Abstract
Several bacteria can grow by using small organic compounds such as trimethylamine oxide (TMAO) as electron acceptors. In Shewanella species, the TMAO reductase respiratory system is encoded by the torECAD operon. We showed that production of the TMAO reductase of S. oneidensis was induced by TMAO and repressed by oxygen, and we noticed that a three-gene cluster (torSTR) encoding a complex two-component regulatory system was present downstream of the torECAD operon. We introduced the torSTR gene cluster into Escherichia coli and showed that this regulatory gene cluster is involved in TMAO induction of the torE promoter but plays no role in the oxygen control. The TorR response regulator was purified, and gel shift and footprinting experiments revealed that TorR binds to a single region located about 70 bases upstream of the transcription start site of the tor structural operon. By deletion analysis, we confirmed that the TorR operator site is required for induction of the tor structural promoter. As the TMAO regulatory system of S. oneidensis is homologous to that of E. coli, we investigated a possible complementation between the TMAO regulatory components of the two bacteria. Interestingly, TorSec, the TMAO sensor of E. coli, was able to transphosphorylate TorRso, the TMAO response regulator of S. oneidensis.
Under anaerobic conditions, many bacteria can grow by using alternative terminal electron acceptors such as nitrate, nitrite, dimethyl sulfoxide, or trimethylamine oxide (TMAO) (4, 27). TMAO is a widespread low-molecular-mass constituent of marine invertebrates and fish, in which it provides protection against stresses such as high concentrations of urea or hydrostatic pressure (36, 37). Various distant species, including enteric, photosynthetic, and marine bacteria, can obtain energy by reduction of TMAO into volatile trimethylamine (3, 11).
TMAO respiratory systems have been widely studied at the molecular level in Escherichia coli and Rhodobacter and Shewanella species (8, 19, 21, 29, 33). A common feature of these systems is the presence of three conserved structural components: a periplasmic molybdenum-containing reductase, a pentahemic c-type cytochrome anchored to the membrane, and a reductase-specific chaperone. Although TMAO is mainly found in the marine environment, few molecular studies have been performed for the TMAO respiratory system of marine bacteria, and little is known about the TMAO reductase system of Vibrio species (8, 26).
In Shewanella massilia, the terminal reductase TorA is encoded by the third gene of the torECAD operon. torC, torD, and torE encode, respectively, TorC, a membrane-anchored pentahemic c-type cytochrome; TorD, a TorA-specific chaperone; and TorE, a small membranous protein homologous to the NapE protein of Thiosphaera pantotropha (8). NapE belongs to the nitrate reductase system, but the roles of NapE and TorE are not yet known (4).
In E. coli, in addition to the torCAD structural operon, the tor locus comprises two regulatory genes, torS and torR, which encode the TorS/TorR two-component regulatory system (15). TorS is the transmembrane sensor that detects the presence of TMAO in the medium, and TorR is the response regulator that, once phosphorylated by TorS, activates the tor operon by binding to three regulatory sites (2, 14, 31). A third regulatory gene, torT, encoding a periplasmic protein similar to the ribose-binding protein, is also found in the tor locus (16). TorT probably binds a still unknown inducer and then interacts with the periplasmic detector region of TorS to stimulate tor induction. In Rhodobacter species, a two-component regulatory system homologous to the TorS/TorR proteins of E. coli is found, but the Rhodobacter system contains no protein similar to TorT (22, 29, 34). In these organisms, the regulatory gene clusters are located upstream of the structural operons (15, 21, 34). In S. massilia, although the Tor system is induced by TMAO, no regulatory gene was found upstream of the torECAD operon (8).
Usually, full induction of the TMAO reductase operons occurs in anaerobiosis. In Rhodobacter species, the anaerobic control is mediated by FnrL, a general anaerobic regulator (22, 38), whereas, in E. coli, it is mediated neither by FNR nor by ArcA, another global anaerobic regulator (32). In Shewanella oneidensis (formerly S. putrefaciens MR1), EtrA, an FNR analog, does not seem to be involved in TMAO reductase gene expression (18).
In this study, we show that the tor structural operon of S. oneidensis is tightly controlled by TMAO and, to a lesser extent, by anaerobiosis. The TMAO regulatory proteins of S. oneidensis constitute a complex two-component system related to that of E. coli, but tor operon induction requires binding of the response regulator to only one operator site.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
The bacterial strains and plasmids used in this work are listed in Table 1. The S. oneidensis strain (23) was grown at 30°C in rich medium with 50 mM TMAO when necessary. To maintain selection for plasmids or for transductants in E. coli, ampicillin and chloramphenicol were added at 50 μg/ml and kanamycin at 10 μg/ml. E. coli was grown at 37°C in Luria broth medium supplemented with 10 mM TMAO and 0.001% arabinose when required. In order to limit torRso expression from pTorRso2, 0.05% glucose was added when indicated. E. coli strain LCB436 was constructed by P1 transduction of the deletion (ΔtorSTRCAD)-insertion (Kmr) of strain BW26416 (7) into strain MC4100. The deletion of the tor locus in strain LCB436 was checked by PCR with appropriate primers.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| E. coli | ||
| MC4100 | araD139 Δ(lacIPOZYA-argF)U169 rpsL thi | M. J. Casadaban |
| LCB621 | MC4100 torR49::mini-Tn 10 (Tcr) | 32 |
| BW26416 | BW25113 Δ(torSTRCAD) Kmr | 7 |
| LCB436 | Δ(torSTRCAD) Kmr derivative of MC4100 | This work |
| S. oneidensis ATCC 700550 | Wild type, formerly S. putrefaciens MR1 | 23 |
| Plasmids | ||
| pBAD24 | Vector containing P BAD promoter with a pBR origin of replication | 13 |
| pBAD33 | Vector containing P BAD promoter with a pACYC origin of replication | 13 |
| pGE593 | Operon fusion vector with a pBR origin of replication | 10 |
| pRSO1 | torR sequence from S. oneidensis inserted into pBAD24 | This work |
| pRSO2 | torR sequence from S. oneidensis inserted into pBAD33 | This work |
| pPTorSO4 | torE promoter (−481 to +217)a from S. oneidensis cloned into pGE593 | This work |
| pPTorSO7 | torE promoter (−84 to +119)a from S. oneidensis cloned into pGE593 | This work |
| pPTorSO9 | torE promoter (−60 to +119)a from S. oneidensis cloned into pGE593 | This work |
| pSTRSO | torSTR sequence from S. oneidensis cloned into pBAD33 | This work |
| pTRSO | pSTRSO but with torS partly deleted | This work |
| pSRSO | pSTRSO but with torT disrupted | This work |
| pSSO | pSTRSO but with torR and part of torT deleted | This work |
Nucleotide positions relative to the transcription start site of torE.
