Abstract
1. The effects of the vagus nerve and of antral gastrin on the rate of histamine formation (histamine forming capacity, HFC, i.e. histidine decarboxylase activity) in the parietal cell region of the gastric mucosa has been investigated in the following stomach preparations: gastric fistula, denervated Heidenhain pouch, antral resection with gastrojejunostomy, gastrojejunostomy with exclusion of the duodenum and in the intact stomach. The determinations of mucosal HFC were made on fasting rats and on re-fed animals when the effect of feeding was studied.
2. In fasting rats with a gastrojejunostomy and the antrum intact the mucosal HFC of the innervated stomach was about 4 times higher than in the corresponding preparation with the antrum resected. In the innervated main stomach the mucosal HFC was about twice as high as in the denervated pouch, indicating that the vagus and endogenous antral gastrin each contribute to maintaining mucosal HFC in the fasting state.
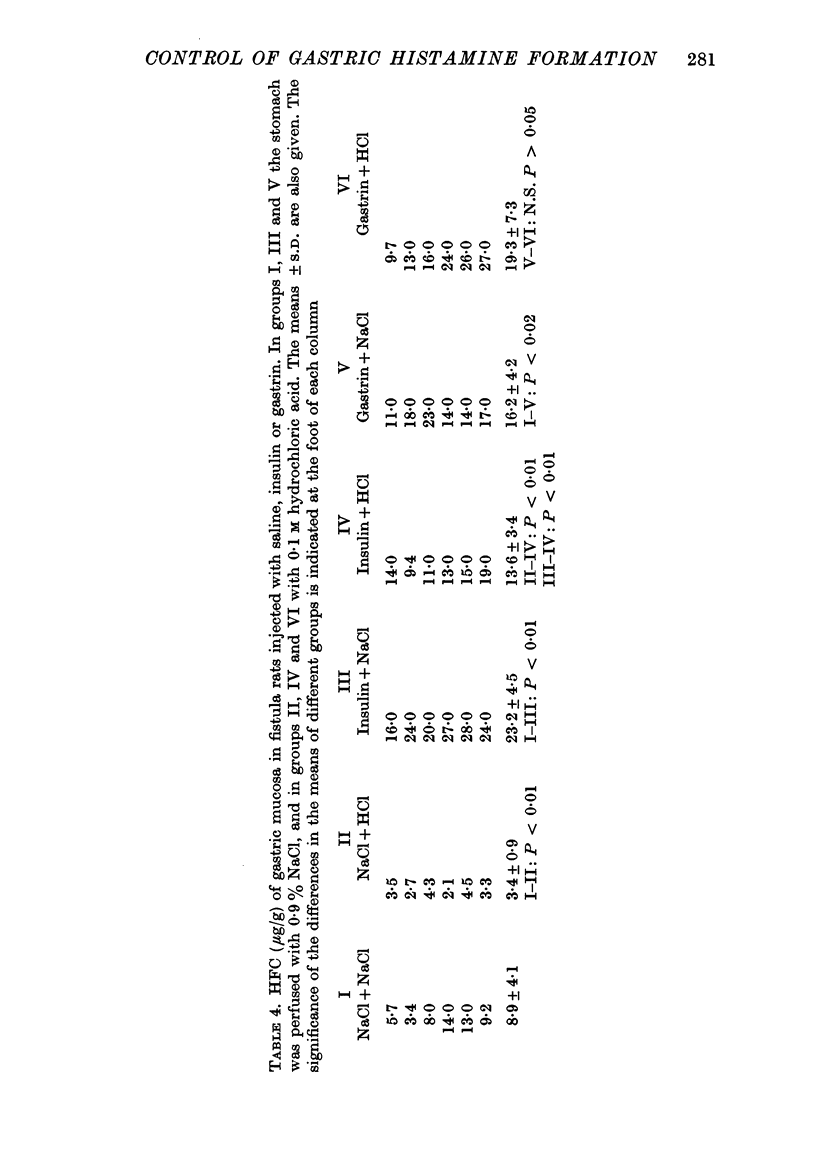
3. Acidifying the stomach caused a substantial lowering of the mucosal HFC presumably by inhibiting antral gastrin release, whereas acid in the stomach did not interfere with the elevation of mucosal HFC evoked by injection of gastrin.
