Abstract
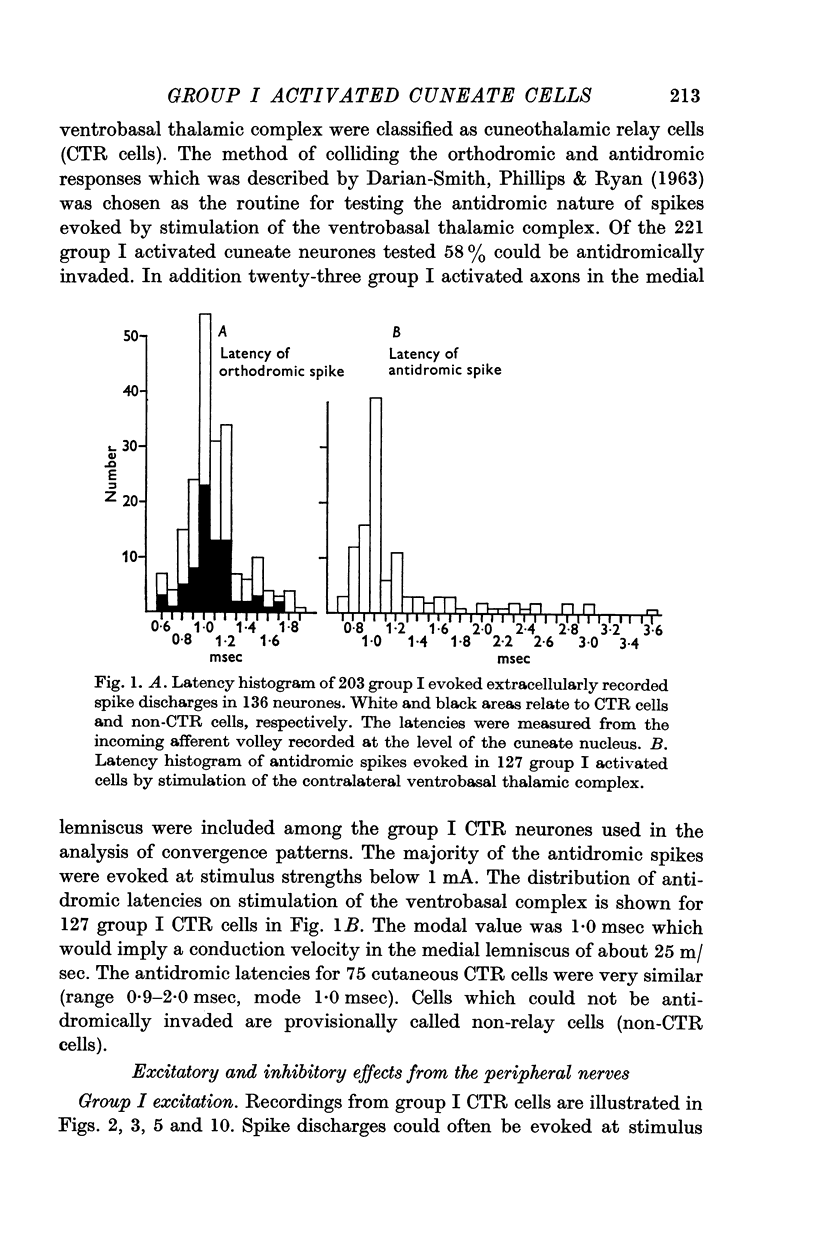
1. Extracellular recordings were made from a total of 240 group I activated cells in the main cuneate nucleus. Cuneothalamic relay neurones (128) were identified by antidromic stimulation of the medial lemniscus in the ventrobasal thalamic complex.