DNA manipulations.
DNA preparations, restriction endonuclease digestions, purifications, and ligations were carried out according to standard procedures (28). Transformations were performed as described by Chung and Miller (5).
Construction of plasmids.
To create plasmid pRso1, we employed PCR with S. oneidensis chromosomal DNA as the template to generate DNA fragments corresponding to the torRso coding sequence with an upstream EcoRI and a downstream SmaI site. After enzymatic hydrolysis, the PCR product was cloned into the same sites of pBAD24 (13). To create plasmid pRso2, pRso1 was digested with BamHI, and the resulting torRso-containing fragment was subcloned into the same sites of pBAD33 (13). In these plasmids, torR is under the control of the arabinose-inducible promoter.
To create plasmid pSTRso, we used PCR with S. oneidensis chromosomal DNA as the template to generate a torSTR-containing DNA fragment (from position −182 relative to the first nucleotide of the initiation codon of torR to the stop codon of torS) with an upstream XbaI and a downstream SmaI site. The PCR product was cloned into the same sites of pBAD33, and the orientation was PBAD-torRTS. To create plasmid pTRso, the torS sequence of plasmid pSTRso was partly deleted (from position +1899 relative to the initiation codon of torS) after PstI digestion, followed by intramolecular ligation. To create plasmid pSRso, the KpnI site of the torT gene of plasmid pSTRso was cleaved and blunted with T4 DNA polymerase (blunting kit from Takara) before ligation, leading to a frameshift at position +582 relative to the initiation codon of torT. To create plasmid pSso, torR and a part of torT were deleted from plasmid pSTRso after KpnI digestion, followed by intramolecular ligation.
We amplified the torE promoter region by PCR from positions −481 to +217, −84 to +119, and −60 to +119 relative to the torE transcription start site to create plasmids pPTorso4, pPTorso7, and pPTorso9, respectively. The DNA fragments were blunted and introduced into plasmid pGE593 (10), previously linearized by SmaI, thus placing the lacZ coding sequence under the control of the putative torE promoter. Except for the large insert of plasmid pSTRso, all the cloned fragments and fusion sites were confirmed by sequencing. To minimize the number of spontaneous mutations, the torSTRso gene cluster was amplified with only 20 cycles, and three independent pSTRso clones were tested.
Analytical procedure.
Crude extracts of S. oneidensis were made as previously described for E. coli (30). The periplasmic fraction was prepared by the procedure of Easter et al. (9). The periplasmic proteins were analyzed by sodium dodecyl sulfate-7.5% polyacrylamide gel electrophoresis (SDS-7.5% PAGE). After electrophoresis, TMAO reductase was visualized either directly by an activity staining method (25) or by immunodetection with antibodies against TorA of S. massilia after protein transfer to a Hybond enhanced chemiluminescence (ECL) nitrocellulose membrane. The ECL-Western blotting system was used as described by the supplier (Amersham Pharmacia Biotech). The protein concentrations were estimated by the technique of Lowry et al. (17).
Primer extension analysis.
Strain LCB436 carrying pPTorso4 and pRso2 and the S. oneidensis strain were grown anaerobically in the presence of TMAO until the culture reached an A600 of 0.5. Total RNA was prepared by using the Qiagen RNA extraction kit. The oligonucleotide 5"-AAGAGTATGAAAATGATGAATCCCAG-3", complementary to a torE coding region, was end labeled with [γ-33P]ATP (2,500 Ci/mmol) by using T4 polynucleotide kinase and purified with the Qiagen Qiaquick nucleotide removal kit. The primer extension reaction was performed with reverse transcriptase (Superscript II from Gibco-BRL). The sequencing ladder was generated with the same oligonucleotide used for the primer extensions.
β-Galactosidase assays.
β-Galactosidase activities were measured on whole cells by the method of Miller (20) either after overnight anaerobic growth or after aerobic growth performed in rotating Erlenmeyer flasks when the culture reached 0.4 to 0.5 A600 unit. Values represent the averages of at least three independent experiments with a variation of no more than 15% from the mean.
Purification of TorR and TorS proteins.
Overproduction of the TorRso protein was achieved by growing 100 ml of strain MC4100 carrying plasmid pRso1. When the culture reached an A600 of 1, overproduction of the TorR protein was induced for 1 h with 0.2% arabinose. The cells were then harvested by centrifugation, and the pellet was resuspended in 5 ml of 40 mM Tris-HCl, pH 7.6. After disruption of the cells by French press, the extract was centrifuged at 14,000 rpm for 10 min. The supernatant was directly loaded on a heparin-Sepharose column (Pharmacia). The proteins were eluted with a step gradient of KCl from 100 mM to 1 M. TorRso was purified to near homogeneity in the 400 mM KCl fraction. The E. coli TorR and TorS726 proteins were purified as described previously (14, 32).
