Abstract
1. Experiments were carried out on cat adrenal glands perfused with Locke solution to study the effects of inhibition of metabolism on calcium-evoked catecholamine release.
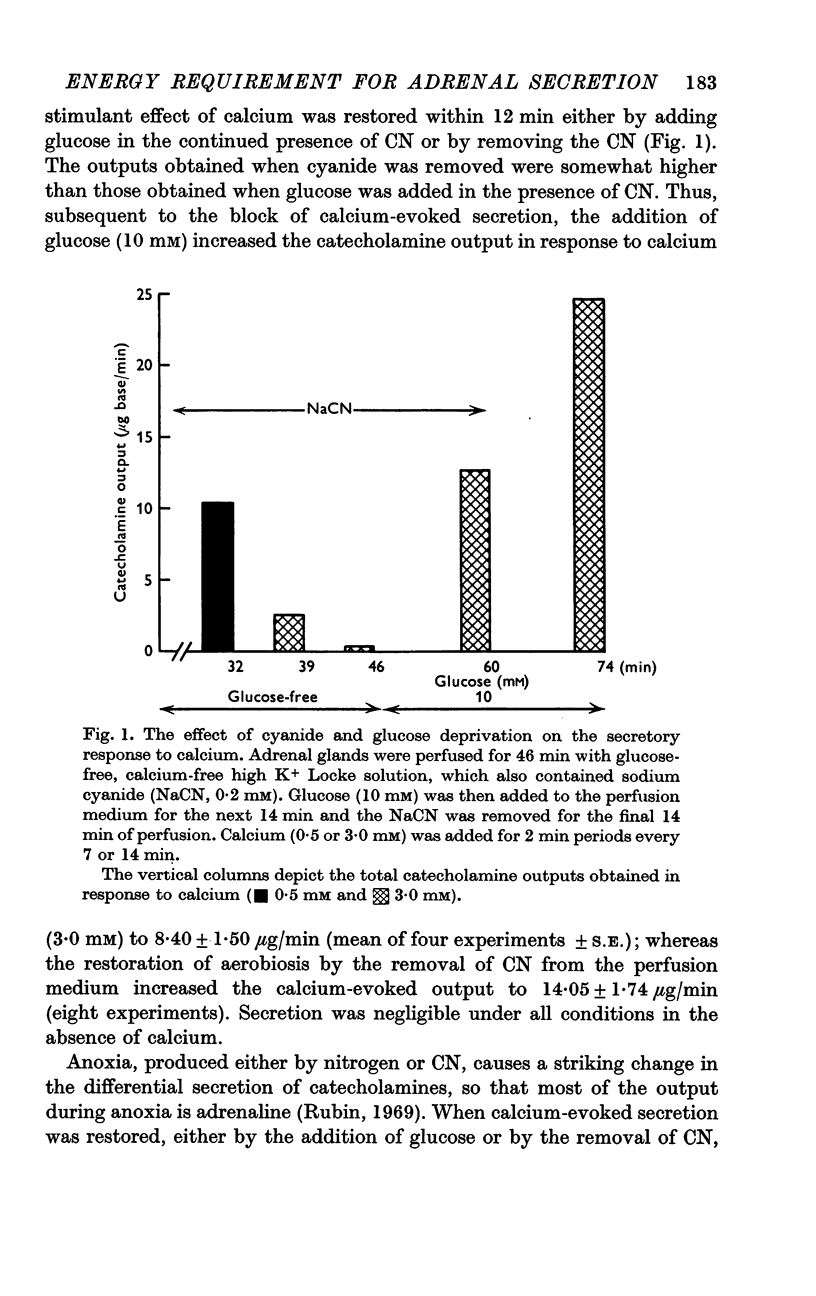
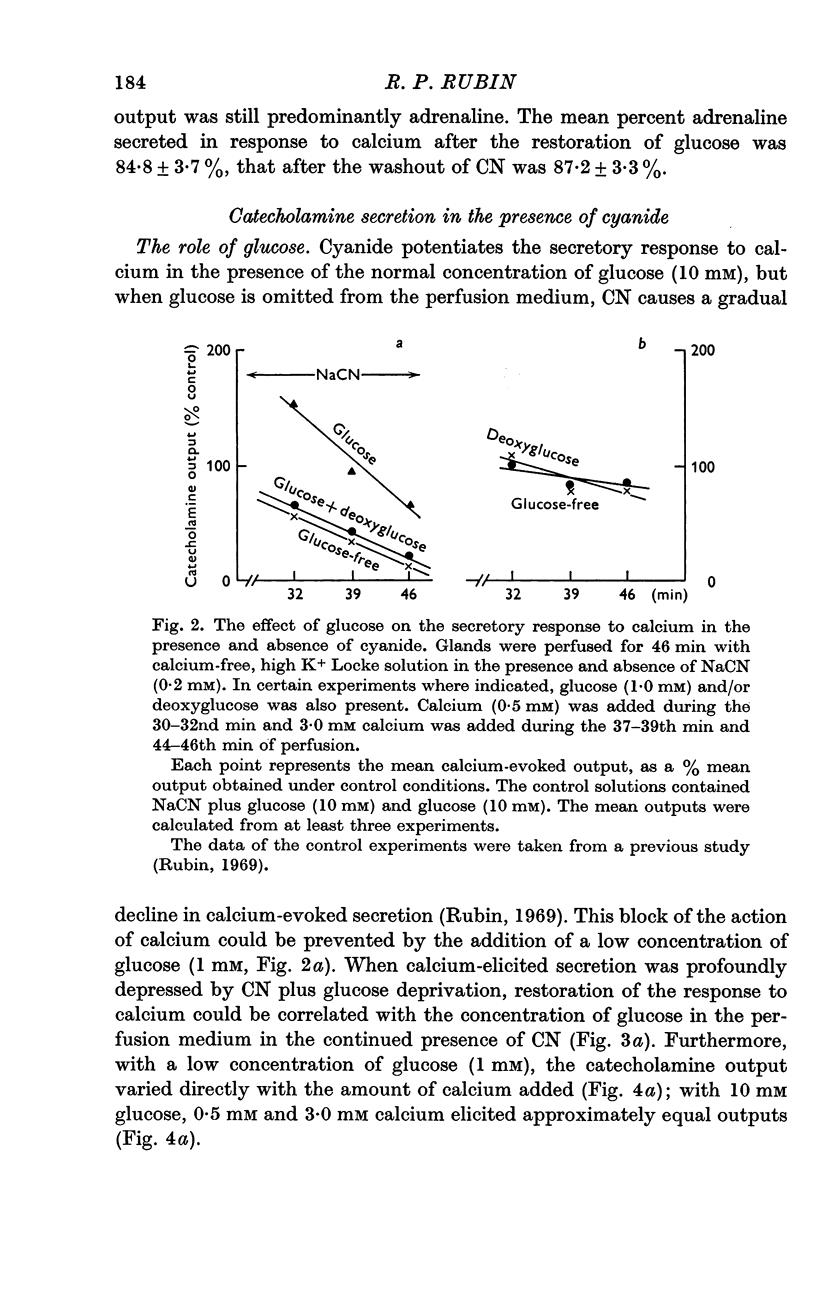
