Abstract
Recently, we observed that Staphylococcus aureus strains newly isolated from patients had twofold-higher aconitase activity than a strain passaged extensively in vitro, leading us to hypothesize that aconitase specific activity decreases over time during in vitro passage. To test this hypothesis, a strain recovered from a patient with toxic shock syndrome was serially passaged for 6 weeks, and the aconitase activity was measured. Aconitase specific activity decreased 38% (P < 0.001) by the sixth week in culture. During serial passage, S. aureus existed as a heterogeneous population with two colony types that had pronounced (wild type) or negligible zones of beta-hemolytic activity. The cell density-sensing accessory gene regulatory (agr) system regulates beta-hemolytic activity. Surprisingly, the percentage of colonies with a wild-type beta-hemolytic phenotype correlated strongly with aconitase specific activity (ρ = 0.96), suggesting a common cause of the decreased aconitase specific activity and the variation in percentage of beta-hemolytic colonies. The loss of the beta-hemolytic phenotype also coincided with the occurrence of mutations in the agrC coding region or the intergenic region between agrC and agrA in the derivative strains. Our results demonstrate that in vitro growth is sufficient to result in mutations within the agr operon. Additionally, our results demonstrate that S. aureus undergoes significant phenotypic and genotypic changes during serial passage and suggest that vigilance should be used when extrapolating data obtained from the study of high-passage strains.
The ability of Staphylococcus aureus to evade the host immune response and cause disease is due to an extensive repertoire of known and putative virulence factors, including four hemolysins, two lipases, several proteases, exotoxins, and enterotoxins. The production of many virulence factors is regulated by the accessory gene regulatory (agr) operon (22, 27) and several other global regulatory loci. The agr locus consists of two divergently transcribed mRNAs designated RNA II and RNA III (19). RNA II encodes a two-component regulatory system (AgrA and AgrC) that senses the level of cyclic thiolactone peptides generated from agrB and agrD, also encoded by RNA II (15). RNA III is an RNA effector molecule that reciprocally regulates the transcription of cell-associated adherence factors and secreted proteins (25). RNA III also regulates the translation of alpha-toxin mRNA (25). Transcription of the agr operon is autoregulated by agrA in a cell density-dependent manner and by at least two other global regulatory proteins: the staphylococcal accessory regulator (SarA) (6) and ArlR, the regulatory moiety of the ArlS-ArlR two-component regulatory system (10). Recently, we determined that aconitase affects the synthesis of several S. aureus virulence factors and the expression of the global gene regulators RNA III and sarA (G. A. Somerville, unpublished data). Aconitase (citrate [isocitrate] hydrolyase, EC 4.2.1.3) is a citric acid cycle enzyme that converts citrate to isocitrate via a cis-aconitate intermediate. During the course of that study, we observed that recent human isolates of S. aureus had higher aconitase activity than did strain RN6390, which has been extensively passaged for many years. Based on this observation, we hypothesized that aconitase specific activity decreases during laboratory passage.
To test this hypothesis, a strain recovered from a patient with toxic shock syndrome was serially passaged for 6 weeks. Consistent with the hypothesis, aconitase activity decreased with increasing time in culture. Inasmuch as aconitase is a bifunctional protein with an enzymatic and regulatory function (1, 30) and has been postulated to be a virulence factor regulator (29), we also examined the effect of serial passage on growth and virulence factor production.
MATERIALS AND METHODS
Bacterial strains and materials.
All S. aureus strains used in this study have been described, and except for strain RN6390, produce toxic shock syndrome toxin 1 (23, 24). Strain SA564 was used for serial passage experiments. This strain was recovered from a patient with toxic shock syndrome and is electrophoretic type 91 (major lineage F1), an abundantly occurring virulent clone (23). It has been passed twice in vitro prior to these studies. Bacteria were grown in tryptic soy broth (TSB; BD Biosciences, Sparks, Md.) or on TSB containing 15 g of agar (TSA) per liter, TSA containing 5% washed rabbit erythrocytes, or mannitol salt agar (MSA; BD Biosciences). All cultures were grown at 37°C, and liquid cultures were aerated at 225 rpm.
Serial passage.
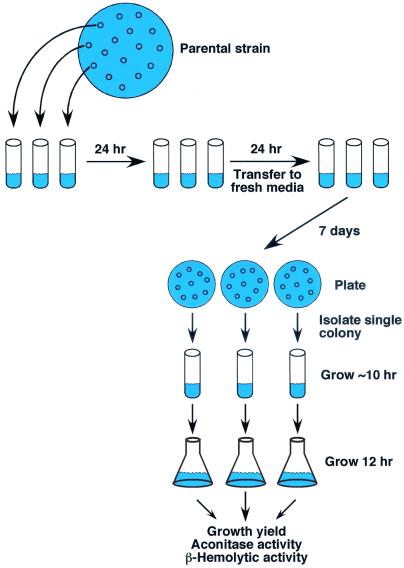
Strain SA564 was plated onto TSA and grown overnight, and three bacterial colonies were isolated (Fig. 1). Each colony was inoculated into a 15-ml round-bottom tube containing 3 ml of TSB. The cultures were incubated in an environmental chamber at a 45° angle to allow for maximum aeration. Once each day for 6 weeks the bacteria were diluted 1:200 into fresh TSB, resulting in approximately 4 h of exponential-phase growth, 7 to 9 h of post-exponential-phase growth, and 11 to 13 h of stationary-phase growth. Once per week an aliquot of the bacterial culture was plated onto TSA and grown overnight, and individual colonies picked at random were inoculated into TSB. Bacterial cultures were grown for approximately 10 h and then inoculated 1:200 (normalized for growth) into fresh media, incubated for 12 h, and used for the determination of the growth yield (A600), aconitase activity, and α-toxin titer.