Preparation of labeled DNA fragments.
A 295-bp and a 271-bp DNA fragment encompassing the tor regulatory region were generated by PCR from pPTorso7 and pPTorso9 with appropriate labeled and unlabeled primers. Labeling was carried out with [γ-32P]ATP (4,000 Ci/mmol) and T4 polynucleotide kinase (Gibco-BRL), and the labeled fragments were separated from unincorporated nucleotides by using a column of the nucleotide removal kit from Qiagen.
Gel retardation assays.
Binding of TorR to labeled DNA fragments (7 nM) was carried out in a 4-μl reaction mixture containing 50 mM Tris-HCl (pH 8), 1.25 mM EDTA, 0.25 M sucrose, 0.025% bromophenol blue, and 0.25 μg of poly(dI-dC)·poly(dI-dC) per μl. After 30 min at room temperature, samples were loaded and run on a native 12.5% polyacrylamide gel (Pharmacia Phast System). The gel was exposed for 3 h at room temperature.
DNase I footprinting.
The footprinting experiments were performed as follows. The labeled DNA fragment was diluted to a concentration of about 1 nM in 50 μl of binding mix [10 mM Tris-HCl (pH 7.5), 50 mM NaCl, 2.5 mM MgCl2, 0.5 mM dithiothreitol, 4% glycerol, and 30 ng of poly(dI-dC)·poly(dI-dC) per μl], and different amounts of TorR were then added. After 30 min of incubation at room temperature, DNase I was added (0.33 U), and the reaction was conducted for 1 min, then stopped by the addition of 140 μl of DNase stop solution (192 mM sodium acetate, 32 mM EDTA, 0.14% SDS, and 64 μg of yeast RNA per ml). After phenol-chloroform extraction and DNA-ethanol precipitation, the pellets were resuspended in loading solution (95% formamide, 20 mM EDTA, 0.05% bromophenol blue, 0.05% xylene cyanol) and loaded on a 6% polyacrylamide-8.3 M urea electrophoresis gel. The location of the protected nucleotides was deduced by running a ladder with the products of the G+A cleavage reaction.
Phosphorylation assays.
For the phosphorylation experiments, proteins TorS726, TorRso, and TorRec (5 μM each) were incubated 10 min with 0.1 mM [γ-32P]-ATP (1 μCi) in 10 μl of buffer containing 50 mM Tris-HCl (pH 7.6), 4 mM dithiothreitol (DTT), 0.5 mM EDTA, 5 mM MgCl2, 10% glycerol, and 60 mM KCl. The reactions were stopped by addition of 5 μl of loading buffer (200 mM Tris-HCl [pH 8.8], 5 mM EDTA, 1 M sucrose, 0.1% bromophenol blue, 6% SDS, 0.1 M DTT, 1.6% β-mercaptoethanol) and incubated at 55°C for 3 min. The samples were analyzed after SDS-PAGE with the Pharmacia Phastsystem apparatus.
RESULTS AND DISCUSSION
Induction of TMAO reductase in S. oneidensis.
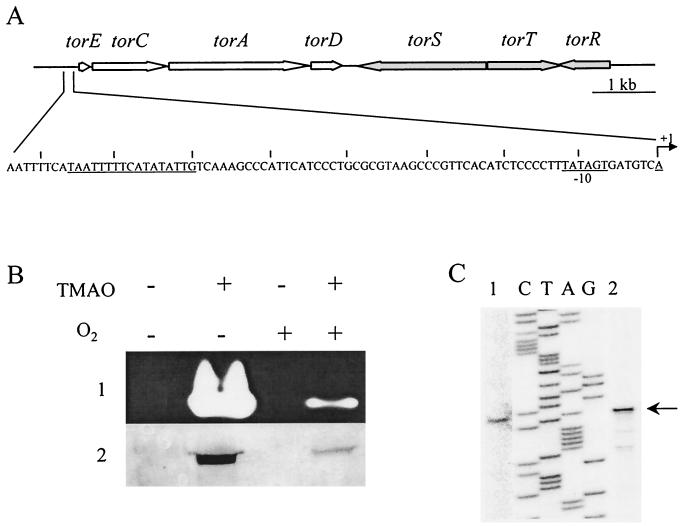
During a structural analysis of the periplasmic TMAO reductase terminal enzyme (TorAsm) of S. massilia, we also sequenced the gene encoding the TMAO reductase (TorAso) of S. oneidensis strain MR1 (formerly S. putrefaciens MR1) (6, 35). Starting from this sequence, we retrieved the surrounding DNA sequence of torAso from the website of the Institute for Genomic Research (http://www.tigr.org). Analysis of the sequence revealed that torAso belongs to a four-gene cluster (Fig. 1A) homologous to the torECAD operon of S. massilia, and the gene products show 83, 98, 93, and 70% identity with TorE, TorC, TorA, and TorD of S. massilia, respectively.
FIG. 1.
(A) Physical map of the tor locus of S. oneidensis. The large arrows show the locations and orientations of the tor genes. The nucleotide sequence of the torECAD regulatory region is indicated. The +1 arrow corresponds to the transcription start site. The putative −10 promoter box and the TorR binding region are underlined. (B) SDS-PAGE analysis of TMAO reductase production of S. oneidensis grown anaerobically (−O2) or aerobically (+O2), in the presence (+) or absence (−) of TMAO. Nonheated (row 1) or heated (row 2) periplasmic extracts (15 μg) were loaded on the gel. After electrophoresis, the presence of TorAso was checked by staining the gel for TMAO reductase activity (row 1) or by a Western blot with anti-TorAsm antibodies (row 2). (C) Localization of the transcription start site of torE. A labeled primer complementary to torE internal sequence was annealed to total RNA from S. oneidensis cells grown anaerobically in the presence of TMAO (lane 1) or to RNA from E. coli LCB436 (pPTor4-pRso2) cells grown anaerobically in the presence of 0.001% arabinose (lane 2), and extended with reverse transcriptase. The primer extension reactions were loaded on each side of a sequencing ladder of the torE region made with the same primer as in the extension reaction.