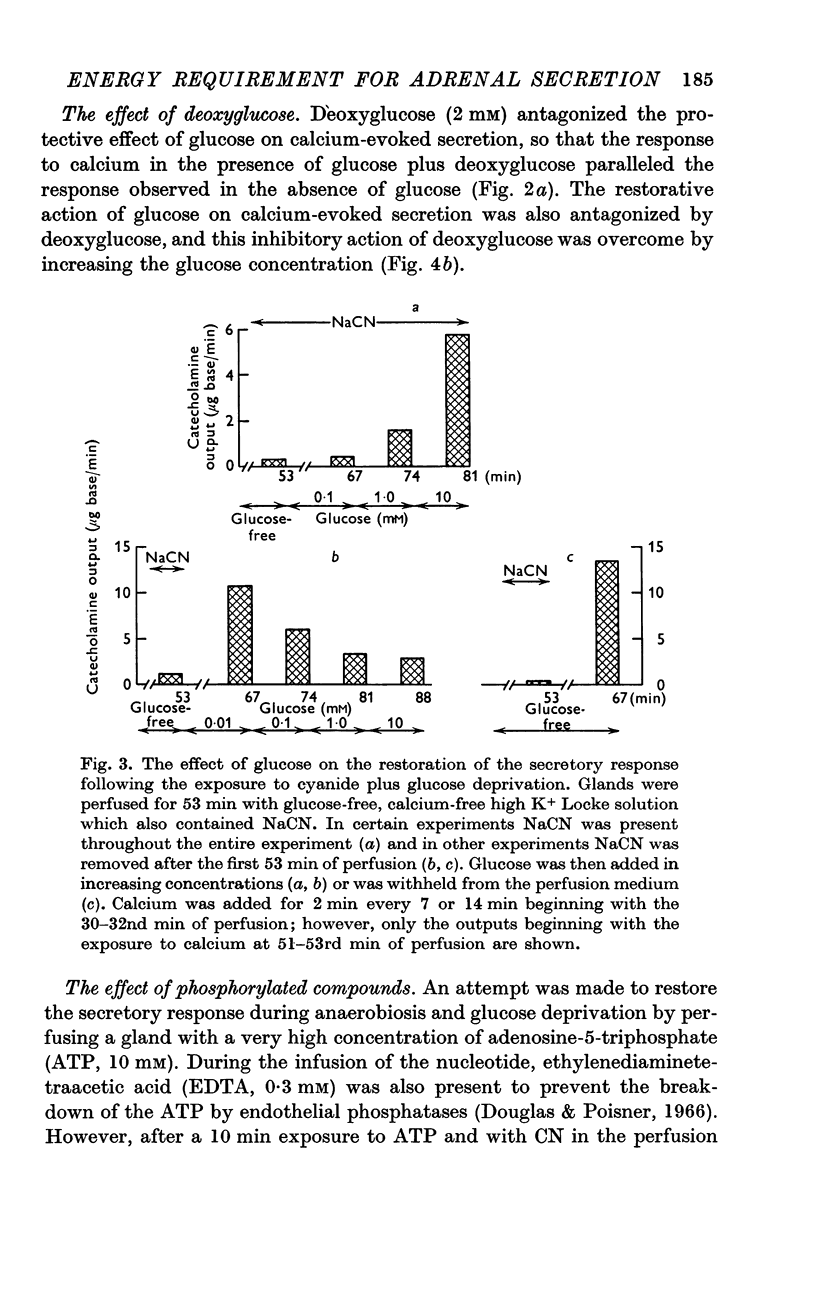
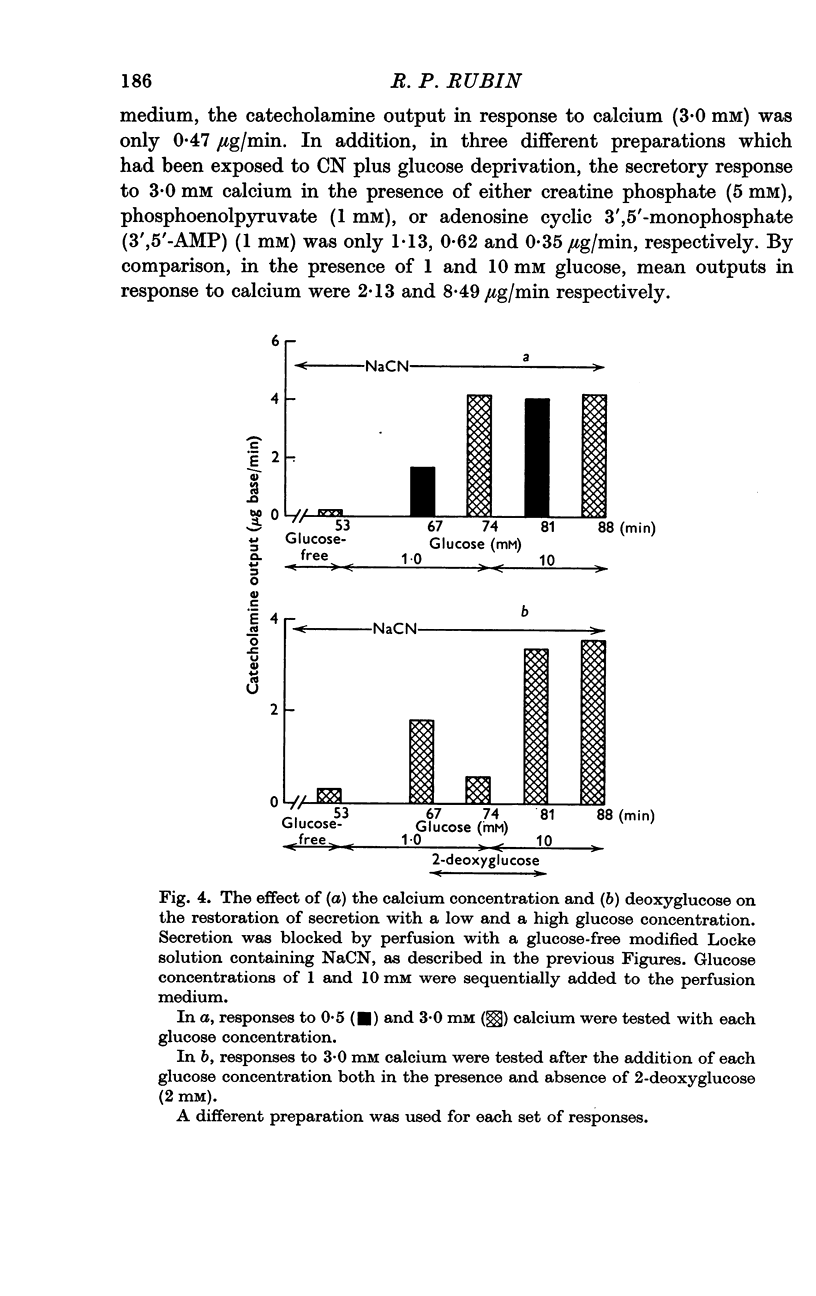
2. In the presence of sodium cyanide (CN, 0·2 mM), a low concentration of glucose (1 mM) prevented the gradual decline in the secretory response to sequential exposures to calcium. Furthermore, when the secretory response was almost completely blocked by perfusing with a glucose-deprived solution containing CN, restoration of secretion was correlated with the glucose concentration in the perfusion medium.
3. In the presence of CN, 2-deoxyglucose blocked both the protective effect and the restorative effect of glucose. The deoxyglucose inhibition of the glucose-dependent restoration of secretion was antagonized by a higher concentration of glucose.
