Abstract
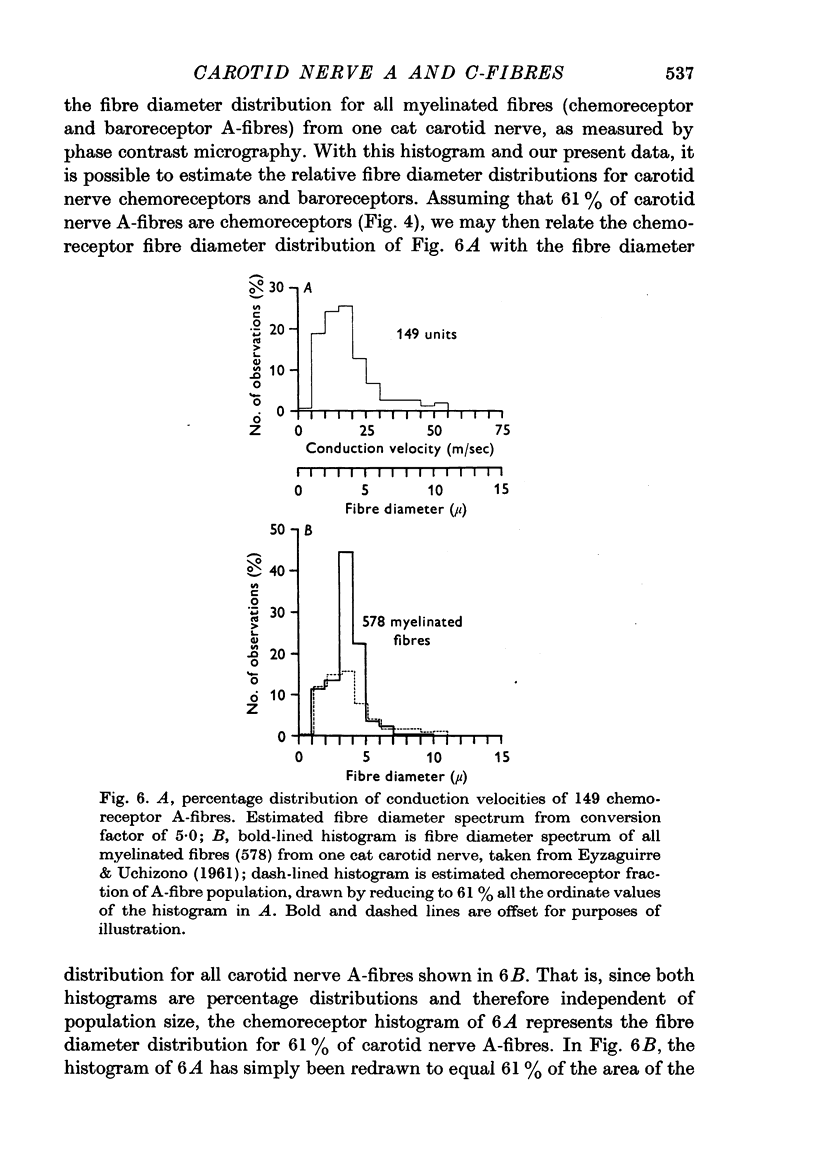
1. 149 A-fibres and 52 C-fibres from the cat carotid nerve were studied in vivo with single-unit recording techniques. These units subserved chemoreceptor and baroreceptor modalities. In addition, half of the C-fibres were determined to be efferent in origin. The estimated fibre diameter spectrum for chemoreceptor and baroreceptor A-fibres is described.