To characterize the pattern of expression of the putative torECAD operon of S. oneidensis, we examined the inducing conditions for the TorAso enzyme. Periplasmic extracts were prepared from S. oneidensis cells grown aerobically or anaerobically in the presence or absence of TMAO, and TMAO reductase activity was detected after loading nonheated samples on an SDS-polyacrylamide gel (Fig. 1B). Maximum activity was observed for the strain grown anaerobically with TMAO, and the mobility of the active band corresponded to that expected for TorA (about 90 kDa). The presence of oxygen during growth strongly reduced the activity level, and no activity was detected in the absence of TMAO.
To confirm that the TMAO reductase active bands corresponded to TorA enzyme, we carried out a Western blot from an equivalent gel with antibodies against TorAsm. TorAsm and TorAso are highly homologous, and the anti-TorAsm antibodies recognized TorAso (Fig. 1B). Antibody binding to TorAso confirmed that the intensity of the TMAO reductase active bands reflects the amount of TorA enzyme. Strict control by the substrate and, to a lesser extent, by anaerobiosis appears to be a general regulatory feature of TMAO respiratory systems, since the same inducing conditions have been observed in various bacteria (8, 15, 22, 26). However, the cis and trans elements involved in the regulation of Shewanella species have not yet been characterized.
To define the promoter of the torECAD operon of S. oneidensis, we carried out a primer extension experiment using RNA prepared from cells grown anaerobically with TMAO (Fig. 1C). The transcription start site was located 33 bases upstream of the torE start codon, and a −10 promoter box (TATAGT) close to the E. coli −10 consensus sequence is correctly positioned relative to the start site. In contrast, no putative −35 box matching the E. coli consensus sequence could be found 16 to 18 bp upstream of the TATA box (Fig. 1A). The absence of a −35 consensus box supports the idea that the S. oneidensis tor promoter is positively regulated and that transcription initiation requires binding of an activator. In E. coli, TorR, the activator of the tor operon, binds to four decameric direct repeats (31), but no such repeats could be found upstream of the TATA box of the S. oneidensis tor promoter. Moreover, no sequence related to the E. coli FNR box (TTGATNNNNATCAA) was seen in the tor regulatory region, in agreement with the fact that EtrA, the S. oneidensis analog of the pleiotropic anaerobic regulator FNR, plays no role in the regulation of the Tor system (18).
Activation of S. oneidensis tor promoter by overproduction of TorR response regulator.
In contrast to the E. coli tor locus (32), no gene encoding a response regulator is present upstream of the tor structural genes in S. oneidensis. However, three genes located just downstream of the torECAD operon and named torS, torT and torR might encode the regulatory proteins of the Tor system (Fig. 1A). Indeed, torS encodes a putative transmembrane protein of 1,010 amino acids homologous to the TorS sensor of E. coli (32% identity), while torR and torT encode a response regulator and a periplasmic protein showing 55 and 34% identity with the TorR response regulator and the TorT regulatory protein of E. coli, respectively.
As genetic tools for S. oneidensis are poorly developed, we decided to investigate the regulation of the torECAD promoter in E. coli. To avoid interference between the Tor systems of the two strains, we used an E. coli strain (LCB436) deleted of the entire tor locus. A DNA fragment containing torE and the untranscribed region upstream of torE was fused to the lacZ coding sequence of the operon fusion vector pGE593, and the torRso coding sequence was cloned downstream of the arabinose-inducible PBAD promoter of plasmid pBAD33. The two plasmids (pPTorso4 and pRso2), which are compatible, were introduced together into strain LCB436, and the effect of TorR overproduction on expression of the torEC"-lacZ fusion was followed.
As shown in Fig. 2, in anaerobiosis, the β-galactosidase activity of the fusion increased strongly when arabinose was added. Overproduction of TorR thus activates expression of the torEC"-lacZ fusion. As overproduction of response regulators has often been employed to mimic regulator activation by phosphorylation (24, 32), this result strongly suggests that TorR is the response regulator responsible for induction of the torECAD operon. However, it does not prove that the same promoter sequence is used in E. coli and in S. oneidensis. To clarify this point, we carried out a primer extension experiment to determine the transcription start site of the torEC"-lacZ fusion (Fig. 1C). RNA was prepared from the strain containing both plasmids grown anaerobically with arabinose, and the primer used was complementary to the torE coding sequence. The same transcription start site was used in E. coli and S. oneidensis, indicating that transcription initiates from the same promoter in both bacteria.
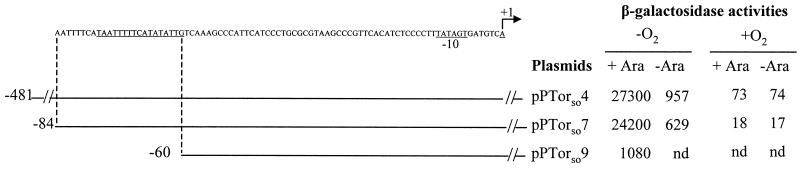
FIG. 2.
Effect of TorRso overproduction and of deletions in the torE promoter region on tor-lacZ fusion expression in E. coli. The TorR binding site and the putative −10 box are underlined, and the transcription start point of torE is indicated by the arrow at +1. The 5" end of the cloned tor sequence is shown, whereas the 3" part of the tor region is not shown and corresponds to position +217 for pPTorso4 and +119 for pPTorso7 and pPTorso9, relative to the torE transcription start site. Strain LCB436 containing plasmid pRso2 (torRso) and a plasmid of the pPTorso series, as indicated, was grown anaerobically (−O2) or aerobically (+O2), in the presence (+) or absence (−) of 0.001% arabinose. β-Galactosidase activities of the plasmid-borne tor-lacZ fusions are expressed in Miller units. nd, not determined.
