Abstract
1. Segments of rat diaphragms were kept in choline-free media for 4 hr and were then exposed to a physiological concentration of [14C]-choline (30 μM) at 37° C. The synthesis, storage and subsequent release of [14C]acetylcholine by the muscles was assessed by isotopic- and bio-assays after isolation of the transmitter by paper electrophoresis.
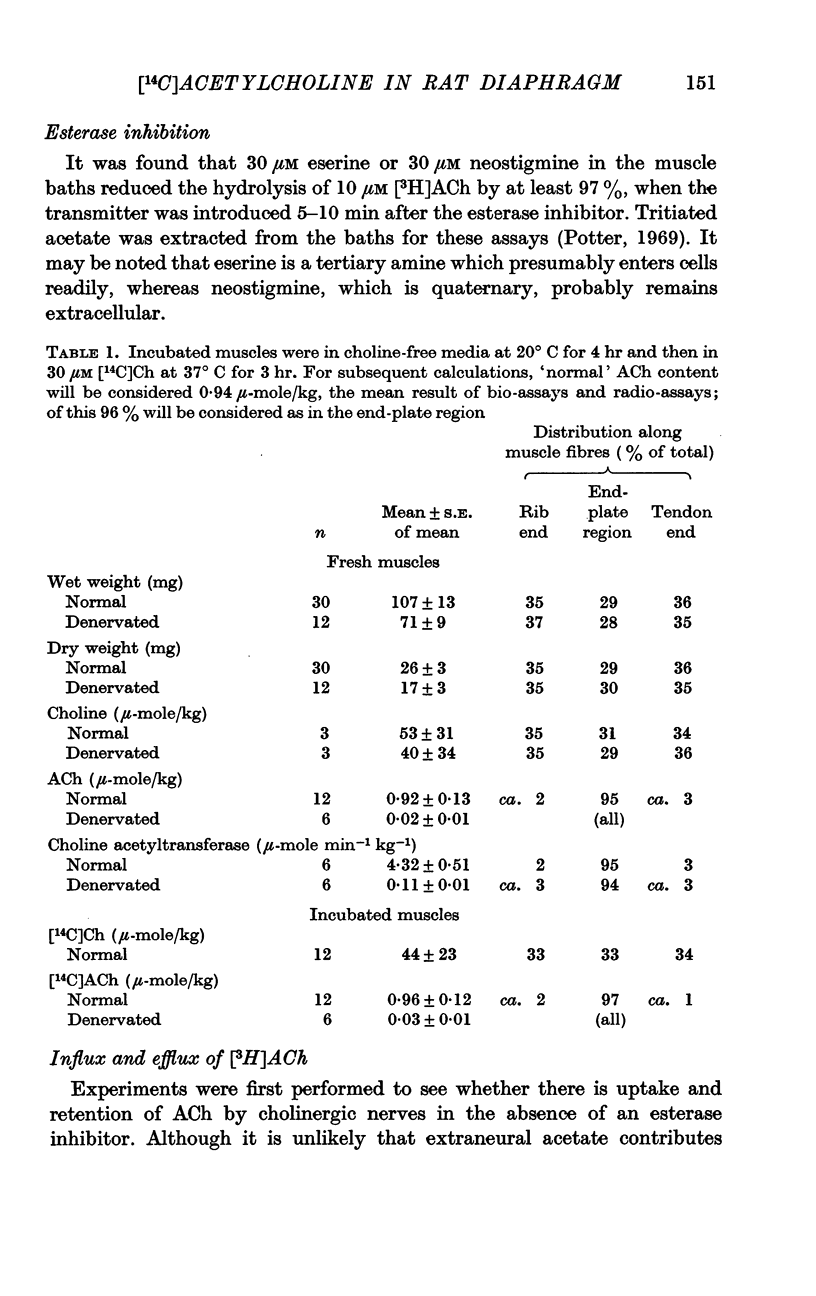
2. Replacement of endogenous acetylcholine (0·92 μ-mole/kg) with labelled acetylcholine proceeded slowly at rest, but rapidly during nerve stimulation. [14C]Acetylcholine accumulated most rapidly when hydrolysis of the released transmitter, and thus the re-use of endogenous choline, was prevented by an esterase inhibitor. Fully replaced stores were maintained during nerve stimulation by synthesis rates sufficient to replenish at least 35% of the store size in 5 min.