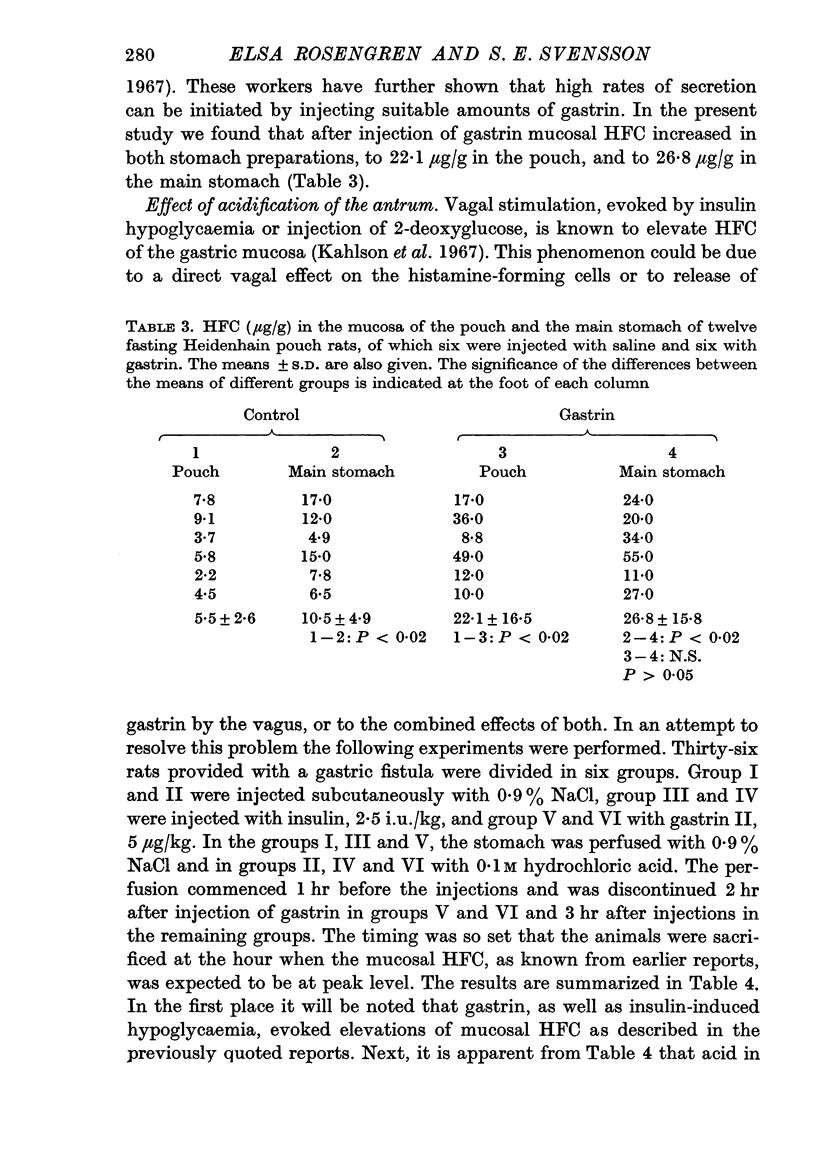
4. Injection of gastrin elevated mucosal HFC in the innervated main stomach and in the denervated pouch to approximately equal levels. With the dose of gastrin employed there was about a fourfold increase in the HFC of the pouch mucosa.