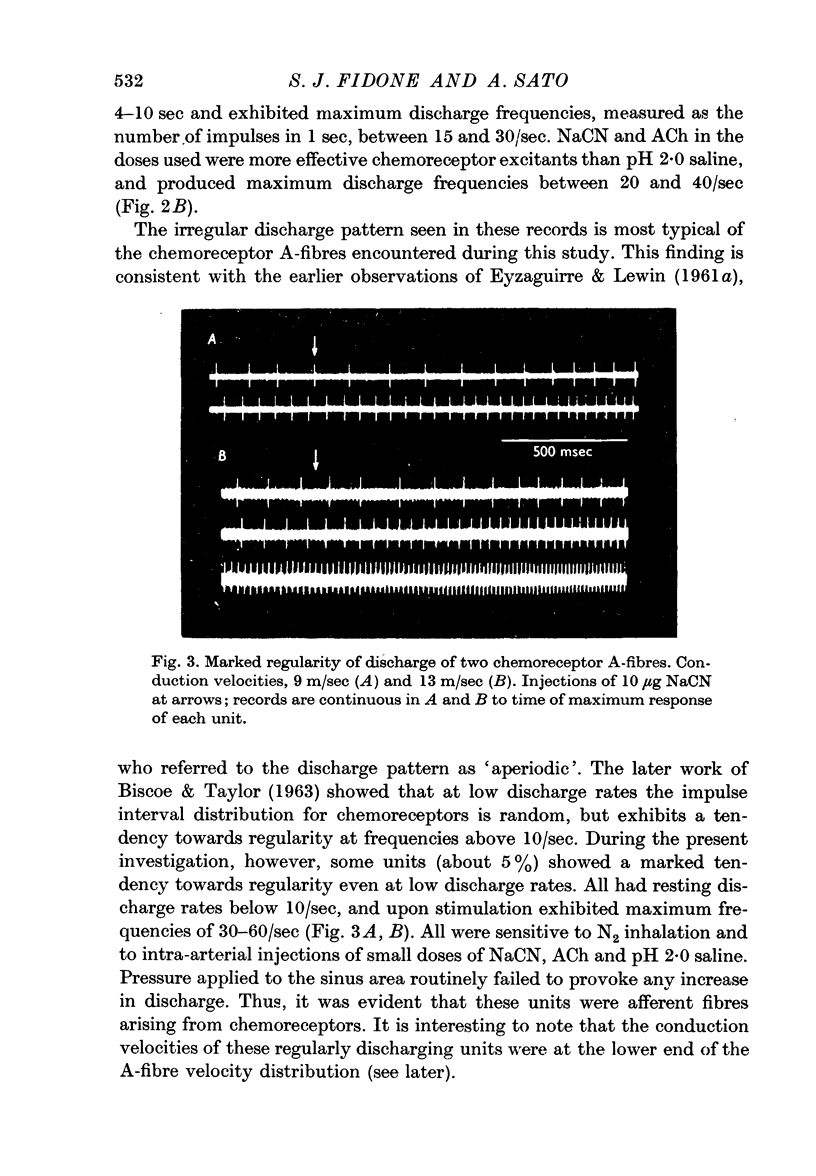
2. The discharge pattern of chemoreceptor A and C-fibres was characteristically irregular both at rest and during activation. However, about 5% of the chemoreceptor A-fibre population