) expression are regulated reciprocally; Φ(nrfA-lacZ) expression is inhibited during anaerobic growth with nitrate and induced during growth with nitrite (17, 43, 64, 67). Thus, we monitored the differential rate of Φ(nrfA-lacZ) expression in a nar+ strain grown in a batch culture started with 5 mM nitrate (Fig. 6). Enzyme synthesis proceeded at a very low rate until the nitrite concentration peaked (i.e., until about the time that the nitrate in the culture medium was exhausted), and then the rate of synthesis was steady and high until the nitrite was exhausted. Again, the results of these time course experiments are completely consistent with previous conclusions.
Thus, in contrast to the complex patterns of Φ(napF-lacZ) expression described above, the patterns of Φ(narG-lacZ), Φ(fdnG-lacZ), and Φ(nrfA-lacZ) expression were simple and readily interpretable.
Epilogue.
Studies of E. coli periplasmic nitrate reductase have continually challenged our view of this organism's physiology. In the context of regulated gene expression, the napF operon control region is unique among known Nar-regulated targets in both its architecture and its response to the transcriptional regulators NarL and NarP. These unique features evidently underlie uniquely complex patterns of transcriptional regulation. Continued acceptance of the challenges presented should reveal additional, perhaps subtle aspects of the Nar regulatory network.
Acknowledgments
We are grateful to Jiarong Shi (Cornell University) and to Janine Lin and Chesi Ho (University of California, Davis) for their efforts in evaluating the ΔnapA5 strains. We thank Francisco Blasco, Malcolm Casadaban, Joachim Fellay, Robert Simons, Karen Skorupski, the late Gordon Stewart, Kerry Tyson, and Barry Wanner for generous gifts of strains and plasmids.
This study was supported by Public Health Service grant GM36877 from the National Institute of General Medical Science.
REFERENCES
- 1.Bedzyk, L., T. Wang, and R. W. Ye. 1999. The periplasmic nitrate reductase in Pseudomonas sp. strain G-179 catalyzes the first step of denitrification. J. Bacteriol. 181:2802-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell, L. C., M. D. Page, B. C. Berks, D. J. Richardson, and S. J. Ferguson. 1993. Insertion of transposon Tn 5 into a structural gene of the membrane-bound nitrate reductase of Thiosphaera pantotropha results in anaerobic overexpression of periplasmic nitrate reductase activity. J. Gen. Microbiol. 139:3205-3214. [DOI] [PubMed] [Google Scholar]
- 3.Bell, L. C., D. J. Richardson, and S. J. Ferguson. 1990. Periplasmic and membrane-bound nitrate reductases in Thiosphaera pantotropha: the periplasmic enzyme catalyzes the first step in aerobic denitrification. FEBS Lett. 265:85-87. [DOI] [PubMed] [Google Scholar]
- 4.Berks, B. C., S. J. Ferguson, J. W. B. Moir, and D. J. Richardson. 1995. Enzymes and associated electron transport systems that catalyse the respiratory reduction of nitrogen oxides and oxyanions. Biochim. Biophys. Acta 1232:97-173. [DOI] [PubMed] [Google Scholar]
- 5.Berks, B. C., D. J. Richardson, C. Robinson, A. Reilly, R. T. Aplin, and S. J. Ferguson. 1994. Purification and characterization of the periplasmic nitrate reductase from Thiosphaera pantotropha. Eur. J. Biochem. 220:117-124. [DOI] [PubMed] [Google Scholar]
- 6.Blasco, F., F. Nunzi, J. Pommier, R. Brasseur, M. Chippaux, and G. Giordano. 1992. Formation of active heterologous nitrate reductases between nitrate reductases A and Z of Escherichia coli. Mol. Microbiol. 6:209-219. [DOI] [PubMed] [Google Scholar]
- 7.Blattner, F. R., G. Plunkett, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1462. [DOI] [PubMed] [Google Scholar]