Location of TorR binding region in tor operon promoter of S. oneidensis.
To confirm that TorR induces expression of the tor operon directly by binding to the tor promoter, we purified TorR and carried out a DNA-binding gel shift assay (Fig. 3A). When the DNA fragment contained the tor region from positions −84 to +119 relative to the transcription initiation site, a retarded band was observed in the presence of small amounts of TorR and the apparent equilibrium binding constant, Keq, was about 250 nM. Increasing the concentration of TorR led to retardation of most of the labeled DNA, but the same retarded band appeared even at the highest TorR concentration, suggesting that TorR binds only one DNA site in the tor promoter.
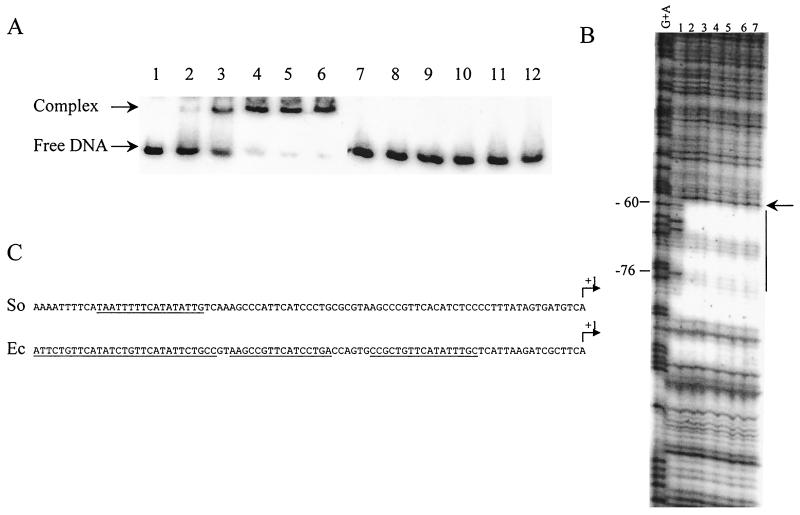
FIG. 3.
Analysis of TorR binding to the torE promoter region by band shift assays (A) and DNase I footprinting experiments (B). (A) Labeled DNA fragments containing the torE region from positions −84 to +119 (lanes 1 to 6) or from −60 to +119 (lanes 7 to 12) were incubated in the presence of the following concentrations of purified TorR protein: lanes 1 and 7, no protein; lanes 2 and 8, 0.05 μM; lanes 3 and 9, 0.25 μM; lanes 4 and 10, 0.5 μM; lanes 5 and 11, 1 μM; and lanes 6 and 12, 2.5 μM. (B) The labeled DNA fragment was prepared by PCR using sense 32P-end-labeled and reverse unlabeled primers and plasmid pPTorso7 as the template. The fragment was digested with 0.33 U of DNase I in the presence of various concentrations of purified TorRso protein: lane 1, no protein; lane 2, 0.25 μM; lane 3, 0.5 μM; lane 4, 1 μM; lane 5, 2.5 μM; lane 6, 5 μM; and lane 7, 10 μM. The G+A sequencing ladder is indicated. Numbering is relative to the transcription start site. Vertical bar indicates the protected region, and the arrow shows a DNase I-hypersensitive site. (C) Nucleotide sequences of the S. oneidensis (So) and E. coli (Ec) tor promoters. The regions protected by TorRso or TorRec are underlined, and transcription start sites are indicated by arrows labeled +1.
We identified the TorR binding region by DNase I footprinting analysis. A 295-bp DNA fragment containing the tor promoter (from positions −84 to +119) was generated by PCR using one labeled oligonucleotide and incubated with increasing concentration (0 to 10 μM) of TorR. Only one region of about 17 bp was protected even at the highest TorR concentrations (Fig. 3B). A DNase I-hypersensitive site appeared at position −59, suggesting that increased exposure of the minor groove to DNase I attack resulted from DNA bending induced by TorR binding. The protected region is A/T rich but contains neither palindromic structures nor direct repeats (Fig. 3C). Almost the same region was protected against DNase I when the DNA fragment was labeled on the opposite strand or when TorR was preincubated with 100 mM acetyl phosphate or carbamoyl phosphate, two small phosphodonor molecules for response regulators (data not shown). These results suggest that TorR activates the torECAD operon by binding to a single site centered at position −68 and contrast with the situation observed for the torCAD promoter of E. coli, in which three distinct regions were protected by TorRec (31) (Fig. 3C).
To test whether sequences upstream of the TorR binding site affected promoter function, we amplified a DNA fragment carrying the tor promoter sequence from positions −84 to +119 and cloned it upstream of the lacZ reporter gene of plasmid pGE593 to generate pPTorso7. The β-galactosidase activity measured when TorR was overproduced in anaerobiosis indicated that the tor promoter starting just a few bases upstream of the TorR box was expressed almost as strongly as that starting 400 bases further upstream (Fig. 2). In contrast, deletion of the TorR box (pPTorso9) resulted in both a >20-fold decrease in activity (Fig. 2) and complete absence of DNA retardation (Fig. 3A). The TorR operator site is thus essential for tor operon expression, whereas sequences upstream of position −84 are dispensable.
Involvement of torS and torT in TMAO control of tor operon expression.