FIG. 1.
In vitro serial passage experimental design. Strain SA564 was plated onto TSA and grown overnight, and three bacterial colonies were isolated. Each colony was inoculated into a 15-ml round-bottom tube containing 3 ml of TSB. The cultures were incubated in an environmental chamber at a 45° angle to allow for maximum aeration. Once each day for 6 weeks the bacteria were diluted 1:200 into fresh TSB. Once per week an aliquot of the bacterial culture was plated onto TSA and grown overnight, and individual colonies picked at random were inoculated into TSB. Bacterial cultures were grown for ca. 10 h and then inoculated 1:200 (normalized for growth) into fresh media, incubated for 12 h, and used for the determination of growth yield (A600), aconitase activity, and alpha-toxin titer.
Aconitase activity assay.
Cell-free lysates of S. aureus were prepared as follows. Aliquots (3.0 ml) were harvested; centrifuged; resuspended in 1.5 ml of lysis buffer containing 90 mM Tris (pH 8.0), 100 μM fluorocitrate, and 50 μg of lysostaphin (Sigma) per ml; incubated at 37°C for 10 min; and passed through a French press (two times at 15,000 lb/in2). The lysate was centrifuged for 5 min at 14,000 rpm at 4°C, and the aconitase activity in the cell-free lysate was measured by the method of Kennedy et al. (17).
Determination of alpha-toxin titer and the percentage of beta-hemolytic colonies.
To determine beta-hemolytic activity, twofold serial dilutions of culture supernatants were mixed with an equal volume of 2% washed rabbit erythrocytes in U-bottom microtiter plates. The plates were incubated at 37°C for 30 min, followed by incubation at 4°C overnight. The alpha-toxin titer is defined as the inverse of the highest dilution for which 50% of the erythrocytes remain intact after the overnight incubation (8). The percentage of beta-hemolytic colonies was determined by plating serial dilutions of exponentially growing, serially passaged cultures on TSA plates containing 5% washed rabbit erythrocytes. These plates were incubated overnight at 37°C, and the total number of colonies and the number of colonies with a beta-hemolytic phenotype were counted. For ease of description, a colony was defined as nonhemolytic when the zone of erythrocyte clearing was <0.5 mm from the edge of the colony.
Analysis of secreted and cell-associated proteins.
Culture supernatants from the parental strain and the three derivative strains obtained at week 6 were used to qualitatively determine the effect of serial passage on the level of secreted proteins. Bacteria were grown for 12 h and removed by centrifugation. The volume of culture supernatant loaded was normalized for growth (A600), and electrophoresis was performed on a 10% polyacrylamide gel (Bio-Rad). Protein bands were visualized by SYPRO Ruby staining (Genomics Solutions, Chelmsford, Mass.). Western immunoblots were performed as described elsewhere (28) with antibodies to α-toxin (Sigma) and staphylococcal enterotoxin C (SEC; IGEN International, Inc., Gaithersburg, Md.).
To determine protein A levels, bacteria from 12-h cultures (10 ml) were harvested by centrifugation, washed once with phosphate-buffered saline (PBS), resuspended in PBS (4 ml) containing 10 mM MgCl2, 50 μg of lysostaphin per ml, and 200 units of DNase. Protein concentrations were determined by the Lowry method (20). Electrophoresis was carried out as described above with 50 μg of total protein. Visualization of protein A bands was carried out with a secondary mouse anti-immunoglobulin G antibody conjugated to horseradish peroxidase.
Production of ROS.
Bacteria (109 CFU/ml) were equilibrated with either 25 μM 2",7"-dihydrodichlorofluorescein (DCF) or 50 μM dihydroethidine (DE) (both from Molecular Probes, Inc., Eugene, Oreg.) for 30 to 45 min on ice. Bacteria (108 CFU/ml) were subsequently transferred to wells of a 96-well microtiter plate, and the oxidation of DCF or DE by reactive oxygen species (ROS) was measured at 37°C for 2 h by using a Spectramax Gemini microplate spectrofluorometer (Molecular Devices, Sunnyvale, Calif.). DCF fluorescence was detected by using excitation and emission wavelengths of 485 and 538 nm, respectively. For DE fluorescence, the excitation and emission wavelengths were 473 and 593 nm, respectively, and a cutoff filter was set at 530 nm. Fluorescence measurements were collected once per minute, and the Vmax was calculated as the maximum rate of fluorescence change over a 5- to 10-min period by using Softmax Pro, version 3.1, software (Molecular Devices).
Statistical analysis.
The statistical significance of physiological changes (e.g., aconitase activity) in the week 6 derivative strains relative to the parental strain was assessed with the Student's t test. To determine whether a correlation existed between two physiological parameters, a correlation coefficient (ρ) was calculated and the percentage covariance was determined from ρ2.