- 8.Butler, C. S., J. M. Charnock, B. Bennett, H. J. Sears, A. J. Reilly, S. J. Ferguson, C. D. Garner, D. J. Lowe, A. J. Thomson, B. C. Berks, and D. J. Richardson. 1999. Models for molybdenum coordination during the catalytic cycle of periplasmic nitrate reductase from Paracoccus denitrificans derived from EPR and EXAFS spectroscopy. Biochemistry 38:9000-9012. [DOI] [PubMed] [Google Scholar]
- 9.Castilho, B. A., P. Olfson, and M. J. Casadaban. 1984. Plasmid insertion mutagenesis and lac gene fusion with mini-Mu bacteriophage transposons. J. Bacteriol. 158:488-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, L., L. I. Wei, J. P. Audia, R. A. Morton, and H. E. Schellhorn. 1999. Expression of the Escherichia coli NRZ nitrate reductase is highly growth phase dependent and is controlled by RpoS, the alternative vegetative sigma factor. Mol. Microbiol. 34:756-766. [DOI] [PubMed] [Google Scholar]
- 11.Choe, M., and W. S. Reznikoff. 1991. Anaerobically expressed Escherichia coli genes identified by operon fusion techniques. J. Bacteriol. 173:6139-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choe, M., and W. S. Reznikoff. 1993. Identification of the regulatory sequence of anaerobically expressed locus aeg-46.5. J. Bacteriol. 175:1165-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole, J. 1996. Nitrate reduction to ammonia by enteric bacteria: redundancy, or a strategy for survival during oxygen starvation? FEMS Microbiol. Lett. 136:1-11. [DOI] [PubMed] [Google Scholar]
- 14.Craske, A., and S. J. Ferguson. 1986. The respiratory nitrate reductase from Paracoccus denitrificans. Molecular characterisation and kinetic properties. Eur. J. Biochem. 158:429-436. [DOI] [PubMed] [Google Scholar]
- 15.Darwin, A. J., and V. Stewart. 1996. The NAR modulon systems: nitrate and nitrite regulation of anaerobic gene expression, p. 343-359. In E. C. C. Lin and A. S. Lynch (ed.), Regulation of gene expression in Escherichia coli. R. G. Landes Co., Georgetown, Tex.
- 16.Darwin, A. J., and V. Stewart. 1995. Nitrate and nitrite regulation of the Fnr-dependent aeg-46.5 promoter of Escherichia coli K-12 is mediated by competition between homologous response regulators (NarL and NarP) for a common DNA-binding site. J. Mol. Biol. 251:15-29. [DOI] [PubMed] [Google Scholar]
- 17.Darwin, A. J., K. L. Tyson, S. J. W. Busby, and V. Stewart. 1997. Differential regulation by the homologous response regulators NarL and NarP of Escherichia coli K-12 depends on DNA binding site arrangement. Mol. Microbiol. 25:583-595. [DOI] [PubMed] [Google Scholar]
- 18.Darwin, A. J., E. C. Ziegelhoffer, P. J. Kiley, and V. Stewart. 1998. Fnr, NarP, and NarL regulation of Escherichia coli K-12 napF (periplasmic nitrate reductase) operon transcription in vitro. J. Bacteriol. 180:4192-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeMoss, J. A., and P. Y. Hsu. 1991. NarK enhances nitrate uptake and nitrite excretion in Escherichia coli. J. Bacteriol. 173:3303-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fellay, R., J. Frey, and H. Krisch. 1987. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of Gram-negative bacteria. Gene 52:147-154. [DOI] [PubMed] [Google Scholar]
- 21.Gennis, R. B., and V. Stewart. 1996. Respiration, p. 217-261. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella. Cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 22.Goldman, B. S., and R. G. Kranz. 2001. ABC transporters associated with cytochrome c biogenesis. Res. Microbiol. 152:323-329. [DOI] [PubMed] [Google Scholar]
- 23.Grove, J., S. Busby, and J. Cole. 1996. The role of the genes nrfEFG and ccmFH in cytochrome c biosynthesis in Escherichia coli. Mol. Gen. Genet. 252:332-341. [DOI] [PubMed] [Google Scholar]
- 24.Grove, J., S. Tanapongpipat, G. Thomas, L. Griffiths, H. Crooke, and J. Cole. 1996. Escherichia coli K-12 genes essential for the synthesis of c-type cytochromes and a third nitrate reductase located in the periplasm. Mol. Microbiol. 19:467-481. [DOI] [PubMed] [Google Scholar]