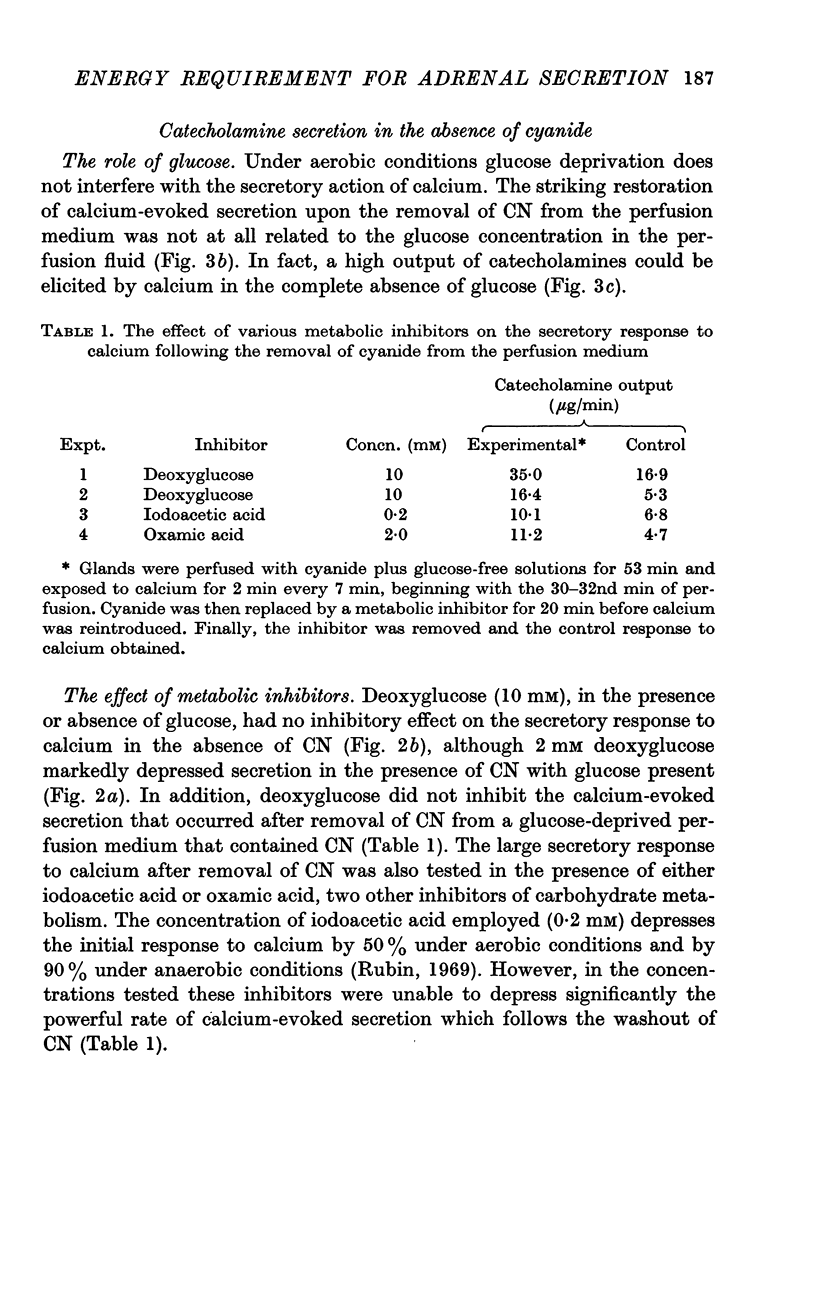
4. Restoration of calcium-evoked secretion was also observed after the washout of CN. The extent of this restoration was not at all related to the glucose concentration and was not affected by various inhibitors of carbohydrate metabolism, including deoxyglucose.