DNA microarray analysis.
S. aureus strains were grown overnight in TSB and DNA was isolated with the Total Genomic DNA kit (Edge Biosystems, Gaithersburg, Md.) according to the manufacturer's instructions. Construction of the S. aureus microarray and DNA hybridizations were carried out as described earlier (9). The DNA microarray was constructed to represent only open reading frames greater than 100 bp in length; therefore, only deletions of greater than 100 bp in length would be detected.
DNA sequence analysis.
The agr operon from agrA to RNA III was amplified by high-fidelity PCR from genomic DNA. Resultant amplicons were sequenced by using fluorescent dye terminator chemistry from both strands with the primers listed in Table 1. Sequence data was acquired on an Applied Biosystems 3700 automated sequencer, assembled using Sequencher (GeneCodes, Ann Arbor, Mich.) and analyzed by using Lasergene (DNASTAR, Madison, Wis.).
TABLE 1.
Primers used in the sequencing of the agr operon of strain SA564 and derivative strains
| Primera | Orientation | Sequence (5"→3") |
|---|---|---|
| AGR_1∗ | Reverse | GGAGGGGCTCACGACCAT |
| AGR_2 | Reverse | GATATCTTGTGCCATTGA |
| AGR_6 | Reverse | GAAATTGAGACCTATTAC |
| AGR_8 | Reverse | GTATCAAATTAGGCAGTG |
| AGR_11∗ | Forward | AAATAGGTAAGTTCACTG |
| AGR_12 | Forward | GACCTAAACCACGACCTT |
| AGR_13 | Forward | CATAATCATGTCGGAACT |
| AGR_17 | Forward | TTAAGGAAGGAGTGATTT |
| AGR_20 | Forward | ATCAATACGGCTCTACTT |
| AGR_21 | Reverse | CAACTTTTTCATCTATCA |
| AGR_22 | Reverse | GTCGCAGTATTGGTATTA |
| AGR_23 | Reverse | TTAAAATTAAACAACTCA |
| AGR_24 | Reverse | ACGCTATAATGCACATGG |
| AGR_25 | Reverse | CTAAAGTACCCGCTGAAT |
| AGR_26 | Reverse | GGCCAGTAAATAAGTAAA |
Primers used for PCR amplification of the agr locus are indicated with an asterisk.
RESULTS
Decreased aconitase specific activity of S. aureus during serial passage.
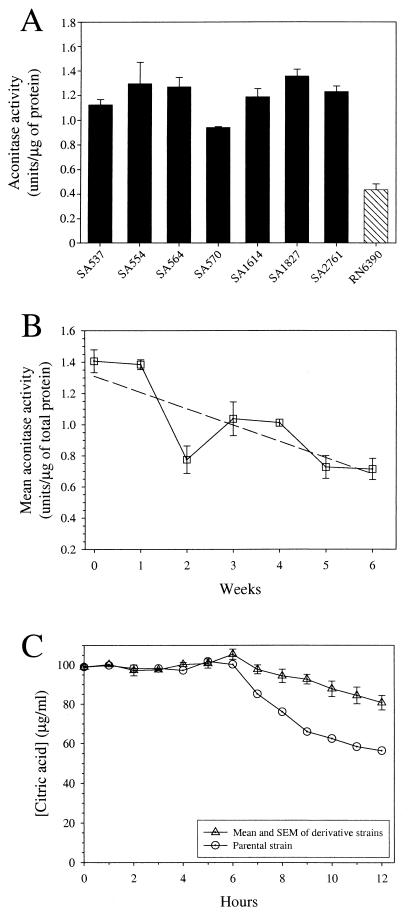
We recently observed that seven clonally diverse recent human isolates of S. aureus had at least twofold-greater aconitase activity than the laboratory passaged strain RN6390 (Fig. 2A). This observation led us to hypothesize that aconitase specific activity decreased during laboratory passage. To test this hypothesis, strain SA564 was serially passaged for 6 weeks, and the aconitase activity was measured (Fig. 2B). The mean aconitase activity in the derivative strains varied over the 6 weeks in culture, but after the first week it was consistently less than the level made by the parental strain. By the sixth week in culture, the mean aconitase activity in the derivative strains had decreased 38% (P < 0.001) relative to the parental strain. Additionally, the depletion of endogenous citrate in the culture medium by the derivative strains was decreased relative to the parental strain (Fig. 2C), a finding consistent with the decrease in aconitase activity. Taken together, these results confirm that aconitase activity decreased significantly during serial passage.
FIG. 2.
Aconitase activity of S. aureus isolates and serially passaged strains. (A) Aconitase activity of clonally diverse S. aureus strains and the highly passaged laboratory strain RN6390. Data are presented as the average of triplicate determinations, and error bars represent the standard deviation. (B) Mean aconitase activity of the parental strain (SA564) and the serially passaged derivative strains over 6 weeks. The dashed line represents a linear regression through the data and is only intended to show the trend in the data. The data represent the mean aconitase activity and the standard error of the mean (SEM) for the parental strain (week 0) and three individual progeny for each week (weeks 1 to 6). (C) Depletion of citric acid from the culture media of the parental strain and the week 6 derivative strains. The data are presented as the mean and SEM for three week 6 progeny, and a representative profile for the parental strain is shown.