- 25.Itoh, T., H. Aiba, T. Baba, K. Fujita, K. Hayashi, T. Inada, K. Isono, H. Kasai, S. Kimura, M. Kitakawa, M. Kitagawa, K. Makino, T. Miki, K. Mizobuchi, H. Mori, T. Mori, K. Motomura, S. Nakade, Y. Nakamura, H. Nashimoto, Y. Nishio, T. Oshima, N. Saito, G. Sampei, Y. Seki, S. Sivasundaram, H. Tagami, J. Takeda, K. Takemoto, C. Wada, Y. Yamamoto, and T. Horiuchi. 1996. A 460-kb DNA sequence of the Escherichia coli K-12 genome corresponding to the 40.1-50.0 min region on the linkage map. DNA Res. 3:379-392. [DOI] [PubMed] [Google Scholar]
- 26.Kepes, A. 1969. Transcription and translation in the lactose operon of Escherichia coli studied by in vivo kinetics. Prog. Biophys. Mol. Biol. 19:201-236. [DOI] [PubMed] [Google Scholar]
- 27.Kohara, Y., K. Akiyama, and K. Isono. 1987. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell 50:495-508. [DOI] [PubMed] [Google Scholar]
- 28.Kuritzkes, D. R., X.-Y. Zhang, and E. C. C. Lin. 1984. Use of Φ(glp-lac) in studies of respiratory regulation of the Escherichia coli anaerobic sn-glycerol-3-phosphate dehydrogenase genes (glpAB). J. Bacteriol. 157:591-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin, J. T., and V. Stewart. 1998. Nitrate assimilation by bacteria. Adv. Microb. Physiol. 38:1-30. [DOI] [PubMed] [Google Scholar]
- 30.Liu, H. P., S. Takio, T. Satoh, and I. Yamamoto. 1999. Involvement in denitrification of the napKEFDABC genes encoding the periplasmic nitrate reductase system in the denitrifying phototrophic bacterium Rhodobacter sphaeroides f. sp. denitrificans. Biosci. Biotechnol. Biochem. 63:530-536. [DOI] [PubMed] [Google Scholar]
- 31.MacGregor, C. H., C. A. Schnaitman, D. E. Normansell, and M. G. Hodgins. 1974. Purification and properties of nitrate reductase from Escherichia coli K-12. J. Biol. Chem. 249:5321-5327. [PubMed] [Google Scholar]
- 32.Maloy, S. R., V. J. Stewart, and R. K. Taylor. 1996. Genetic analysis of pathogeneic bacteria. A laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Metcalf, W. W., and B. L. Wanner. 1993. Construction of new β-glucuronidase cassettes for making transcriptional fusions and their use with new methods for allele replacement. Gene 129:17-25. [DOI] [PubMed] [Google Scholar]
- 34.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 35.Monod, J., A. M. Pappenheimer, Jr., and G. Cohen-Bazire. 1952. La cinétique de la biosynthèse de la β-galactosidase chez Escherichia coli considérée comme fonction de la croissance. Biochim. Biophys. Acta 9:648-660. [DOI] [PubMed]
- 36.Moreno-Vivián, C., and S. J. Ferguson. 1998. Definition and distinction between assimilatory, dissimilatory and respiratory pathways. Mol. Microbiol. 29:664-666. [DOI] [PubMed] [Google Scholar]
- 37.Postma, P. W., and J. W. Lengeler. 1985. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol. Rev. 49:232-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Potter, L., H. Angove, D. J. Richardson, and J. Cole. 2001. Nitrate reduction in the periplasm of gram-negative bacteria. Adv. Microb. Physiol. 45:51-112. [DOI] [PubMed] [Google Scholar]
- 39.Potter, L. C., P. Millington, L. Griffiths, G. H. Thomas, and J. A. Cole. 1999. Competition between Escherichia coli strains expressing either a periplasmic or a membrane-bound nitrate reductase: does Nap confer a selective advantage during nitrate-limited growth? Biochem J. 344:77-84. [PMC free article] [PubMed] [Google Scholar]
- 40.Potter, L. C., P. D. Millington, G. H. Thomas, R. A. Rothery, G. Giordano, and J. A. Cole. 2000. Novel growth characteristics and high rates of nitrate reduction of an Escherichia coli strain, LCB2048, that expresses only a periplasmic nitrate reductase. FEMS Microbiol. Lett. 185:51-57. [DOI] [PubMed] [Google Scholar]