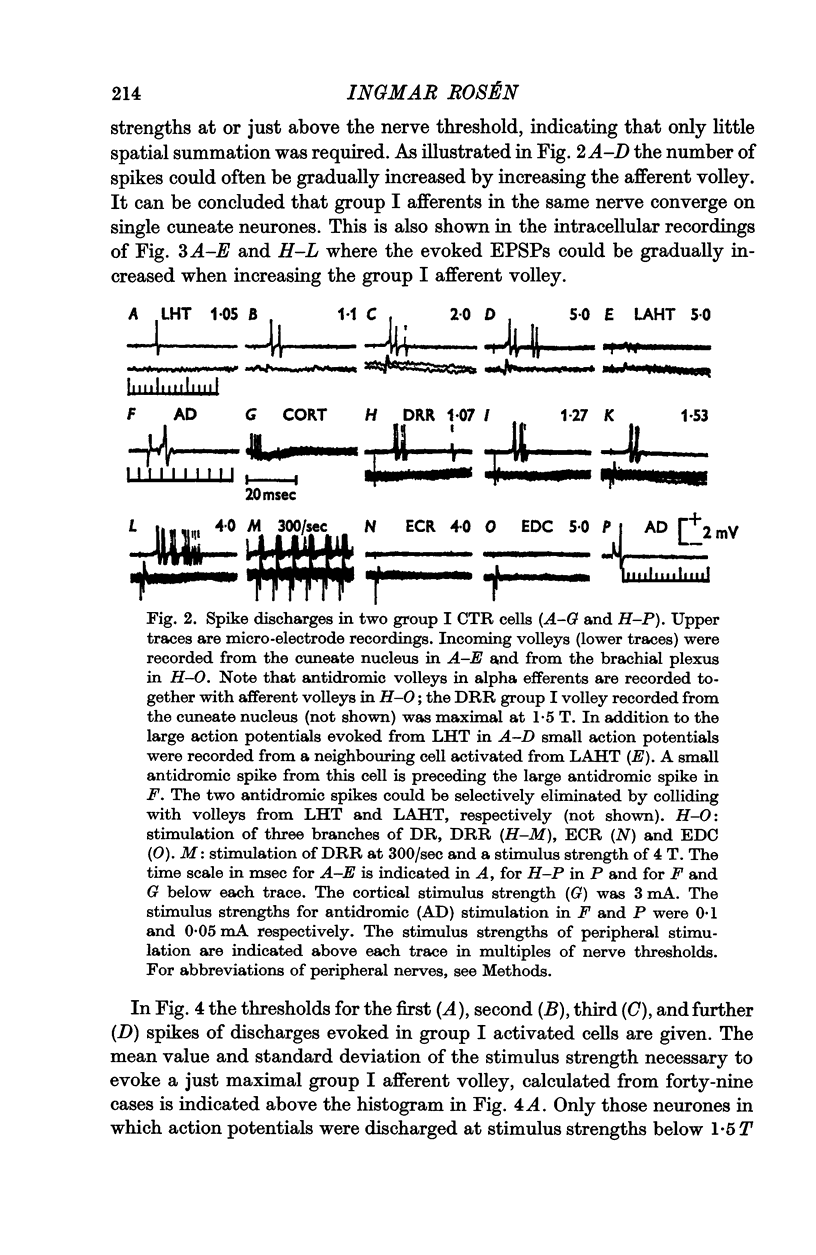
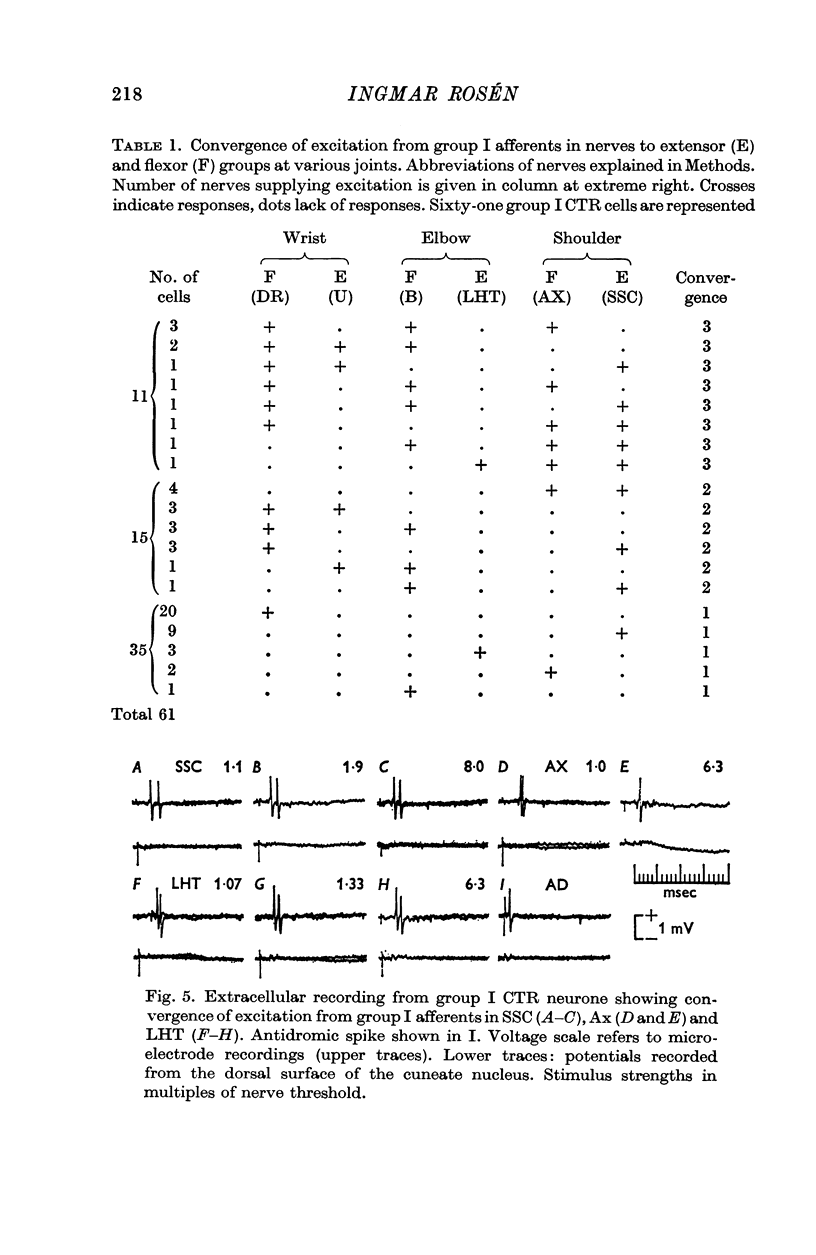
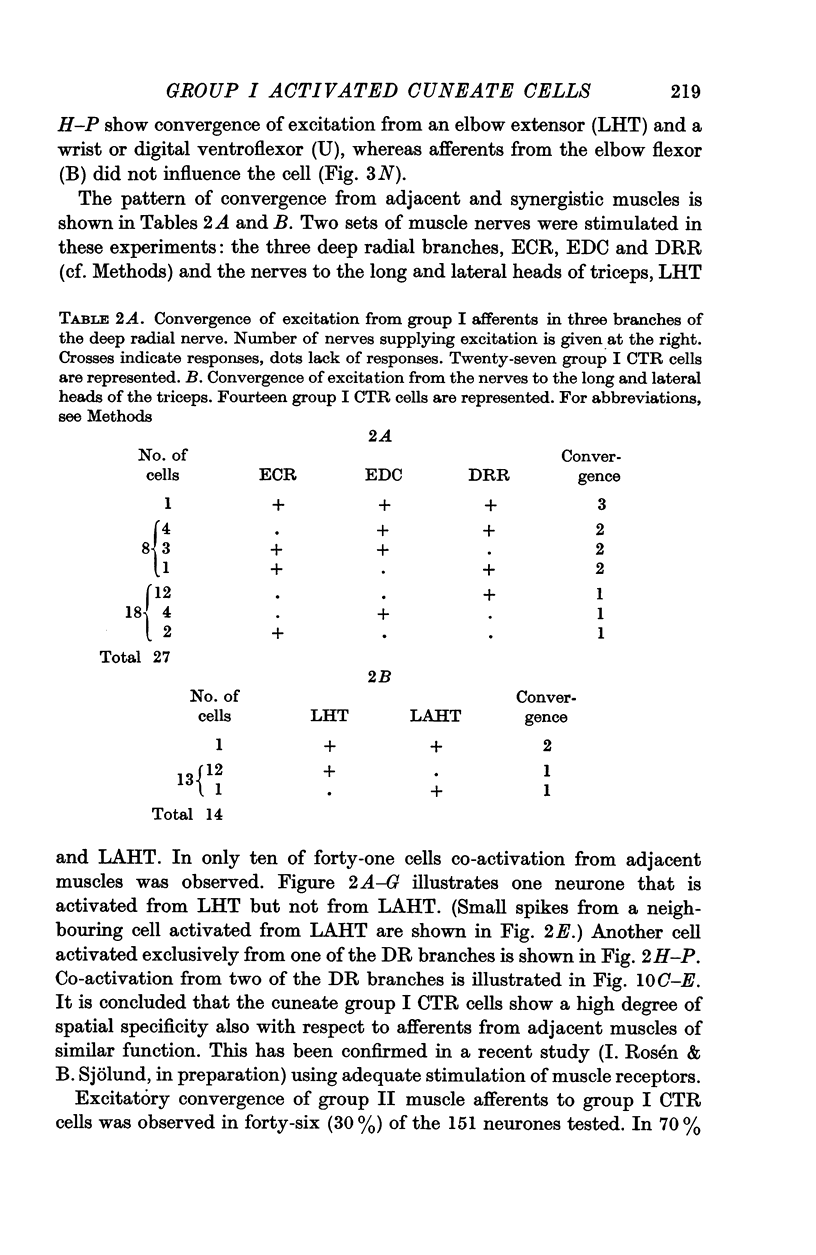
2. A majority of the relay neurones were activated by afferents in only one of six dissected forelimb nerves innervating