Change in growth characteristics of S. aureus during serial passage.
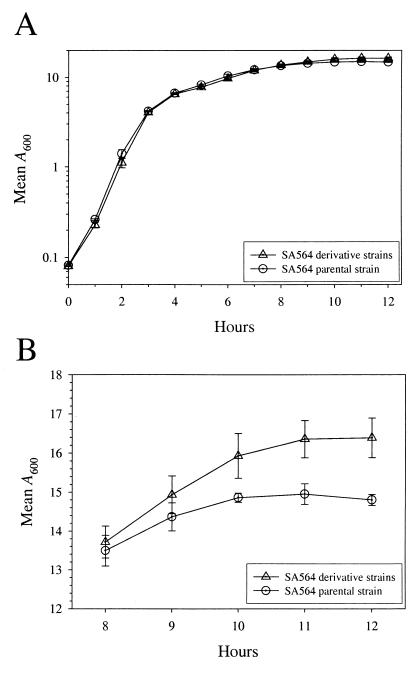
Aconitase is a citric acid cycle enzyme that converts citrate to isocitrate. Therefore, decreased aconitase activity could detrimentally alter growth. To assess the affects of serial passage on growth, we measured growth yields over a 6-week time period (Fig. 3A). The growth yields of derivative strains increased after only 1 week in serial passage and were consistently higher than the parental strain throughout the growth study. After 6 weeks of serial passage, the derivative strains had a significantly increased mean growth yield of 2 A600 units (∼2 × 109 CFU/ml), representing a 12% (P = 0.012; n = 6) increase compared to the parental strain. However, in contrast to the results of a previous report (4), we found no significant change in the growth rate of the derivative strains relative to parental strain SA564 (Fig. 4). These data indicated that increased growth was due to an enhanced ability to acquire or more efficiently utilize available nutrients during postexponential growth. An increase in cell density during in vitro growth might also be achieved by diverting metabolites from nonessential functions, such as the production of secreted virulence factors, to growth, an idea put forth previously (4, 31).
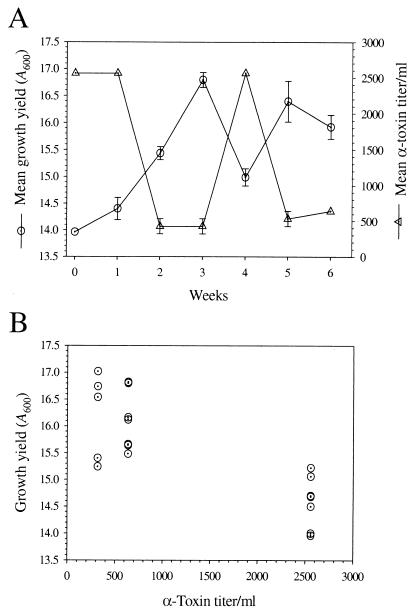
FIG. 3.
Beta-hemolytic activity and growth yield inversely correlate. (A) The mean and SEM for the growth yield (A600) and beta-hemolytic activity of the parental strain (week 0) and three individual progeny for each week (weeks 1 to 6). (B) Plot of the growth yield as a function of beta-hemolytic titer.
FIG. 4.
Growth yields increase during serial passage but not growth rates. (A) Growth curve of the parental strain and three week 6 progeny. (B) The post-exponential-growth phases shown in panel A demonstrating the increase in growth yield. Note that the scale in panel B is presented as linear. The data are presented as the mean and SEM of three independent cultures and are representative of growth studies performed at least twice.
Alpha-toxin and protein A production during serial passage.
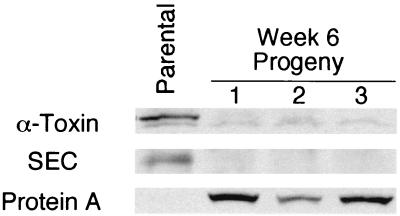
Aconitase has been shown to have both an enzymatic and regulatory function (1, 30) and has been postulated to be involved in virulence factor regulation (29). These observations and the decreased aconitase activity present in the derivative strains led us to examine the affect of serial passage on alpha-toxin production. The mean alpha-toxin titer of the derivative strains fluctuated during serial passage but was predominantly lower relative to the parental strain (Fig. 3A). To determine whether a correlation existed between aconitase activity and alpha-toxin titer, the hemolytic titer was plotted as a function of aconitase activity (data not shown), but no correlation was found (ρ2 = 1.3 %), demonstrating that aconitase activity and alpha-toxin production are not linked. However, an overlay of the mean growth yields and the mean alpha-toxin titers suggested that the two parameters were correlated (Fig. 3A). Plotting the growth yield as a function of alpha-hemolytic titers revealed that an inverse correlation existed between growth yield and alpha-toxin titers (ρ2 = 64.1%) (Fig. 2B). Western immunoblotting confirmed a significant decrease in the production of the secreted proteins alpha-toxin and SEC in the week 6 derivative strains relative to the parental strain (Fig. 5). Secreted proteins are reciprocally regulated with cell-associated proteins, including protein A, by the agr cell density-sensing system. The level of the cell-associated protein A increased concomitantly with the decrease in secreted proteins (Fig. 5), confirming a reciprocal regulation. Taken together, these data suggest that an alteration in cell density sensing had occurred in the derivative strains during serial passage, an idea consistent with previously published observations with S. aureus (21).