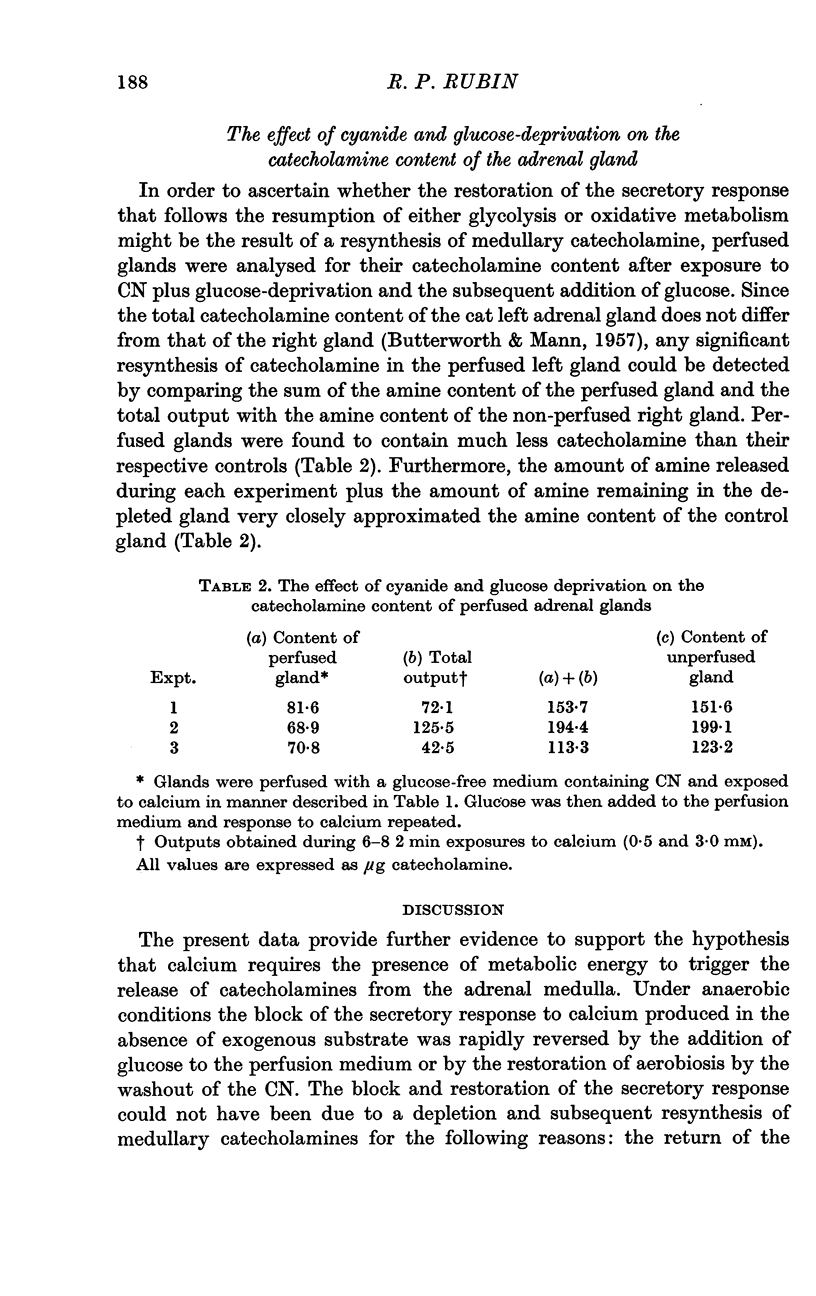
5. Analysis of adrenal glands which had been perfused first with a glucose-free solution containing CN and subsequently with the normal medium indicated that no discernible synthesis of catecholamines had taken place during the experimental procedures.
6. The data provide further evidence that the action of calcium to trigger medullary secretion requires the presence of metabolic energy and support the hypothesis that an interaction between calcium and high-energy nucleotides is a step in the sequence of events leading to the extrusion of catecholamines from the chromaffin cell.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- BLASCHKO H., BORN G. V., D'IORIO A., EADE N. R. Observations on the distribution of catechol amines and adenosinetriphosphate in the bovine adrenal medulla. J Physiol. 1956 Sep 27;133(3):548–557. doi: 10.1113/jphysiol.1956.sp005607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTTERWORTH K. R., MANN M. A quantitative comparison of the sympathominetic amine content of the left and right adrenal glands of the cat. J Physiol. 1957 Apr 30;136(2):294–299. doi: 10.1113/jphysiol.1957.sp005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty N. Further observations on the inhibition of histamine release by 2-deoxyglucose. Acta Physiol Scand. 1968 Apr;72(4):425–432. doi: 10.1111/j.1748-1716.1968.tb03867.x. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. THE EFFECTS OF ALKALINE EARTHS AND OTHER DIVALENT CATIONS ON ADRENAL MEDULLARY SECRETION. J Physiol. 1964 Dec;175:231–241. doi: 10.1113/jphysiol.1964.sp007514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamant B., Krüger P. G. Histamine release from isolated rat peritoneal mast cells induced by adenosine-5'-triphosphate. Acta Physiol Scand. 1967 Dec;71(4):291–302. doi: 10.1111/j.1748-1716.1967.tb03736.x. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. On the relation between ATP splitting and secretion in the adrenal chromaffin cell: extrusion of ATP (unhydrolysed) during release of catecholamines. J Physiol. 1966 Mar;183(1):249–256. doi: 10.1113/jphysiol.1966.sp007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Rubin R. P. The mechanism of catecholamine release from the adrenal medulla and the role of calcium in stimulus-secretion coupling. J Physiol. 1963 Jul;167(2):288–310. doi: 10.1113/jphysiol.1963.sp007150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER R. B., WILLIAMSON J. R. The oxygen uptake of the perfused rat heart. J Physiol. 1961 Sep;158:86–101. doi: 10.1113/jphysiol.1961.sp006756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLARP N. A., NILSON B., HOGBERG B. Adenosine triphosphate in the adrenal medulla of the cow. Nature. 1955 Nov 26;176(4491):1032–1033. doi: 10.1038/1761032a0. [DOI] [PubMed] [Google Scholar]