muscle groups at various joints. Even among afferents from adjacent synergistic muscles, convergence to individual neurones was infrequent.
3. Some of the relay neurones received excitation from group II muscle afferents in the same nerve that provided group I excitation. Excitation from group II muscle afferents in other nerves was uncommon. Some neurones were weakly excited by cutaneous volleys.
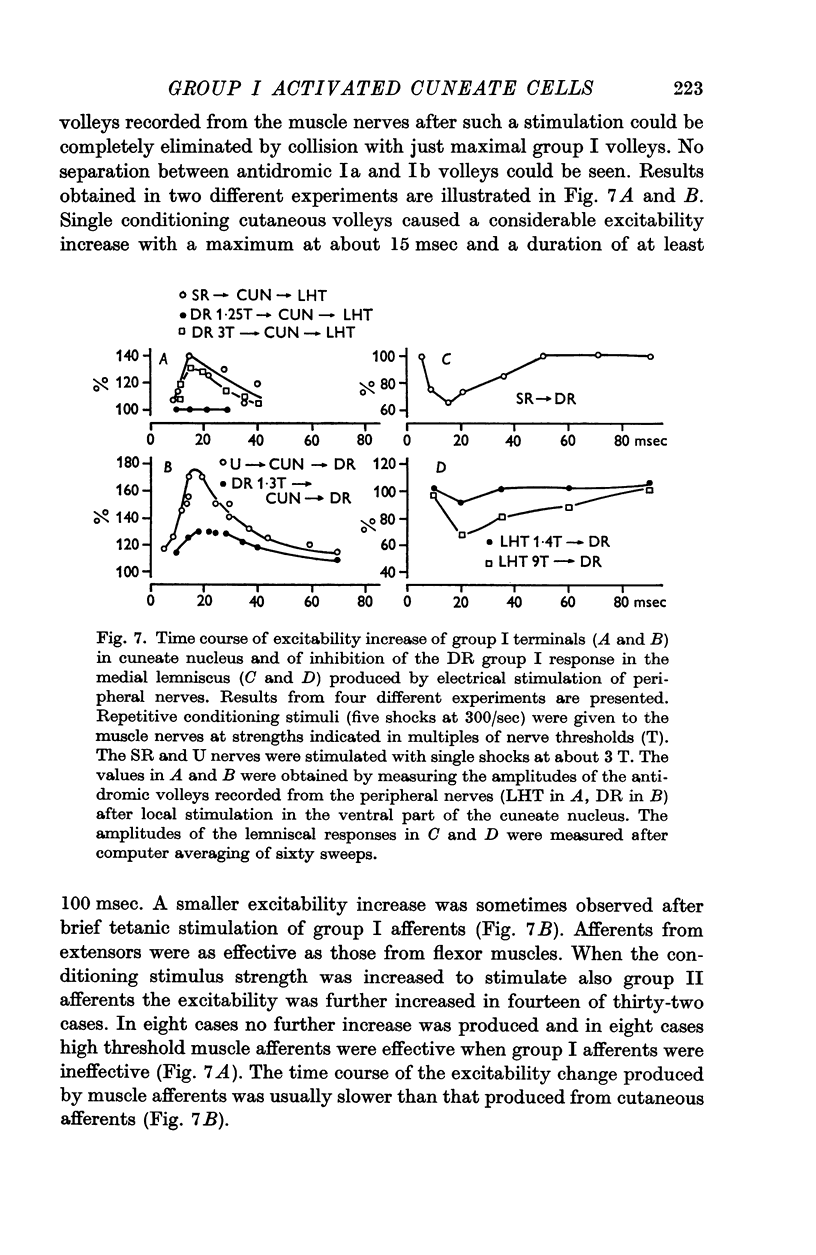
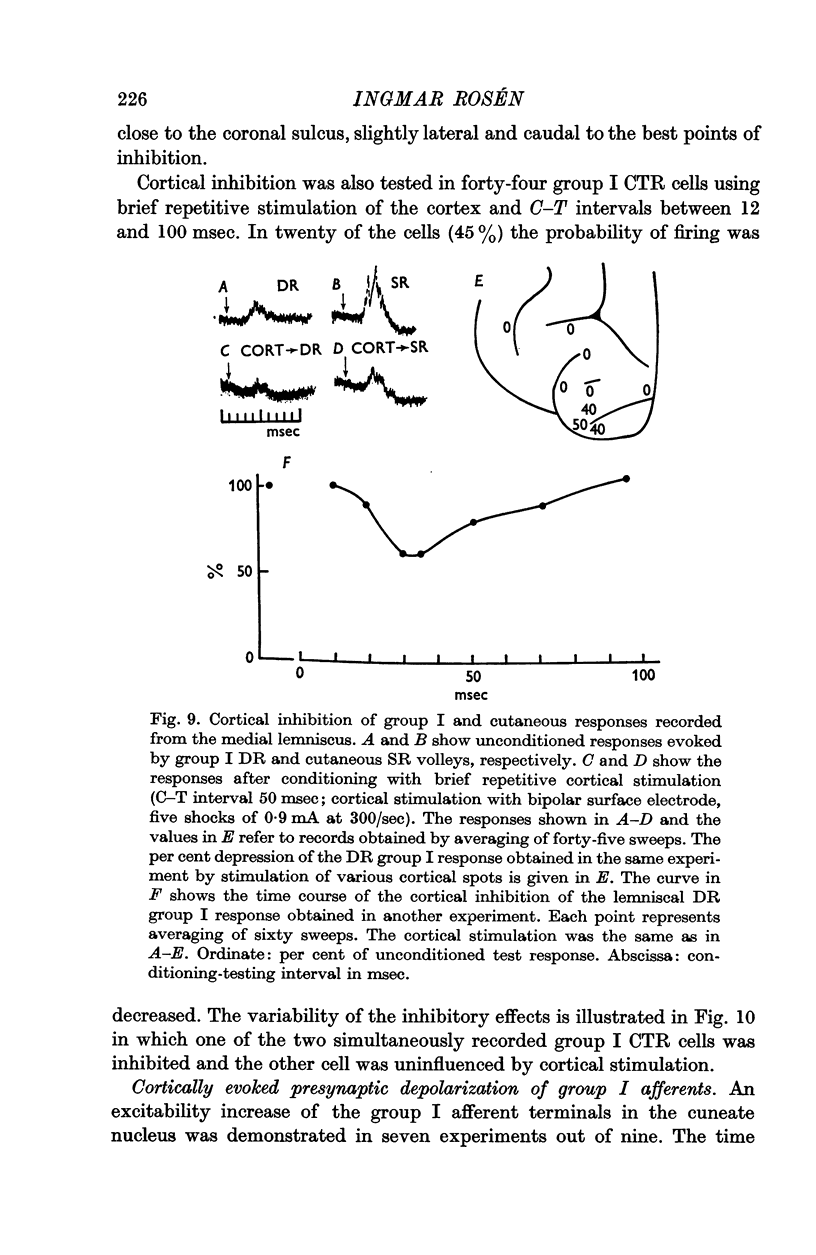