3 In the presence of hemicholinium-3, which inhibits choline uptake, acetylcholine stores declined rapidly during stimulation, and residual synthesis was slight, indicating little intraneural choline. Net choline uptake into nerve terminals was estimated from the highest observed synthesis rate and from previous measurements of the number and size of terminals, as 3-6 p-mole/cm2 sec.
4. Transmitter synthesis was localized in the region of end-plates, and was reduced to a few per cent of normal 6 weeks after phrenic nerve section. Release experiments suggested that at least half of the acetylcholine in phrenic nerves is in their terminals; from this content and the morphology of the terminals, the average concentration of transmitter in the whole endings would appear to be about 50 m-mole/l. Homogenization of the muscles freed choline acetyltransferase into solution, but left some [14C]acetylcholine associated with small particles, presumably synaptic vesicles.
5. Resting transmitter release was about 0·013% of stores/sec. With 360 nerve impulses at 1-20/sec, release increased up to 0·43% of stores/sec, and amounted to 3·5-7 × 10-18 moles per end-plate per impulse. The release rate was unaffected by the doubling of store size which occurred with eserine, but the extra transmitter did help to maintain releasable stores during prolonged stimulation. Experiments with fractional store labelling indicated that newly synthesized acetylcholine was preferentially released.
6. Preformed [3H]acetylcholine was not taken up and retained by muscle or nerve cells in the absence of an esterase inhibitor. With eserine present, labelled acetylcholine was taken up uniformly by muscle segments; when eserine was then removed, radioactive acetylcholine remained only near neuromuscular junctions.
Full text
PDF




















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH J. The level of free choline in plasma. J Physiol. 1952 Jun;117(2):234–240. doi: 10.1113/jphysiol.1952.sp004743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOWMAN W. C., HEMSWORTH B. A. EFFECTS OF TRIETHYLCHOLINE ON THE OUTPUT OF ACETYLCHOLINE FROM THE ISOLATED DIAPHRAGM OF THE RAT. Br J Pharmacol Chemother. 1965 Feb;24:110–118. doi: 10.1111/j.1476-5381.1965.tb02084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOKS V. B., THIES R. E. Reduction of quantum content during neuromuscular transmission. J Physiol. 1962 Jul;162:298–310. doi: 10.1113/jphysiol.1962.sp006934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning E. T., Schulman M. P. (14C) acetylcholine synthesis by cortex slices of rat brain. J Neurochem. 1968 Dec;15(12):1391–1405. doi: 10.1111/j.1471-4159.1968.tb05921.x. [DOI] [PubMed] [Google Scholar]
- COLE W. V. Structural variations of nerve endings in the striated muscles of the rat. J Comp Neurol. 1957 Dec;108(3):445–463. doi: 10.1002/cne.901080306. [DOI] [PubMed] [Google Scholar]
- ELMQVIST D., QUASTEL D. M. PRESYNAPTIC ACTION OF HEMICHOLINIUM AT THE NEUROMUSCULAR JUNCTION. J Physiol. 1965 Apr;177:463–482. doi: 10.1113/jphysiol.1965.sp007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F. Choline acetyltransferase binding to and release from membranes. Biochem J. 1968 Sep;109(3):389–398. doi: 10.1042/bj1090389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen A. J., Ling G. M., Nagai M. Choline and phospholipid-choline in a sympathetic ganglion and their relationship to acetylcholine synthesis. Nature. 1967 May 13;214(5089):722–724. doi: 10.1038/214722a0. [DOI] [PubMed] [Google Scholar]