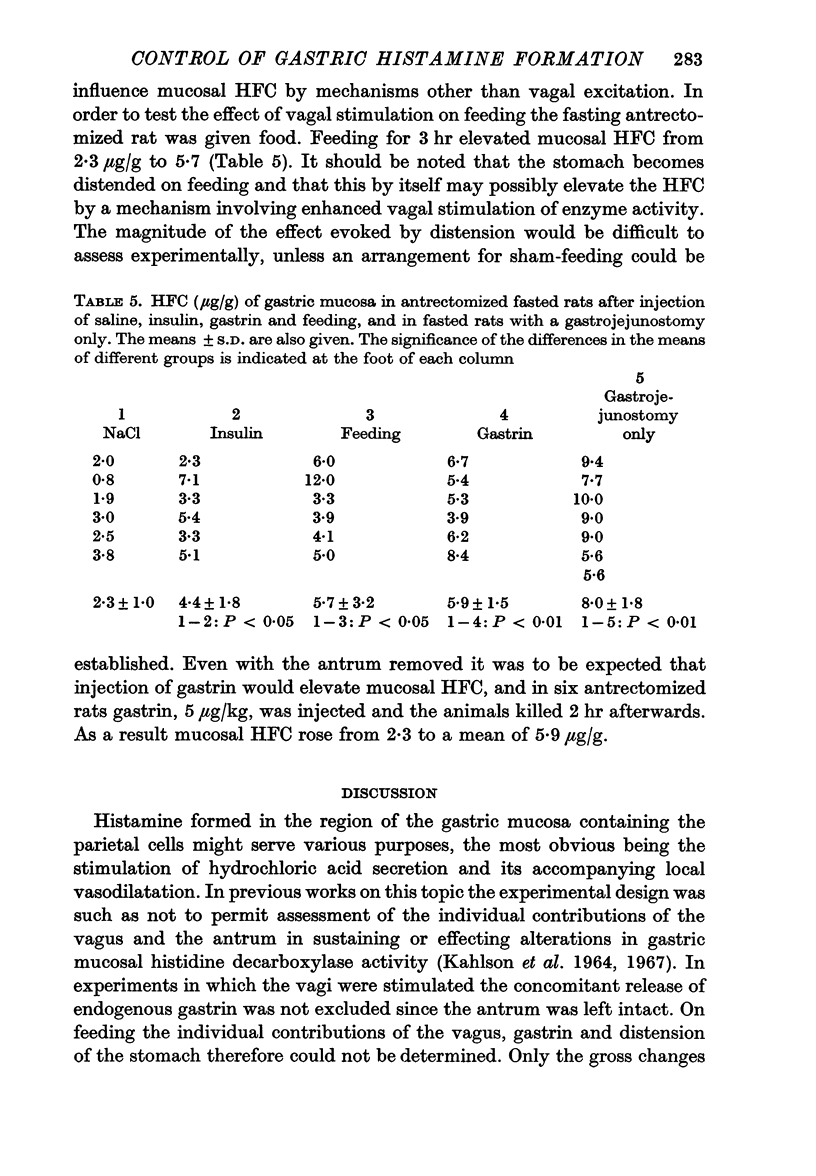
5. In antrectomized rats enhanced vagal influence, evoked by insulin injection or by feeding, raised the mucosal HFC. In rats with the antrum intact and the stomach acidified, insulin injection produced an increased HFC. Thus, a vagal effect on mucosal HFC exists independent of participation of antral gastrin.
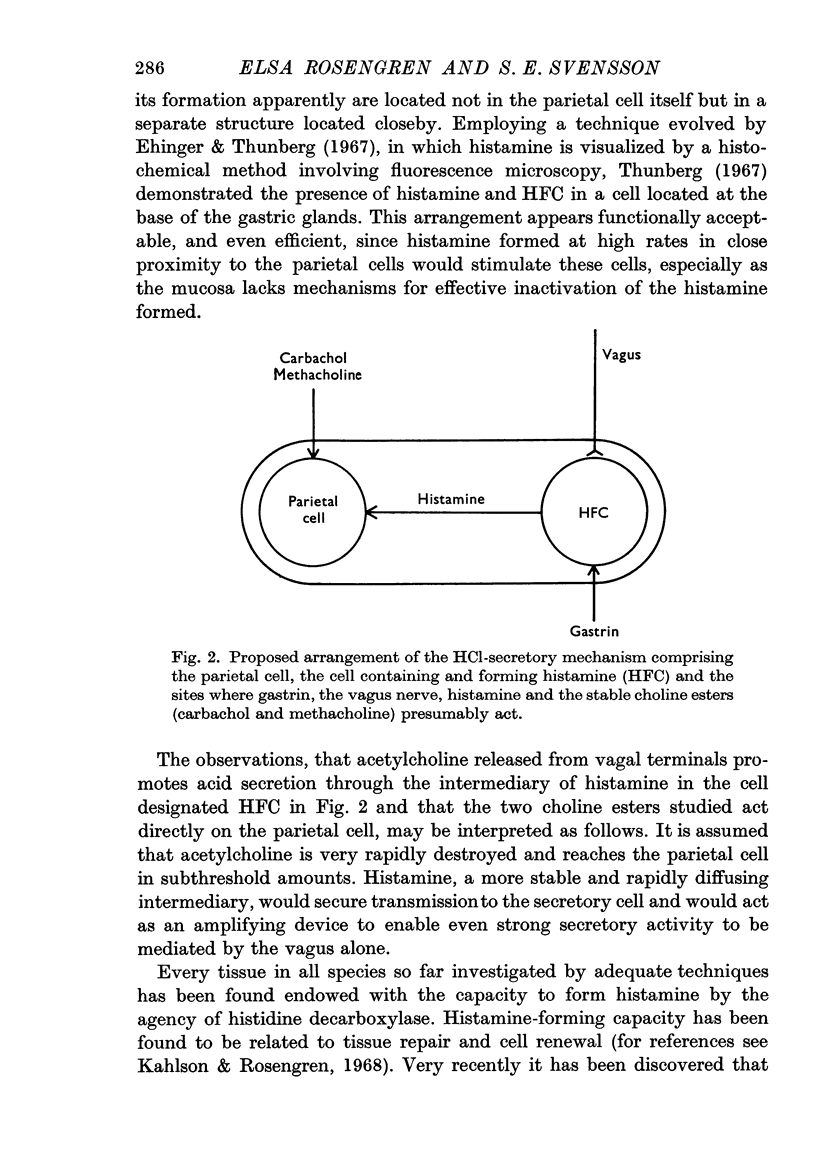
6. The stable choline esters carbachol and methacholine act directly on the parietal cell without involving mucosal HFC. The vagus nerve and gastrin, however, are assumed to provide secretory stimulation by means of accelerated histamine formation.