FIG. 5.
Western blot analysis of secreted alpha-toxin and SEC and of cell-associated protein A. Western blots of supernatants from cultures of the parental strain and three week 6 progeny. The amount of culture supernatant loaded in each lane was normalized for growth. For the determination of protein A levels, 50 μg of total protein from cell-free lysates was loaded per lane.
Microarray analysis of S. aureus genomic DNA.
Serial passage of bacteria can result in genomic diversity of derivative strains relative to the parental strain due to single nucleotide changes, changes in repetitive DNA, recombination, and insertion and deletion events. We used DNA microarray analysis to screen for gene deletions that correlated with the changes in physiology and virulence factor production in the derivative strains. No deletions of open reading frames were found (data not shown), demonstrating that the observed physiologic changes are due to other genetic alterations.
Sequencing of the agr operon.
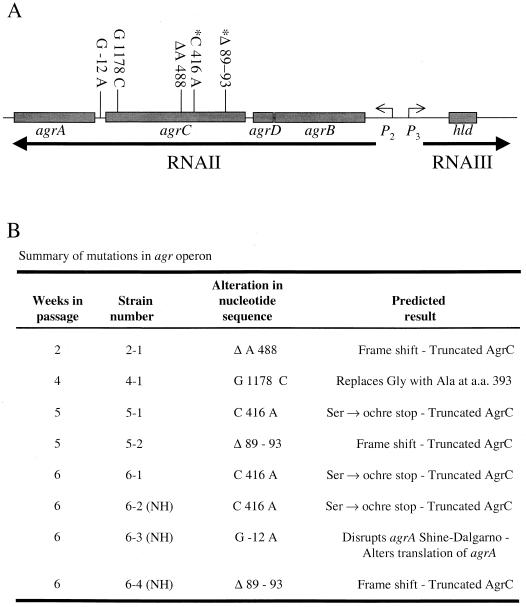
Two types of nucleotide variation have been reported in the agr operon: variation that defines an agr specificity group (13, 14) and spontaneous mutations arising during genetic manipulation (21). To determine if the variation in alpha-toxin activity that occurred during serial passage correlated with nucleotide variation in agr, we sequenced the agr operon in the parental strain, 18 randomly chosen derivative strains (3 from each week of passage; beta-hemolytic phenotype not known), and week 6 progeny for which the beta-hemolytic phenotype was known (3 hemolytic and 3 nonhemolytic). No nucleotide substitutions were present in the agr operon of 13 of the 18 randomly chosen progeny or the 3 beta-hemolytic colonies from the week 6 derivative strains. In striking contrast, all three nonhemolytic week 6 derivative strains had mutations in the coding region of agrC or in the intergenic region between agrC and agrA (Fig. 6). In addition to mutations in these nonhemolytic strains, mutations were identified in 5 of 18 randomly chosen derivative strains (Fig. 6). The nonhemolytic derivative 6-2 had a point mutation at nucleotide position 416 of the agrC coding region that replaced a cytidine with an adenosine, resulting in the conversion of a serine codon (UCA) to an ochre stop codon (UAA). Nonhemolytic derivative 6-3 had a point mutation in the intergenic region between agrC and agrA, altering the predicted agrA Shine-Dalgarno region. The third nonhemolytic week 6 derivative (6-4) had a 5-nucleotide deletion (i.e., nucleotides 89 to 93 of the agrC coding sequence) that resulted in a frameshift mutation generating numerous stop codons. This nucleotide deletion was first identified in a randomly chosen derivative strain from week 5 culture 3 (the progenitor culture of 6-4), suggesting that the mutation was stable. In addition, this mutation increased the growth yield, thereby enhancing the mutant's ability to place progeny into the next generation. Similarly, the point mutation in the nonhemolytic derivative 6-2 was also found in the randomly chosen week 5 and week 6 derivatives (5-1 and 6-1, respectively), also suggesting the mutation was stable and enhanced the mutant's ability to propagate the mutation. Interestingly, all mutations were predicted to code for a truncated or mutated AgrC or to prevent the translation of agrA, and there was never more than one mutation in any derivative strain.
FIG. 6.
Mutations identified by DNA sequencing of the agr operon. (A) The numbers at the top of the figure refer to the nucleotide sequence position relative to the agrC start codon (+1), except the G −12 A mutation, which occurs in the putative Shine-Dalgarno site, and is relative to the agrA start site. The asterisks indicate that a particular mutation was identified more than once. (B) Summary of the nucleotide changes and predicted outcome of the mutations identified in the agr operon. The data represent the results of DNA sequencing of the agr operon from 24 derivative strains. The strain number is an arbitrary designation. Strains 5-1 and 5-2 were obtained from separate derivative cultures. NH, nonhemolytic.
Changes in the occurrence of beta-hemolytic colonies within a population.