- 41.Powell, B. S., M. P. Rivas, D. L. Court, Y. Nakamura, and C. L. Turnbough. 1994. Rapid confirmation of single copy lambda prophage integration by PCR. Nucleic Acids Res. 22:5765. (Erratum, 23:1278, 1995.) [DOI] [PMC free article] [PubMed]
- 42.Rabin, R. S., L. A. Collins, and V. Stewart. 1992. In vivo requirement of integration host factor for nitrate reductase (nar) operon expression in Escherichia coli K-12. Proc. Natl. Acad. Sci. USA 89:8701-8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabin, R. S., and V. Stewart. 1993. Dual response regulators (NarL and NarP) interact with dual sensors (NarX and NarQ) to control nitrate- and nitrite-regulated gene expression in Escherichia coli K-12. J. Bacteriol. 175:3259-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ralt, D., J. S. Wishnok, R. Fitts, and S. R. Tannenbaum. 1988. Bacterial catalysis of nitrosation: involvement of the nar operon of Escherichia coli. J. Bacteriol. 170:359-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richardson, D. J. 2000. Bacterial respiration: a flexible process for a changing environment. Microbiology 146:551-571. [DOI] [PubMed] [Google Scholar]
- 46.Richardson, D. J., and S. J. Ferguson. 1992. The influence of carbon substrate on the activity of the periplasmic nitrate reductase in aerobically grown Thiosphaera pantotropha. Arch. Microbiol. 157:535-537. [Google Scholar]
- 47.Richardson, D. J., G. F. King, D. J. Kelly, A. G. McEwan, S. J. Ferguson, and J. B. Jackson. 1988. The role of auxiliary oxidants in maintaining redox balance during phototrophic growth of Rhodobacter capsulatus on propionate or butyrate. Arch. Microbiol. 150:131-137. [Google Scholar]
- 48.Rowe, J. J., T. Ubbink-Kok, D. Molenaar, W. N. Konings, and A. J. M. Driessen. 1994. NarK is a nitrite-extrusion system involved in anaerobic nitrate respiration by Escherichia coli. Mol. Microbiol. 12:579-586. [DOI] [PubMed] [Google Scholar]
- 49.Sears, H. J., G. Sawers, B. C. Berks, S. J. Ferguson, and D. J. Richardson. 2000. Control of periplasmic nitrate reductase gene expression (napEDABC) from Paracoccus pantotrophus in response to oxygen and carbon substrates. Microbiology 146:2977-2985. [DOI] [PubMed] [Google Scholar]
- 50.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 51.Skorupski, K., and R. K. Taylor. 1996. Positive selection vectors for allelic exchange. Gene 169:47-52. [DOI] [PubMed] [Google Scholar]
- 52.Stewart, G. S. A. B., S. Lubinsky-Mink, C. G. Jackson, A. Kassel, and J. Kuhn. 1986. pHG165: a pBR322 copy number derivative of pUC8 for cloning and expression. Plasmid 15:172-181. [DOI] [PubMed] [Google Scholar]
- 53.Stewart, V. 1988. Nitrate respiration in relation to facultative metabolism in enterobacteria. Microbiol. Rev. 52:190-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stewart, V. 1982. Requirement of Fnr and NarL functions for nitrate reductase expression in Escherichia coli K-12. J. Bacteriol. 151:1320-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stewart, V., and C. H. MacGregor. 1982. Nitrate reductase in Escherichia coli: involvement of chlC, chlE, and chlG loci. J. Bacteriol. 151:788-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stewart, V., and J. Parales, Jr. 1988. Identification and expression of genes narL and narX of the nar (nitrate reductase) locus in Escherichia coli K-12. J. Bacteriol. 170:1589-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stewart, V., and R. S. Rabin. 1995. Dual sensors and dual response regulators interact to control nitrate- and nitrite-responsive gene expression in Escherichia coli, p. 233-252. In J. A. Hoch and T. J. Silhavy (ed.), Two-component signal transduction. ASM Press, Washington, D.C.