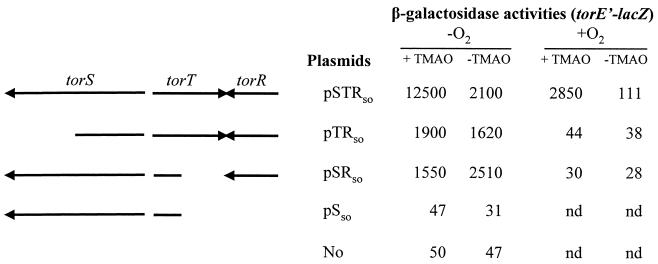
To determine whether torS and torT, the two genes located between torD and torR (Fig. 1A), play a role in tor operon regulation, we amplified the putative regulatory gene cluster torSTR by PCR and cloned it into pBAD33. The resulting plasmid, pSTRso, was introduced together with pPTorso7 into strain LCB436, and expression of the plasmid-borne torE"-lacZ fusion was monitored in various growth conditions (Fig. 4). Strikingly, addition of TMAO led to a strong increase in the fusion activity in both aerobiosis and anaerobiosis. The torSTR gene cluster might thus be involved in TMAO induction of the tor operon. The torE"-lacZ fusion was no longer induced by TMAO when torS or torT was inactivated. In anaerobiosis, the significant activity levels measured in the absence of TorS or TorT probably came from expression of the multicopy torR gene, which was cloned with its promoter sequence. In the absence of TorR, the activity of the fusion dropped to the background level, confirming that TorR is essential for induction of the tor operon.
FIG. 4.
Effect of the torS, torT, and torR genes of S. oneidensis on torE"-lacZ fusion expression in E. coli. The arrows show the locations and orientations of the cloned genes, and disruption and deletions of the reading frames are also indicated. Strain LCB436 containing pPTorso7 and plasmid pSTRso or a pSTRso derivative, as indicated, was grown anaerobically (−O2) or aerobically (+O2), in the presence (+) or absence (−) of TMAO. β-Galactosidase activities are expressed in Miller units. nd, not determined.
Together these results support the idea that TorS, TorT, and TorR constitute the TMAO regulatory system responsible for tor operon expression. As this probable TMAO regulatory system comprises three components homologous to those of the TMAO phosphorelay system of E. coli (1, 14), we propose a similar mechanism of signal transduction in which sensor TorS detects both TorT and TMAO and transphosphorylates response regulator TorR via a four-step phosphorelay. However, although the TorS sensors of both strains contain three phosphorylation sites, their N-terminal detector regions show no similarity (data not shown). TMAO detection as well as TorT interaction could thus implicate unrelated detector sites.
In E. coli, neither FNR, ArcB, nor TorR plays the role of the Tor anaerobic regulator (32). In S. oneidensis, the Tor anaerobic control does not seem to involve EtrA (18), the FNR homologue, and TorR appears to be implicated in the TMAO regulatory pathway. Surprisingly, when expressed in E. coli, the tor operon promoter of S. oneidensis was still under the control of anaerobiosis (Fig. 2 and 4). The TMAO regulatory proteins seem to play no role in this control, since anaerobic regulation still occurred in the absence of TorS or TorT, but the function of TorRso itself might be oxygen labile. Another possibility is that this anaerobic control involves essential proteins highly conserved among these strains. Alternatively, DNA supercoiling could play a role in the tor anaerobic control of both strains.
Phosphotransfer from the E. coli TorS sensor to the S. oneidensis TorR response regulator.
As the TorR proteins of S. oneidensis and E. coli are homologous (62.5 and 52% identity for the receiver and DNA-binding domains, respectively), we tested whether E. coli TorR could activate the tor operon of S. oneidensis. To answer this question, we introduced pPTorso7 (torE"-lacZ) into an E. coli strain containing the wild-type chromosomal tor locus. The activity from the fusion remained very low when TMAO was added (Fig. 5A). The phosphorylated form of E. coli TorR (TorR-P) was thus incapable of activating the tor operon promoter of S. oneidensis. This is not surprising, because the TorR binding site of the S. oneidensis tor promoter is different from that of the E. coli tor operon promoter (Fig. 3C). Therefore, TorR-P from E. coli was probably unable to bind the tor promoter of S. oneidensis. Functional complementation of torTso by the TorT protein of E. coli was also unsuccessful (data not shown).
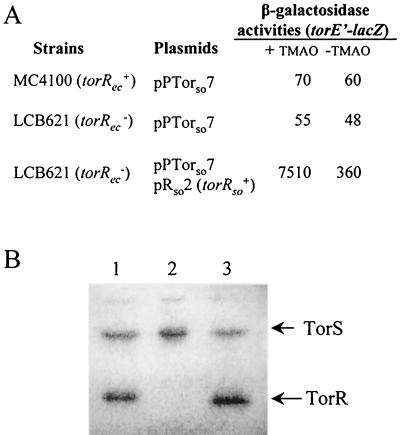
FIG. 5.
Signal transduction from TorS of E. coli to TorR of S. oneidensis. (A) Effect of E. coli and S. oneidensis response regulator TorR on torE"-lacZ fusion expression in E. coli. Strain MC4100 (torRec+) carrying pPTorso7 (torE"-lacZ) and strain LCB621 (torRec) carrying pPTorso7 alone or with pRso2 (torRso+) were grown anaerobically in LB medium supplemented with glucose to limit torRso expression, in the presence (+) or absence (−) of TMAO. β-Galactosidase activities of the plasmid-borne torE"-lacZ fusion are expressed in Miller units. (B) Autophosphorylation of the E. coli TorS726 sensor and transphosphorylation of the TorR response regulators from S. oneidensis and E. coli. TorS726 alone (lane 2) or with TorRso (lane 1) or TorRec (lane 3) was incubated at room temperature for 10 min in the presence of [γ-32P]ATP, and the reaction mixtures were analyzed on SDS-20% PAGE. The autoradiogram of the gel is shown. The positions on the gel of the TorR and TorS726 proteins are indicated.