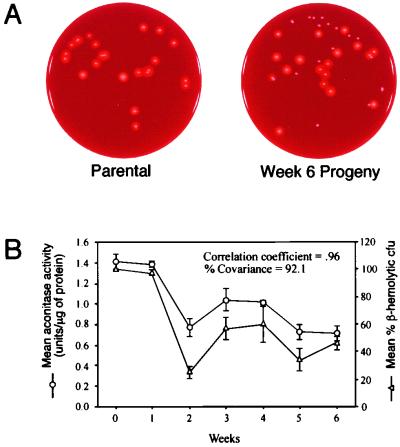
The observed variation in the growth yield, aconitase activity, and beta-hemolytic activity during serial passage suggested that the derivative strains existed as a heterogeneous population. To establish the extent of population variation in a single parameter, the percentage of beta-hemolytic colonies present in the culture over the 6 weeks of serial passage was determined (Fig. 7A). The percentage of beta-hemolytic colonies decreased by 50% during serial passage, but a mixed population was maintained. The latter fact is surprising because the increased growth yield of nonhemolytic bacteria increased their fitness (i.e., the ability to place progeny into the next generation), suggesting that within a limited number of in vitro serial passages the population should contain only nonhemolytic bacteria. One possible explanation for the maintenance of a mixed population is that a symbiotic relationship exists between the two populations. Interestingly, a 92.1% covariance between aconitase activity and the percentage of beta-hemolytic colonies was found, confirming a strong correlation (Fig. 7B). These data suggested a common cause for the decreased aconitase activity and the variation in percentage beta-hemolytic colonies.
FIG. 7.
Determination of the percentage of beta-hemolytic colonies during serial passage. (A) Examples of the rabbit blood agar plates used for the determination of the percentage of beta-hemolytic CFU in panel B. (B) The mean and SEM for aconitase activity and the percentage of beta-hemolytic CFU by the parental strain (week 0) and the progeny (weeks 1 to 6) are plotted for the 6 weeks of serial passage.
Changes in oxidative stress during serial passage.
Aconitase activity (i.e., the conversion of citrate to isocitrate) is mediated by an essential redox inactive iron-sulfur center. The [4Fe-4S] center is necessary for the binding of the C-2 carboxyl of citrate or cis-aconitate and for the binding of the C-1 carboxyl of isocitrate (18). This coordination of binding occurs through the labile fourth iron atom designated Fea. In the absence of substrate, the Fea of the iron-sulfur cluster is susceptible to inactivation by oxygen radicals (11). The oxidative loss of the Fea atom leads to the reversible inactivation of aconitase, leaving the enzyme in the [3Fe-4S] state. The enzyme can be further disassembled by loss of the remaining iron-sulfur center, thus generating the apoaconitase form of the enzyme. This means that aconitase can exist in three forms, [4Fe-4S], [3Fe-4S], and apoaconitase. However, only the first form is enzymatically active. Taken together, these data suggest that decreased aconitase specific activity in the derivative strains could be the result of a reduction in the availability of iron. However, the media was not iron limited, leading us to measure the maximum rate (Vmax) of production of ROS by the parental strain and the week 6 derivative strains. The rate of ROS production by the derivative strains was significantly greater than the parental strain (∼11%, P = 0.013), thus creating an intracellular environment known to inactivate aconitase (26).
DISCUSSION
Aconitase activity and growth.
Serial passage of a S. aureus human isolate resulted in significantly decreased aconitase activity (Fig. 2B), eventually reaching a level of activity similar to that of the laboratory-passaged strain RN6390. A decrease in aconitase activity would reduce the availability of citric acid cycle intermediates for amino acid biosynthesis. Therefore, the reduction in aconitase activity and the decreased ability of the derivative strains to utilize citrate during passage suggests that serial passage decreases the growth rate and/or growth yield of the derivative strains. However, we found that the mean growth rate of the parental and derivative strains was equivalent and that the mean growth yield of the derivative strains was significantly increased (Fig. 4). In addition to establishing that aconitase specific activity decreased during serial passage, these data suggested that the citric acid cycle was being used to generate energy for virulence factor production in postexponential growth.
agr operon.
Serial passage of bacteria can result in increased genomic diversity of derivative strains relative to the parental strain (3). Genomic diversity can result from the accumulation of mutations, DNA deletions or insertions, or changes in repetitive sequences. No large deletions were found that accounted for the physiologic or virulence changes in the derivative strains (data not shown). The bacterial cultures were started from single colonies, and therefore the total DNA is common to all bacteria in the culture. Hence, the possibility is remote that an insertion event would result in the gain of function (e.g., enhanced ability to acquire or utilize additional carbon sources). Thus, it is unlikely that the phenotypic changes occurred as a consequence of large DNA deletion or insertion events.
Transposon mutagenesis of S. aureus has been associated with spontaneous mutations arising in the agr operon, an observation resulting in the agr operon being described as a region of “genetic instability” (21). Our results demonstrate that genetic manipulation alone is not required to generate mutations in this region.