- 58.Stewart, V., and C. Yanofsky. 1986. Role of leader peptide synthesis in tryptophanase operon expression in Escherichia coli K-12. J. Bacteriol. 167:383-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanapongpipat, S., E. Reid, J. A. Cole, and H. Crooke. 1998. Transcriptional control and essential roles of the Escherichia coli ccm gene products in formate-dependent nitrite reduction and cytochrome c synthesis. Biochem. J. 334:355-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thomas, G., L. Potter, and J. A. Cole. 1999. The periplasmic nitrate reductase from Escherichia coli: a heterodimeric molybdoprotein with a double-arginine signal sequence and an unusual leader peptide cleavage site. FEMS Microbiol. Lett. 174:167-171. [DOI] [PubMed] [Google Scholar]
- 61.Thöny-Meyer, L. 2000. Haem-polypeptide interactions during cytochrome c maturation. Biochim. Biophys. Acta 1459:316-324. [DOI] [PubMed] [Google Scholar]
- 62.Tomsett, A. B., and R. H. Garrett. 1980. The isolation and characterization of mutants defective in nitrate assimilation in Neurospora crassa. Genetics 95:649-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tran, Q. H., J. Bongaerts, D. Vlad, and G. Unden. 1997. Requirement for the proton-pumping NADH dehydrogenase I of Escherichia coli in respiration of NADH to fumarate and its bioenergetic implications. Eur. J. Biochem. 244:155-160. [DOI] [PubMed] [Google Scholar]
- 64.Tyson, K. L., J. A. Cole, and S. J. W. Busby. 1994. Nitrite and nitrate regulation at the promoters of two Escherichia coli operons encoding nitrite reductase: identification of common target heptamers for both NarP- and NarL-dependent regulation. Mol. Microbiol. 13:1045-1055. [DOI] [PubMed] [Google Scholar]
- 65.Unden, G., and J. Bongaerts. 1997. Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim. Biophys. Acta 1320:217-234. [DOI] [PubMed] [Google Scholar]
- 66.Vieira, J., and J. Messing. 1982. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259-268. [DOI] [PubMed] [Google Scholar]
- 67.Wang, H., and R. P. Gunsalus. 2000. The nrfA and nirB nitrite reductase operons in Escherichia coli are expressed differently in response to nitrate than to nitrite. J. Bacteriol. 182:5813-5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang, H., C.-P. Tseng, and R. P. Gunsalus. 1999. The napF and narG nitrate reductase operons in Escherichia coli are differentially expressed in response to submicromolar concentrations of nitrate but not nitrite. J. Bacteriol. 181:5303-5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Winans, S. C., S. J. Elledge, J. H. Krueger, and G. C. Walker. 1985. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J. Bacteriol. 161:1219-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu, Q., and V. Stewart. 1998. NasFED proteins mediate assimilatory nitrate and nitrite transport in Klebsiella oxytoca (pneumoniae) M5al. J. Bacteriol. 180:1311-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]