Although the E. coli TorR and TorT proteins cannot substitute for the S. oneidensis homologs, we wondered whether the TorS sensor of E. coli could replace that of S. oneidensis. Plasmids pRso2 and pPTorso7 were introduced together into a torS+ torR E. coli strain, and the activity of the torE"-lacZ fusion was measured in cells producing a small amount of TorRso. The activity of the fusion was low without TMAO but strongly increased in the presence of TMAO (Fig. 5A). This result suggests that the E. coli TorS sensor can transphosphorylate the S. oneidensis TorR response regulator, although the histidine phosphotransfer domain of E. coli TorS has only 26.3% identity with that of S. oneidensis.
To show that E. coli TorS can directly transphosphorylate S. oneidensis TorR, we performed an in vitro experiment with mutant TorS726, a constitutively active E. coli TorS protein (14), and purified S. oneidensis TorR. In the presence of [γ-32P]ATP, TorS726 was labeled, and after addition of TorRso, part of the TorS radioactivity was transferred to TorR (Fig. 5B). The transfer was quite efficient compared to the control experiment with TorR from E. coli. This biochemical analysis thus confirms the genetic data and shows that E. coli TorS can activate S. oneidensis TorR by transphosphorylation. The C-terminal domain of TorS, which is the phosphodonor site for its cognate response regulator, probably does not discriminate between the two TorR proteins.
In conclusion, the S. oneidensis torSTR gene cluster encodes a regulatory system involved in induction of the tor structural operon in response to TMAO. This TMAO regulatory system might regulate expression of additional genes. In particular, the gene encoding the soluble fumarate reductase of a Shewanella species is strongly downregulated by TMAO (12), and TorR might play the role of a repressor in this case.
Acknowledgments
We thank C. Iobbi-Nivol and J. Demoss for reviewing the manuscript and C. R. Myers and B. L. Wanner for the kind gift of strains and plasmids. We are also indebted to the Institute for Genomic Research for genome sequence data.
This work was supported by grants from the Centre National de la Recherche Scientifique and the Université de la Méditerranée. S.G. was supported by grants from the MENRT and from the Fondation pour la Recherche Médicale (FRM).
REFERENCES
- 1.Ansaldi, M., C. Jourlin-Castelli, M. Lepelletier, L. Théraulaz, and V. Méjean. 2001. Rapid dephosphorylation of the TorR response regulator by the TorS unorthodox sensor in Escherichia coli. J. Bacteriol. 183:2691-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansaldi, M., G. Simon, M. Lepelletier, and V. Méjean. 2000. The TorR high-affinity binding site plays a key role in both torR autoregulation and torCAD operon expression in Escherichia coli. J. Bacteriol. 182:961-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett, E. L., and H. S. Kwan. 1985. Bacterial reduction of trimethylamine oxide. Annu. Rev. Microbiol. 39:131-149. [DOI] [PubMed] [Google Scholar]
- 4.Berks, B. C., S. J. Ferguson, J. W. Moir, and D. J. Richardson. 1995. Enzymes and associated electron transport systems that catalyse the respiratory reduction of nitrogen oxides and oxyanions. Biochim. Biophys. Acta 1232:97-173. [DOI] [PubMed] [Google Scholar]
- 5.Chung, C. T., and R. H. Miller. 1988. A rapid and convenient method for the preparation and storage of competent bacterial cells. Nucleic Acids Res. 16:3580.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czjzek, M., Dos Santos, J. P., J. Pommier, G. Giordano, V. Méjean, and R. Haser. 1998. Crystal structure of oxidized trimethylamine N-oxide reductase from Shewanella massilia at 2.5 Å resolution. J. Mol. Biol. 284:435-447. [DOI] [PubMed] [Google Scholar]
- 7.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dos Santos, J. P., C. Iobbi-Nivol, C. Couillault, G. Giordano, and V. Méjean. 1998. Molecular analysis of the trimethylamine N-oxide (TMAO) reductase respiratory system from a Shewanella species. J. Mol. Biol. 284:421-433. [DOI] [PubMed] [Google Scholar]
- 9.Easter, M. C., D. M. Gibson, and F. B. Ward. 1983. The induction and location of trimethylamine N-oxide reductase in Alteromonas sp. NCMB 400. J. Gen. Microbiol. 129:3689-3696. [Google Scholar]
- 10.Eraso, J. M., and G. M. Weinstock. 1992. Anaerobic control of colicin E1 production. J. Bacteriol. 174:5101-5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gennis, R. B., and V. Stewart. 1996. Respiration, p. 217-261. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 12.Gordon, E. H., S. L. Pealing, S. K. Chapman, F. B. Ward, and G. A. Reid. 1998. Physiological function and regulation of flavocytochrome c3, the soluble fumarate reductase from Shewanella putrefaciens NCIMB 400. Microbiology 144:937-945. [DOI] [PubMed] [Google Scholar]
- 13.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jourlin, C., M. Ansaldi, and V. Méjean. 1997. Transphosphorylation of the TorR response regulator requires the three phosphorylation sites of the TorS unorthodox sensor in Escherichia coli. J. Mol. Biol. 267:770-777. [DOI] [PubMed] [Google Scholar]
- 15.Jourlin, C., A. Bengrine, M. Chippaux, and V. Méjean. 1996. An unorthodox sensor protein (TorS) mediates the induction of the tor structural genes in response to trimethylamine N-oxide in Escherichia coli. Mol. Microbiol. 20:1297-1306. [DOI] [PubMed] [Google Scholar]