exhibited a very regular discharge pattern, even at low rates of firing.
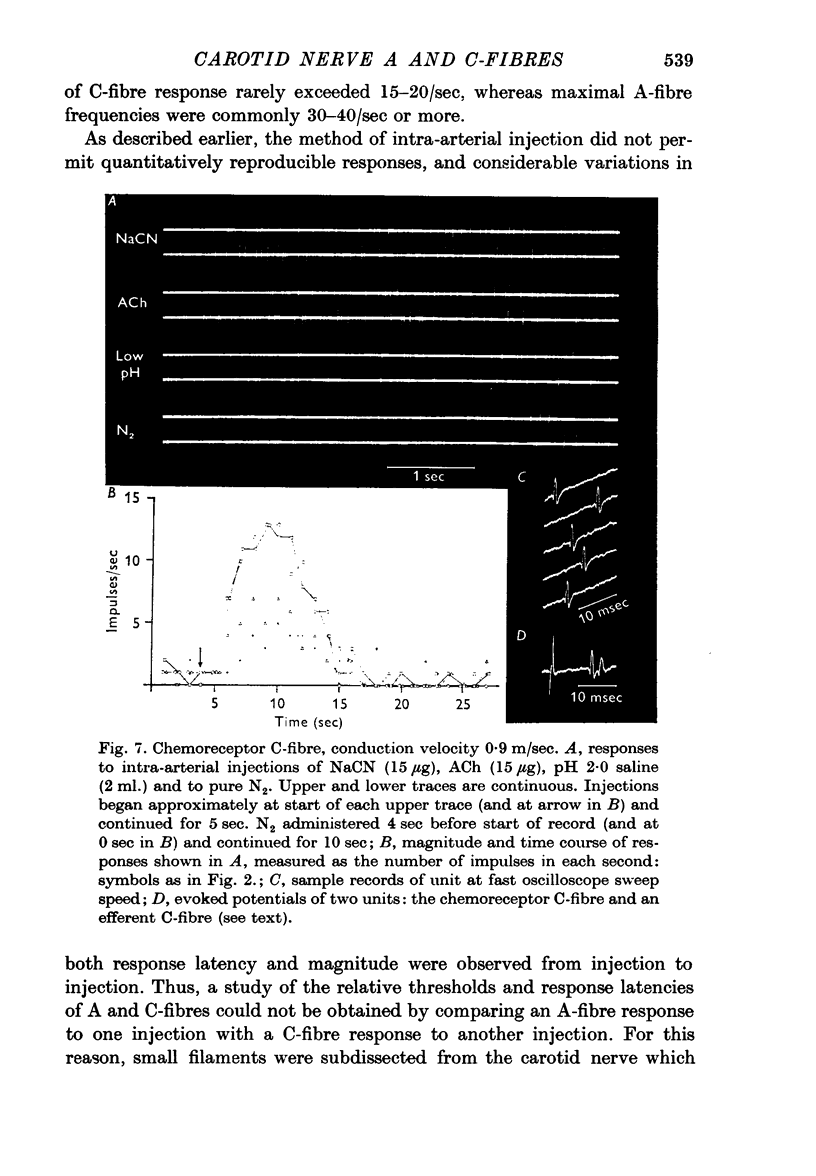
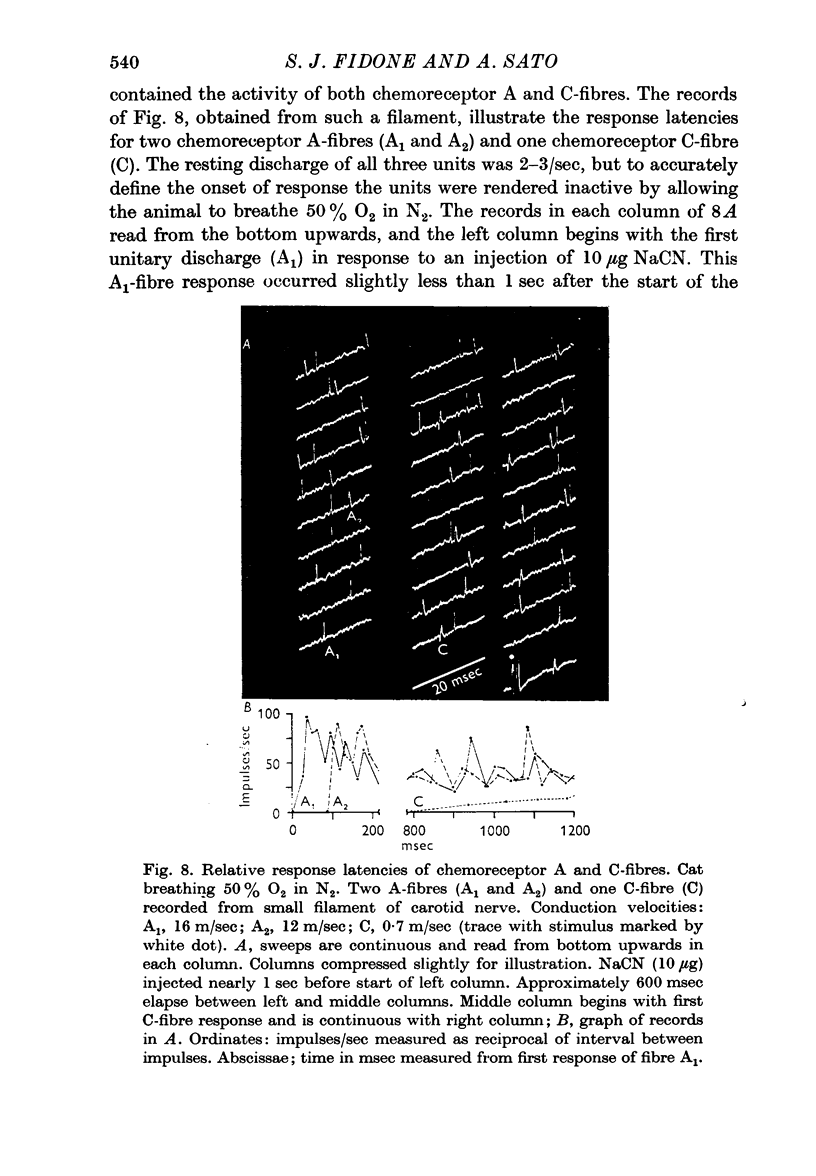
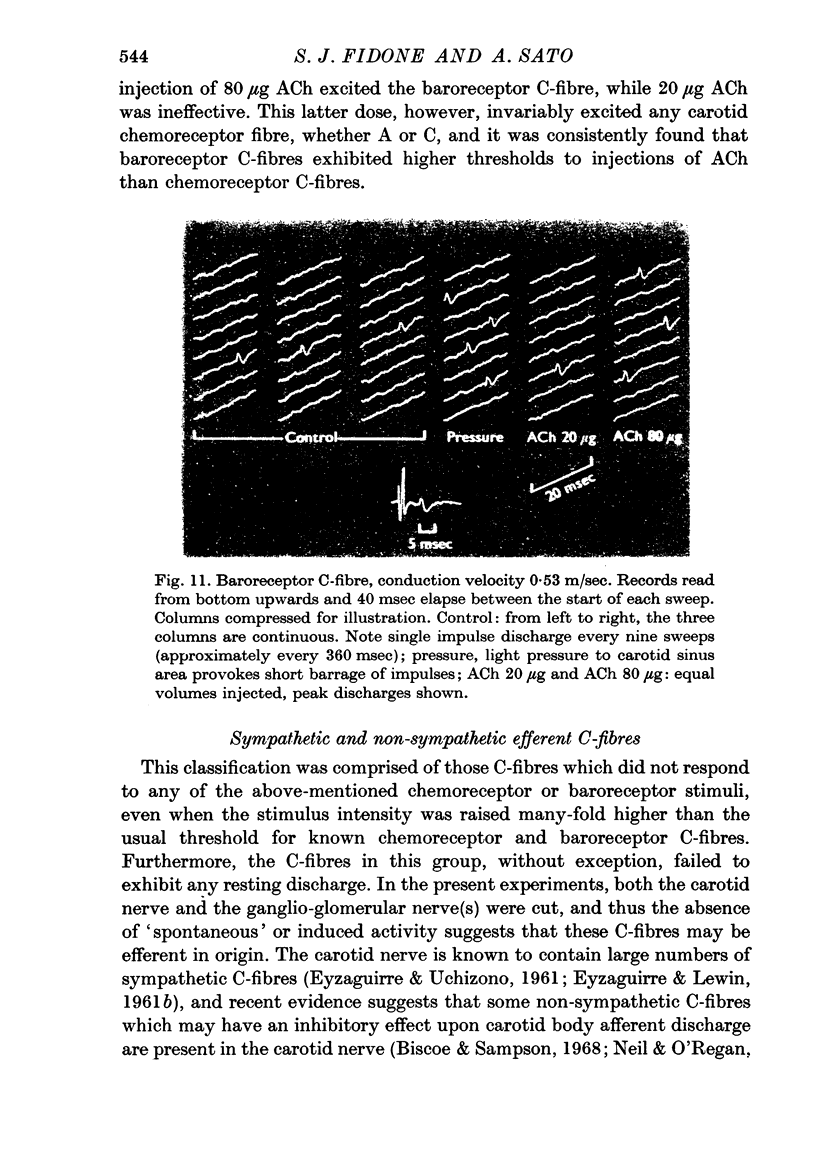
3. In comparing A and C-fibres, it was found that chemoreceptor and baroreceptor A-fibres had lower thresholds, shorter response latencies, more rapid acceleration of discharge and higher discharge frequencies than their C-fibre counterparts.