Our data revealed that all of the nucleotide changes that occurred were predicted to yield a truncated or mutated AgrC or to prevent the translation of agrA transcripts. All of these mutations would be expected to inactivate quorum sensing, producing pronounced phenotypic changes. These mutations decreased the production of secreted virulence factors and increased the growth yield, thereby increasing the fitness of the agr mutants, suggesting that there was selection for these mutations. However, it is reasonable to assume that some phenotypic differences are due to mutations in genes other than those encoded by agr. Evidence for in vivo selection of agr mutants has been suggested by an agr functionality screen (32). In that study, it was found that approximately 25% of 105 recent human isolates were deficient in the production of delta-toxin, indicating that agr mediated regulation was disrupted.
Implications for agr variation in vivo.
S. aureus can invade epithelial cell lines resulting in apoptosis (2, 16), or it can invade and persist intracellularly (12, 33). The organism can also invade and survive in neutrophils, a situation believed to contribute to the spread of infection (12). Compared to the isogenic wild-type strain, agr mutants are internalized more efficiently by epithelial cells in vitro, are better able to persist, and do not induce apoptosis (33). agr-mediated cell density sensing reciprocally regulates the expression of cell-associated adhesion proteins and secreted virulence factors (25). Therefore, the increased uptake and persistence are likely due to an increase in the expression of cell-associated adhesion proteins (e.g., fibronectin-binding protein). Taken together, these observations suggest that in certain circumstances inactivation of agr can be advantageous to S. aureus.
During growth in aerobic conditions, mutations arise in agr (Fig. 6), creating a mixed bacterial population (e.g., beta-hemolytic and nonhemolytic). It is reasonable to assume that in a mixed population of S. aureus, a percentage of the cells express colonization factors (e.g., fibronectin-binding protein), some express secreted virulence proteins (e.g., alpha-toxin), some express neither, and some express both. A diverse bacterial population allows for mutants present in a low percentage under one set of environmental conditions to become the predominant progeny following an environmental shift. Spontaneously arising mutations within the csrR-csrS two-component virulence regulatory system in Streptococcus pyogenes significantly enhance the pathogenicity of wild-type bacteria during coinfection (7). We hypothesize, based on these observations, that mutations of agr create a mixed bacterial population and that the ecological niche and host immune, response select for the S. aureus agr variant(s) best able to adapt to a particular environment.
Conclusions.
Serial passage of S. aureus caused significant phenotypic changes and alteration of the global regulator agr. Our data demonstrate that mutations in agr result in the generation of a diverse population of bacteria. We speculate that a diverse population of bacteria enhances the likelihood of subsequent selection of the S. aureus agr variant(s) most capable of surviving a specific environment. Lastly, our results demonstrate that caution should be used when data generated with high-passage strains are extrapolated to low-passage, recent clinical isolates.
Acknowledgments
We thank G. Sylva for technical assistance, G. Hettrick and A. Mora for help with figure preparation, and M. Otto, J. Hinnebusch, and S. Reid for critical review of the manuscript.
REFERENCES
- 1.Alen, C., and A. L. Sonenshein. 1999. Bacillus subtilis aconitase is an RNA-binding protein. Proc. Natl. Acad. Sci. USA 96:10412-10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayles, K. W., C. A. Wesson, L. E. Liou, L. K. Fox, G. A. Bohach, and W. R. Trumble. 1998. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect. Immun. 66:336-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. [DOI] [PubMed] [Google Scholar]
- 4.Bjorklind, A., and S. Arvidson. 1980. Mutants of Staphylococcus aureus affected in the regulation of exoprotein synthesis. FEMS Microbiol. Lett. 7:203-206. [Google Scholar]
- 5.Chan, P. F., and S. J. Foster. 1998. The role of environmental factors in the regulation of virulence-determinant expression in Staphylococcus aureus 8325-4. Microbiology 144:2469-2479. [DOI] [PubMed] [Google Scholar]
- 6.Cheung, A. L., M. G. Bayer, and J. H. Heinrichs. 1997. sar genetic determinants necessary for transcription of RNAII and RNAIII in the agr locus of Staphylococcus aureus. J. Bacteriol. 179:3963-3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engleberg, N. C., A. Heath, A. Miller, C. Rivera, and V. J. DiRita. 2001. Spontaneous mutations in the CsrRS two-component regulatory system of Streptococcus pyogenes result in enhanced virulence in a murine model of skin and soft tissue infection. J. Infect. Dis. 183:1043-1054. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald, J. R., P. J. Hartigan, W. J. Meaney, and C. J. Smyth. 2000. Molecular population and virulence factor analysis of Staphylococcus aureus from bovine intramammary infection. J. Appl. Microbiol. 88:1028-1037. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald, J. R., D. E. Sturdevant, S. M. Mackie, S. R. Gill, and J. M. Musser. 2001. Evolutionary genomics of Staphylococcus aureus: insights into the origin of methicillin-resistant strains and the toxic shock syndrome epidemic. Proc. Natl. Acad. Sci. USA 98:8821-8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fournier, B., A. Klier, and G. Rapoport. 2001. The two-component system ArlS-ArlR is a regulator of virulence gene expression in Staphylococcus aureus. Mol. Microbiol. 41:247-261. [DOI] [PubMed] [Google Scholar]