- 16.Jourlin, C., G. Simon, J. Pommier, M. Chippaux, and V. Méjean. 1996. The periplasmic TorT protein is required for trimethylamine N-oxide reductase gene induction in Escherichia coli. J. Bacteriol. 178:1219-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowry, O. L., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein determination with the Folin phenol reaction. J. Biol. Chem. 93:265-273. [PubMed] [Google Scholar]
- 18.Maier, T. M., and C. R. Myers. 2001. Isolation and characterization of a Shewanella putrefaciens MR-1 electron transport regulator etrA mutant: reassessment of the role of EtrA. J. Bacteriol. 183:4918-4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Méjean, V., C. Iobbi-Nivol, M. Lepelletier, G. Giordano, M. Chippaux, and M. C. Pascal. 1994. TMAO anaerobic respiration in Escherichia coli: involvement of the tor operon. Mol. Microbiol. 11:1169-1179. [DOI] [PubMed] [Google Scholar]
- 20.Miller, J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 21.Mouncey, N. J., M. Choudhary, and S. Kaplan. 1997. Characterization of genes encoding dimethyl sulfoxide reductase of Rhodobacter sphaeroides 2.4.1T: an essential metabolic gene function encoded on chromosome II. J. Bacteriol. 179:7617-7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mouncey, N. J., and S. Kaplan. 1998. Cascade regulation of dimethyl sulfoxide reductase (dor) gene expression in the facultative phototroph Rhodobacter sphaeroides 2.4.1T. J. Bacteriol. 180:2924-2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers, C. R., and K. H. Nealson. 1988. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240:1319-1321. [DOI] [PubMed] [Google Scholar]
- 24.Olekhnovich, I. N., J. L. Dahl, and R. J. Kadner. 1999. Separate contributions of UhpA and CAP to activation of transcription of the uhpT promoter of Escherichia coli. J. Mol. Biol. 292:973-986. [DOI] [PubMed] [Google Scholar]
- 25.Pommier, J., V. Méjean, G. Giordano, and C. Iobbi-Nivol. 1998. TorD, a cytoplasmic chaperone that interacts with the unfolded trimethylamine N-oxide reductase enzyme (TorA) in Escherichia coli. J. Biol. Chem. 273:16615-16620. [DOI] [PubMed] [Google Scholar]
- 26.Proctor, L. M., and R. P. Gunsalus. 2000. Anaerobic respiratory growth of Vibrio harveyi, Vibrio fischeri and Photobacterium leiognathi with trimethylamine N-oxide, nitrate and fumarate: ecological implications. Environ. Microbiol. 2:399-406. [DOI] [PubMed] [Google Scholar]
- 27.Richardson, D. J. 2000. Bacterial respiration: a flexible process for a changing environment. Microbiology 146:551-571. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 29.Shaw, A. L., A. Hochkoeppler, P. Bonora, D. Zannoni, G. R. Hanson, and A. G. McEwan. 1999. Characterization of DorC from Rhodobacter capsulatus, a c-type cytochrome involved in electron transfer to dimethyl sulfoxide reductase. J. Biol. Chem. 274:9911-9914. [DOI] [PubMed] [Google Scholar]
- 30.Silvestro, A., J. Pommier, M. C. Pascal, and G. Giordano. 1989. The inducible trimethylamine N-oxide reductase of Escherichia coli K12: its localization and inducers. Biochim. Biophys. Acta 999:208-216. [DOI] [PubMed] [Google Scholar]
- 31.Simon, G., C. Jourlin, M. Ansaldi, M. C. Pascal, M. Chippaux, and V. Méjean. 1995. Binding of the TorR regulator to cis-acting direct repeats activates tor operon expression. Mol. Microbiol. 17:971-980. [DOI] [PubMed] [Google Scholar]
- 32.Simon, G., V. Méjean, C. Jourlin, M. Chippaux, and M. C. Pascal. 1994. The torR gene of Escherichia coli encodes a response regulator protein involved in the expression of the trimethylamine N-oxide reductase genes. J. Bacteriol. 176:5601-5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ujiiye, T., I. Yamamoto, H. Nakama, A. Okubo, S. Yamazaki, and T. Satoh. 1996. Nucleotide sequence of the genes, encoding the pentaheme cytochrome (dmsC) and the transmembrane protein (dmsB), involved in dimethyl sulfoxide respiration from Rhodobacter sphaeroides f. sp . denitrificans. Biochim. Biophys. Acta 1277:1-5. [DOI] [PubMed] [Google Scholar]
- 34.Ujiiye, T., I. Yamamoto, and T. Satoh. 1997. The dmsR gene encoding a dimethyl sulfoxide-responsive regulator for expression of dmsCBA (dimethyl sulfoxide respiration genes) in Rhodobacter sphaeroides f. sp. denitrificans. Biochim. Biophys. Acta 1353:84-92. [DOI] [PubMed] [Google Scholar]
- 35.Venkateswaran, K., D. P. Moser, M. E. Dollhopf, D. P. Lies, D. A. Saffarini, B. J. MacGregor, et al. 1999. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int. J. Syst. Bacteriol. 49:705-724. [DOI] [PubMed] [Google Scholar]
- 36.Wang, A., and D. W. Bolen. 1997. A naturally occurring protective system in urea-rich cells: mechanism of osmolyte protection of proteins against urea denaturation. Biochemistry 36:9101-9108. [DOI] [PubMed] [Google Scholar]
- 37.Yancey, P. H., A. L. Fyfe-Johnson, R. H. Kelly, V. P. Walker, and M. T. Aunon. 2001. Trimethylamine oxide counteracts effects of hydrostatic pressure on proteins of deep-sea teleosts. J. Exp. Zool. 289:172-176. [DOI] [PubMed] [Google Scholar]
- 38.Zeilstra-Ryalls, J. H., K. Gabbert, N. J. Mouncey, S. Kaplan, and R. G. Kranz. 1997. Analysis of the fnrL gene and its function in Rhodobacter capsulatus. J. Bacteriol. 179:7264-7273. [DOI] [PMC free article] [PubMed] [Google Scholar]