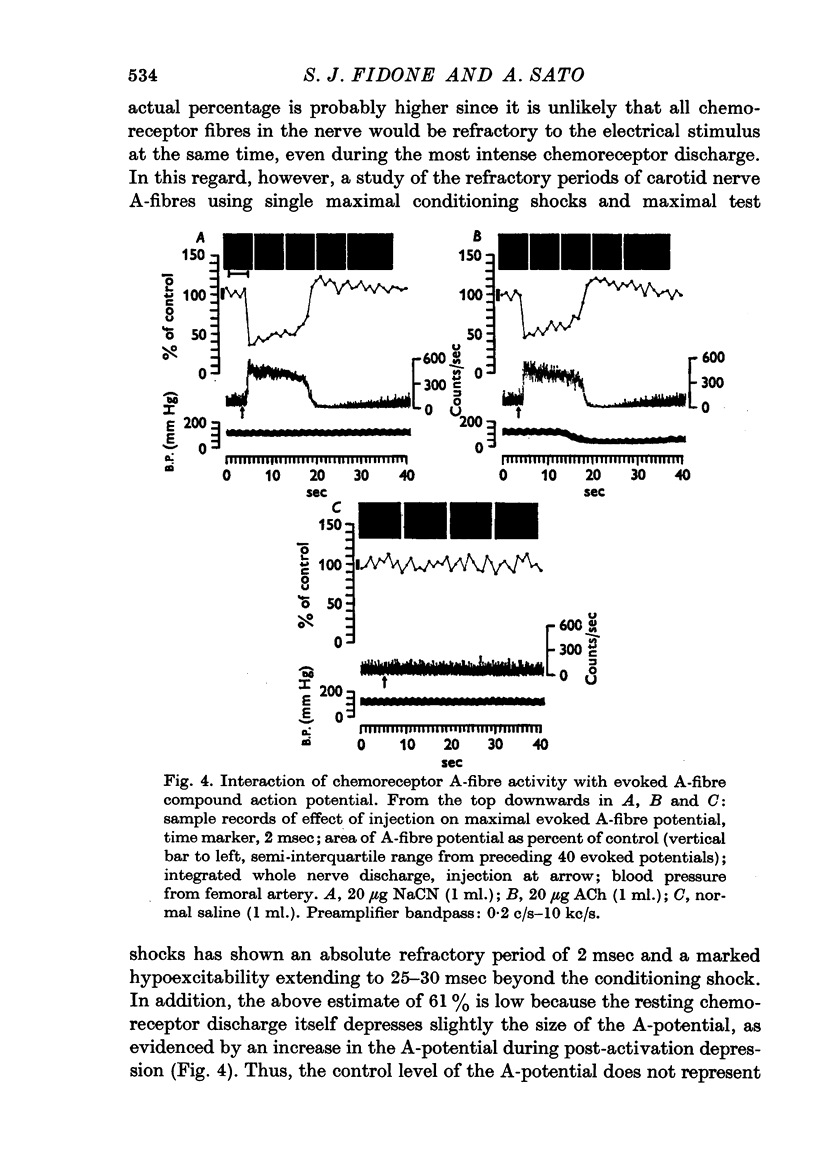
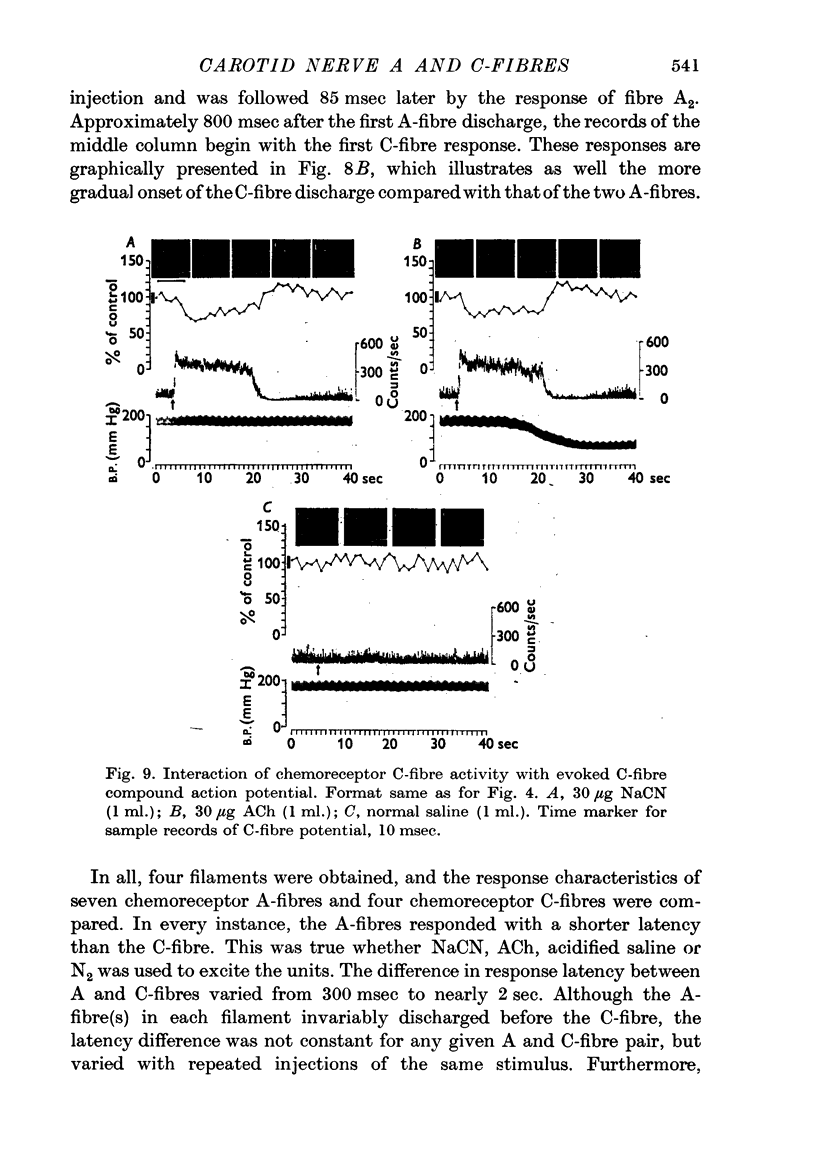
4. During strong chemoreceptor or baroreceptor stimulation, interaction of the `spontaneous' whole nerve activity with the evoked A and C-fibre compound action potentials provided a method of estimating the relative proportions of chemoreceptors and baroreceptors in the A and C-fibre populations of the carotid nerve. The A-fibre population was found to be comprised of approximately 2/3 chemoreceptors, 1/3 baroreceptors. The reverse was true for the C-fibre population, i.e. 2/3 baroreceptors, 1/3 chemoreceptors.
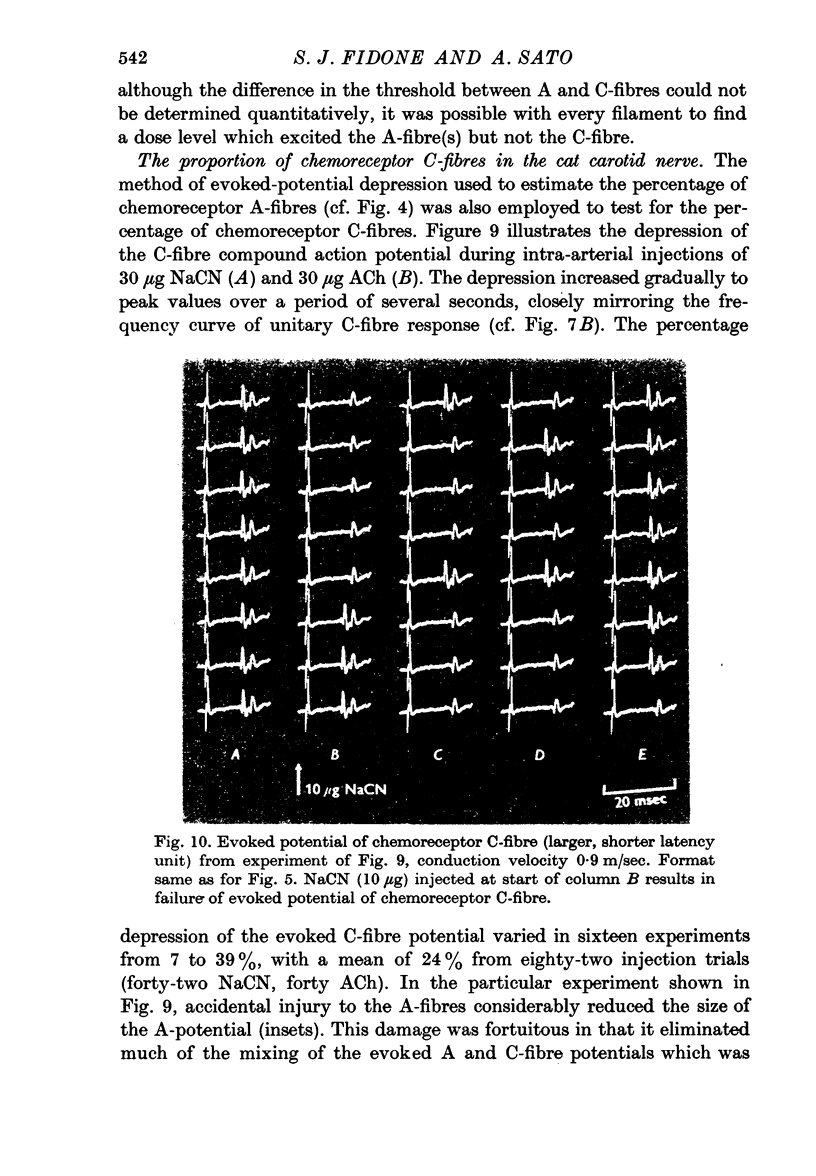
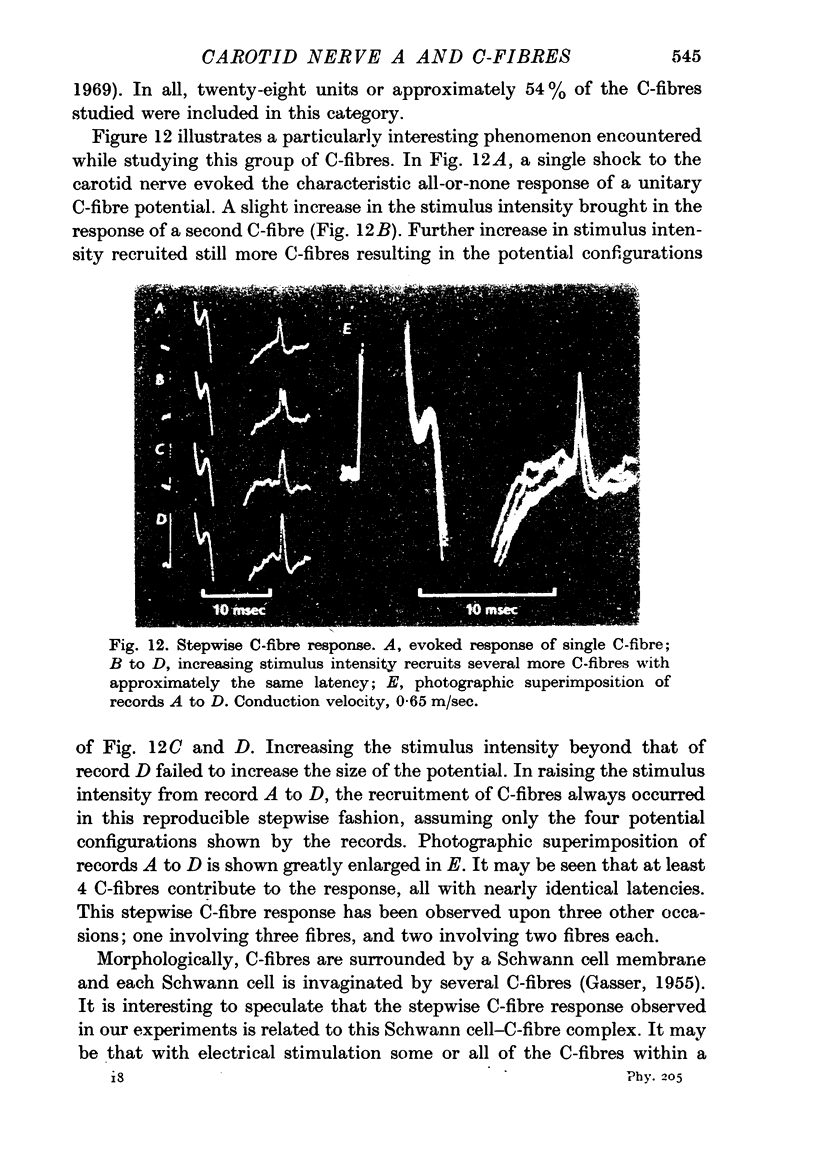
5. A stepwise C-fibre response is described which may arise from the several C-fibres within a single Schwann cell.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMETT C. J., RITCHIE J. M. The action of acetylcholine on conduction in mammalian non-myelinated fibres and its prevention by an anticholinesterase. J Physiol. 