- 11.Gardner, P. R., and I. Fridovich. 1992. Inactivation-reactivation of aconitase in Escherichia coli. A sensitive measure of superoxide radical. J. Biol. Chem. 267:8757-8763. [PubMed] [Google Scholar]
- 12.Gresham, H. D., J. H. Lowrance, T. E. Caver, B. S. Wilson, A. L. Cheung, and F. P. Lindberg. 2000. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J. Immunol. 164:3713-3722. [DOI] [PubMed] [Google Scholar]
- 13.Jarraud, S., G. J. Lyon, A. M. Figueiredo, L. Gerard, F. Vandenesch, J. Etienne, T. W. Muir, and R. P. Novick. 2000. Exfoliatin-producing strains define a fourth agr specificity group in Staphylococcus aureus. J. Bacteriol. 182:6517-6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji, G., R. Beavis, and R. P. Novick. 1997. Bacterial interference caused by autoinducing peptide variants. Science 276:2027-2030. [DOI] [PubMed] [Google Scholar]
- 15.Ji, G., R. C. Beavis, and R. P. Novick. 1995. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc. Natl. Acad. Sci. USA 92:12055-12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahl, B. C., M. Goulian, W. van Wamel, M. Herrmann, S. M. Simon, G. Kaplan, G. Peters, and A. L. Cheung. 2000. Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line. Infect. Immun. 68:5385-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy, M. C., M. H. Emptage, J. L. Dreyer, and H. Beinert. 1983. The role of iron in the activation-inactivation of aconitase. J. Biol. Chem. 258:11098-11105. [PubMed] [Google Scholar]
- 18.Kennedy, M. C., M. Werst, J. Telser, M. H. Emptage, H. Beinert, and B. M. Hoffman. 1987. Mode of substrate carboxyl binding to the [4Fe-4S]+ cluster of reduced aconitase as studied by 17O and 13C electron-nuclear double resonance spectroscopy. Proc. Natl. Acad. Sci. USA 84:8854-8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kornblum, J., B. N. Kreiswirth, S. J. Projan, H. Ross, and R. P. Novick. 1990. Agr: a polycistronic locus regulating exoprotein synthesis in Staphylococcus aureus, p. 373-402. In R. P. Novick (ed.), Molecular biology of the staphylococci. VCH Publishers, Inc., New York, N.Y.
- 20.Lowry, O. H., N. J. Rosebrough, L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:267-275. [PubMed] [Google Scholar]
- 21.McNamara, P. J., and J. J. Iandolo. 1998. Genetic instability of the global regulator agr explains the phenotype of the xpr mutation in Staphylococcus aureus KSI9051. J. Bacteriol. 180:2609-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morfeldt, E., L. Janzon, S. Arvidson, and S. Lofdahl. 1988. Cloning of a chromosomal locus (exp) which regulates the expression of several exoprotein genes in Staphylococcus aureus. Mol. Gen. Genet. 211:435-440. [DOI] [PubMed] [Google Scholar]
- 23.Musser, J. M., and R. K. Selander. 1990. Genetic analysis of natural populations of Staphylococcus aureus, p. 59-67. In R. P. Novick (ed.), Molecular biology of the staphylococci. VCH Publishers, Inc., New York, N.Y.
- 24.Novick, R. P. 1990. The staphylococcus as a molecular genetic system, p. 1-40. In R. P. Novick (ed.), Molecular biology of the staphylococci. VCH Publishers, Inc., New York, N.Y.
- 25.Novick, R. P., H. F. Ross, S. J. Projan, J. Kornblum, B. Kreiswirth, and S. Moghazeh. 1993. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 12:3967-3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paraskeva, E., and M. W. Hentze. 1996. Iron-sulphur clusters as genetic regulatory switches: the bifunctional iron regulatory protein-1. FEBS Lett. 389:40-43. [DOI] [PubMed] [Google Scholar]
- 27.Peng, H. L., R. P. Novick, B. Kreiswirth, J. Kornblum, and P. Schlievert. 1988. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J. Bacteriol. 170:4365-4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook, J., Fritsch, E. F., and Maniatis, T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Plainview, N.Y.
- 29.Somerville, G., C. A. Mikoryak, and L. Reitzer. 1999. Physiological characterization of Pseudomonas aeruginosa during exotoxin A synthesis: glutamate, iron limitation, and aconitase activity. J. Bacteriol. 181:1072-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang, Y., and J. R. Guest. 1999. Direct evidence for mRNA binding and post-transcriptional regulation by Escherichia coli aconitases. Microbiology 145:3069-3079. [DOI] [PubMed] [Google Scholar]
- 31.Timmins, B. S., and K. T. Holland. 1999. Shift-down in growth rate rather than high cell density induces toxic shock syndrome toxin-1 gene expression in Staphylococcus aureus. FEMS Microbiol. Lett. 172:173-177. [DOI] [PubMed] [Google Scholar]
- 32.Vuong, C., H. L. Saenz, F. Gotz, and M. Otto. 2000. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus. J. Infect. Dis. 182:1688-1693. [DOI] [PubMed] [Google Scholar]
- 33.Wesson, C. A., L. E. Liou, K. M. Todd, G. A. Bohach, W. R. Trumble, and K. W. Bayles. 1998. Staphylococcus aureus Agr and Sar global regulators influence internalization and induction of apoptosis. Infect. Immun. 66:5238-5243. [DOI] [PMC free article] [PubMed] [Google Scholar]