- HUMPHREY J. H., JAQUES R. The release of histamine and 5-hydroxytryptamine (serotonin) from platelets by antigen-antibody reactions (in vitro). J Physiol. 1955 Apr 28;128(1):9–27. doi: 10.1113/jphysiol.1955.sp005289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARRABEE M. G., BRONK D. W. [Metabolic requirements of sympathetic neurons]. Cold Spring Harb Symp Quant Biol. 1952;17:245–266. doi: 10.1101/sqb.1952.017.01.023. [DOI] [PubMed] [Google Scholar]
- MONGAR J. L., SCHILD H. O. The effect of calcium and pH on the anaphylactic reaction. J Physiol. 1958 Feb 17;140(2):272–284. doi: 10.1113/jphysiol.1958.sp005933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN H. E., HENDERSON M. J., REGEN D. M., PARK C. R. Regulation of glucose uptake in muscle. I. The effects of insulin and anoxia on glucose transport and phosphorylation in the isolated, perfused heart of normal rats. J Biol Chem. 1961 Feb;236:253–261. [PubMed] [Google Scholar]
- Oka M., Ouchi T., Yoshida H., Imaizumi R. Stimulatory effect of adenosine triphosphate and magensium on the release of catecholamines from adrenal medullary granules. Jpn J Pharmacol. 1967 Jun;17(2):199–207. doi: 10.1254/jjp.17.199. [DOI] [PubMed] [Google Scholar]
- Poisner A. M., Trifaró J. M. The role of ATP and ATPase in the release of catecholamines from the adrenal medulla. I. ATP-evoked release of catecholamines, ATP, and protein from isolated chromaffin granules. Mol Pharmacol. 1967 Nov;3(6):561–571. [PubMed] [Google Scholar]
- Rubin R. P., Jaanus S. D. A study of the release of catecholamines from the adrenal medulla by indirectly acting sympathomimetic amines. Naunyn Schmiedebergs Arch Pharmakol Exp Pathol. 1966;254(2):125–137. doi: 10.1007/BF00535900. [DOI] [PubMed] [Google Scholar]
- Rubin R. P. The metabolic requirements from catecholamine release from the adrenal medulla. J Physiol. 1969 May;202(1):197–209. doi: 10.1113/jphysiol.1969.sp008804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow A. Excitation-contraction coupling in skeletal muscle. Pharmacol Rev. 1965 Sep;17(3):265–320. [PubMed] [Google Scholar]
- Schümann H. J. Second symposium on catecholamines. Properties of adrenergic tissues. Medullary particles. Pharmacol Rev. 1966 Mar;18(1):433–438. [PubMed] [Google Scholar]
- WILLIAMSON J. R. Effects of insulin and diet on the metabolism of L-lactate and glucose by the perfused rat heart. Biochem J. 1962 May;83:377–383. doi: 10.1042/bj0830377. [DOI] [PMC free article] [PubMed] [Google Scholar]