1960 Jun;152:141–158. doi: 10.1113/jphysiol.1960.sp006476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armett C. J., Ritchie J. M. The ionic requirements for the action of acetylcholine on mammalian non-myelinated fibres. J Physiol. 1963 Jan;165(1):141–159. doi: 10.1113/jphysiol.1963.sp007048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISCOE T. J., TAYLOR A. THE DISCHARGE PATTERN RECORDED IN CHEMORECEPTOR AFFERENT FIBRES FROM THE CAT CAROTID BODY WITH NORMAL CIRCULATION AND DURING PERFUSION. J Physiol. 1963 Sep;168:332–344. doi: 10.1113/jphysiol.1963.sp007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Sampson S. R. Rhythmical and non-rhythmical spontaneous activity recorded from the central cut end of the sinus nerve. J Physiol. 1968 May;196(2):327–338. doi: 10.1113/jphysiol.1968.sp008510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE CASTRO F. Sur la structure de la synapse dans les chemocepteurs; leur mécanisme d'excitation et rôle dans la circulation sanguine locale. Acta Physiol Scand. 1951 Feb 21;22(1):14–43. doi: 10.1111/j.1748-1716.1951.tb00747.x. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RITCHIE J. M. A technique for recording functional activity in specific groups of medullated and non-medullated fibres in whole nerve trunks. J Physiol. 1957 Aug 29;138(1):19–30. doi: 10.1113/jphysiol.1957.sp005835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RITCHIE J. M. Cardiovascular reflexes produced by electrical excitation of non-medullated afferents in the vagus, carotid sinus and aortic nerves. J Physiol. 1956 Oct 29;134(1):167–178. doi: 10.1113/jphysiol.1956.sp005632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EYZAGUIRRE C., LEWIN J. Effect of different oxygen tensions on the carotid body in vitro. J Physiol. 1961 Dec;159:238–250. doi: 10.1113/jphysiol.1961.sp006805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EYZAGUIRRE C., LEWIN J. The effect of sympathetic stimulation on carotid nerve activity. J Physiol. 1961 Dec;159:251–267. doi: 10.1113/jphysiol.1961.sp006806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EYZAGUIRRE C., UCHIZONO K. Observations on the fibre content of nerves reaching the carotid body of the cat. J Physiol. 1961 Dec;159:268–281. doi: 10.1113/jphysiol.1961.sp006807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidone S., Sato A., Eyzaguirre C. Acetylcholine activation of carotid body chemoreceptor A fibers. Brain Res. 1968 Jul;9(2):374–376. doi: 10.1016/0006-8993(68)90242-4. [DOI] [PubMed] [Google Scholar]
- GASSER H. S. Properties of dorsal root unmedullated fibers on the two sides of the ganglion. J Gen Physiol. 1955 May 20;38(5):709–728. doi: 10.1085/jgp.38.5.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGGO A. Cutaneous mechanoreceptors with afferent C fibres. J Physiol. 1960 Jul;152:337–353. doi: 10.1113/jphysiol.1960.sp006491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintal A. S. Mechanism of stimulation of aortic chemoreceptors by natural stimuli and chemical substances. J Physiol. 1967 Mar;189(1):63–84. doi: 10.1113/jphysiol.1967.sp008155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A., Fidone S., Eyzaguirre C. Presence of chemoreceptor and baroreceptor C-fibers in the carotid nerve of the cat. Brain Res. 1968 Nov;11(2):459–463. doi: 10.1016/0006-8993(68)90041-3. [DOI] [PubMed] [Google Scholar]