7. The interrelation between increased histamine formation and hydrochloric acid secretion is discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALPHIN R. S., LIN T. M. Preparation of chronic denervated gastric pouches in the rat. Am J Physiol. 1959 Aug;197:257–259. doi: 10.1152/ajplegacy.1959.197.2.257. [DOI] [PubMed] [Google Scholar]
- Ehinger B., Thunberg R. Induction of fluorescence in histamine-containing cells. Exp Cell Res. 1967 Aug;47(1):116–122. doi: 10.1016/0014-4827(67)90215-7. [DOI] [PubMed] [Google Scholar]
- Grahn B., Hughes R., Kahlson G., Rosengren E. Retardation of protein synthesis in rat foetal liver on inhibiting rate of histamine formation. J Physiol. 1969 Feb;200(3):677–685. doi: 10.1113/jphysiol.1969.sp008716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAHLSON G., ROSENGREN E., SVAHN D., THUNBERG R. MOBILIZATION AND FORMATION OF HISTAMINE IN THE GASTRIC MUCOSA AS RELATED TO ACID SECRETION. J Physiol. 1964 Nov;174:400–416. doi: 10.1113/jphysiol.1964.sp007494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAHLSON G., ROSENGREN E., THUNBERG R. OBSERVATIONS ON THE INHIBITION OF HISTAMINE FORMATION. J Physiol. 1963 Dec;169:467–486. doi: 10.1113/jphysiol.1963.sp007273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAHLSON G., ROSENGREN E., WESTLING H. Increased formation of histamine in the pregnant rat. J Physiol. 1958 Aug 29;143(1):91–103. doi: 10.1113/jphysiol.1958.sp006046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlson G., Rosengren E. New approaches to the physiology of histamine. Physiol Rev. 1968 Jan;48(1):155–196. doi: 10.1152/physrev.1968.48.1.155. [DOI] [PubMed] [Google Scholar]
- Kahlson G., Rosengren E., Thunberg R. Accelerated mobilization and formation of histamine in the gastric mucosa evoked by vagal excitation. J Physiol. 1967 Jun;190(3):455–463. doi: 10.1113/jphysiol.1967.sp008221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Ridley P. T., Tuegel C. Effect of insulin on gastric acid secretion, histamine formation, and on the incidence of gastric lesions. Life Sci. 1968 Apr 1;7(7):403–409. doi: 10.1016/0024-3205(68)90011-8. [DOI] [PubMed] [Google Scholar]
- LANE A., IVY A. C., IVY E. K. Response of the chronic gastric fistula rat to histamine. Am J Physiol. 1957 Aug;190(2):221–228. doi: 10.1152/ajplegacy.1957.190.2.221. [DOI] [PubMed] [Google Scholar]
- Lilja B., Svensson S. E. Gastric secretion during pregnancy and lactation in the rat. J Physiol. 1967 May;190(2):261–272. doi: 10.1113/jphysiol.1967.sp008206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAYER R. W. Histidine decarboxylase of rat stomach and other mammalian tissues. Am J Physiol. 1957 Jun;189(3):533–536. doi: 10.1152/ajplegacy.1957.189.3.533. [DOI] [PubMed] [Google Scholar]