- GARDINER J. E. The inhibition of acetylcholine synthesis in brain by a hemicholinium. Biochem J. 1961 Nov;81:297–303. doi: 10.1042/bj0810297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEBB C. O., KRNJEVIC K., SILVER A. ACETYLCHOLINE AND CHOLINE ACETYLTRANSFERASE IN THE DIAPHRAGM OF THE RAT. J Physiol. 1964 Jun;171:504–513. doi: 10.1113/jphysiol.1964.sp007393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel M., Gautron J., Lesbats B. Isolement des vésicules synaptiques de l'organe électrique de la Torpille et localisation de l'acétylcholine à leur niveau. C R Acad Sci Hebd Seances Acad Sci D. 1968 Jan 15;266(3):273–275. [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Acetylcholine in mammalian neuromuscular transmission. Nature. 1958 Sep 20;182(4638):805–806. doi: 10.1038/182805b0. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Presynaptic failure of neuromuscular propagation in rats. J Physiol. 1959 Dec;149:1–22. doi: 10.1113/jphysiol.1959.sp006321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MITCHELL J. F. Diffusion of acetylcholine in agar gels and in the isolated rat diaphragm. J Physiol. 1960 Oct;153:562–572. doi: 10.1113/jphysiol.1960.sp006555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MITCHELL J. F. The release of acetylcholine in the isolated rat diaphragm. J Physiol. 1961 Feb;155:246–262. doi: 10.1113/jphysiol.1961.sp006625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., STRAUGHAN D. W. THE RELEASE OF ACETYLCHOLINE FROM THE DENERVATED RAT DIAPHRAGM. J Physiol. 1964 Mar;170:371–378. doi: 10.1113/jphysiol.1964.sp007337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968 Mar;195(2):481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. The quantal components of the mammalian end-plate potential. J Physiol. 1956 Sep 27;133(3):571–587. doi: 10.1113/jphysiol.1956.sp005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACINTOSH F. C. SYNTHESIS AND STORAGE OF ACETYLCHOLINE IN NERVOUS TISSUE. Can J Biochem Physiol. 1963 Dec;41:2555–2571. [PubMed] [Google Scholar]
- Marchbanks R. M. The uptake of [14C] choline into synaptosomes in vitro. Biochem J. 1968 Dec;110(3):533–541. doi: 10.1042/bj1100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K. Concentrative accumulation of choline by human erythrocytes. J Gen Physiol. 1968 Apr;51(4):497–516. doi: 10.1085/jgp.51.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. Electrophysiology and electron-microscopy of rat neuromuscular junctions after nerve degeneration. Proc R Soc Lond B Biol Sci. 1968 Feb 27;169(1016):289–306. doi: 10.1098/rspb.1968.0012. [DOI] [PubMed] [Google Scholar]
- Mitchell J. F., Silver A. The spontaneous release of acetylcholine from the denervated hemidiaphragm of the rat. J Physiol. 1963 Jan;165(1):117–129. doi: 10.1113/jphysiol.1963.sp007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba T., Grob D. Cholinesterase activity of the motor endplate in isolated muscle membrane. J Neurochem. 1968 Dec;15(12):1445–1454. doi: 10.1111/j.1471-4159.1968.tb05926.x. [DOI] [PubMed] [Google Scholar]
- PERRY W. L. M. Acetylcholine release in the cat's superior cervical ganglion. J Physiol. 1953 Mar;119(4):439–454. doi: 10.1113/jphysiol.1953.sp004858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter L. T., Glover V. A., Saelens J. K. Choline acetyltransferase from rat brain. J Biol Chem. 1968 Jul 25;243(14):3864–3870. [PubMed] [Google Scholar]
- Potter L. T., Murphy W. Electrophoresis of acetylcholine, choline and related compounds. Biochem Pharmacol. 1967 Jul 7;16(7):1386–1388. doi: 10.1016/0006-2952(67)90174-8. [DOI] [PubMed] [Google Scholar]
- Potter L. T. Storage of norepinephrine in sympathetic nerves. Pharmacol Rev. 1966 Mar;18(1):439–451. [PubMed] [Google Scholar]
- SAELENS J. K., STOLL W. R. RADIOCHEMICAL DETERMINATION OF CHOLINE AND ACETYLCHOLINE FLUX FROM ISOLATED TISSUE. J Pharmacol Exp Ther. 1965 Mar;147:336–342. [PubMed] [Google Scholar]
- Sheridan M. N., Whittaker V. P., Israël M. The subcellular fractionation of the electric organ of Torpedo. Z Zellforsch Mikrosk Anat. 1966;74(3):293–307. [PubMed] [Google Scholar]
- Suga N. Analysis of frequency-modulated sounds by auditory neurones of echo-locating bats. J Physiol. 1965 Jul;179(1):26–53. doi: 10.1113/jphysiol.1965.sp007648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A. The long-lasting depression in neuromuscular transmission of frog. Jpn J Physiol. 1958 Jun 15;8(2):102–113. doi: 10.2170/jjphysiol.8.102. [DOI] [PubMed] [Google Scholar]
- THIES R. E. NEUROMUSCULAR DEPRESSION AND THE APPARENT DEPLETION OF TRANSMITTER IN MAMMALIAN MUSCLE. J Neurophysiol. 1965 May;28:428–442. doi: 10.1152/jn.1965.28.3.427. [DOI] [PubMed] [Google Scholar]