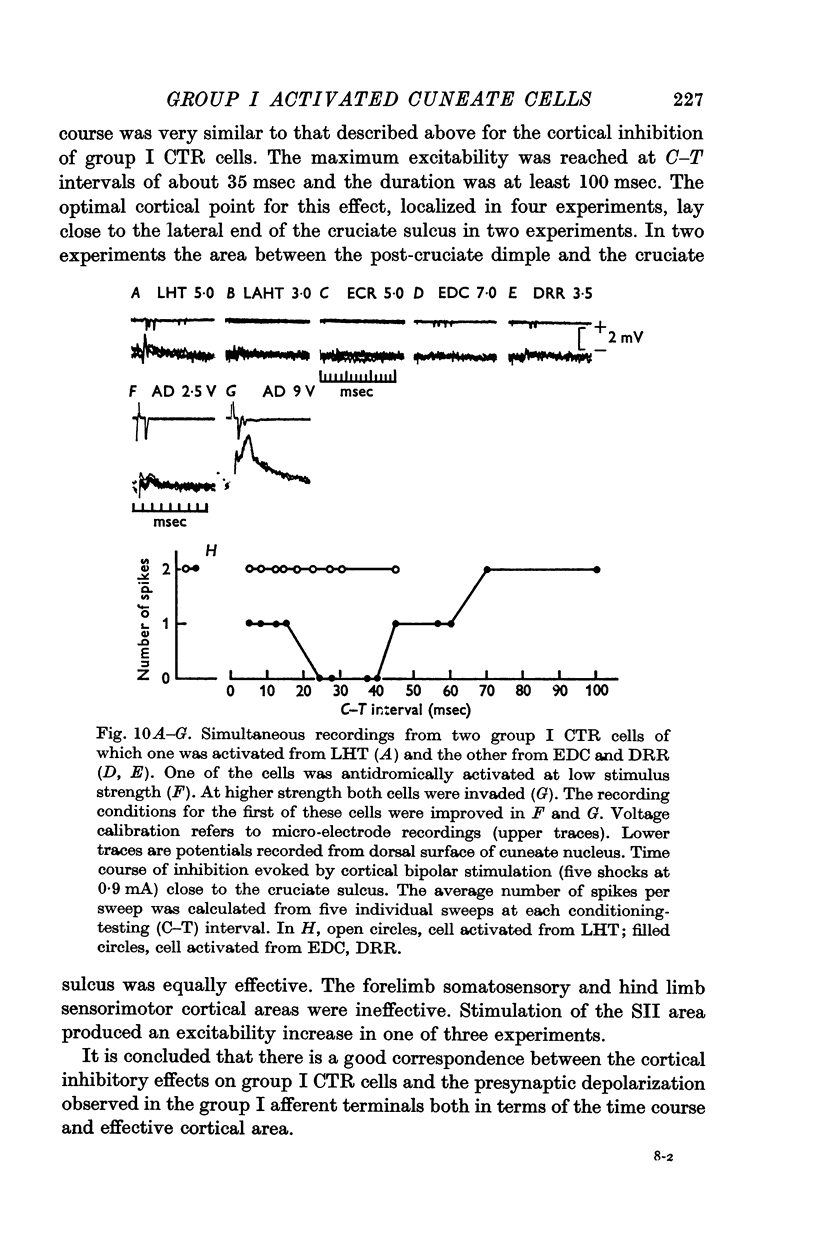
4. Inhibition of group I relay cells was produced from cutaneous afferents and group II muscle afferents. Weak inhibition was sometimes observed from group I afferents. The relay cells were also inhibited by stimulation of the cerebral cortex with a focus around the lateral end of the cruciate sulcus. A good correspondence was found between the inhibition and the depolarization of group I afferent terminals in the cuneate nucleus.
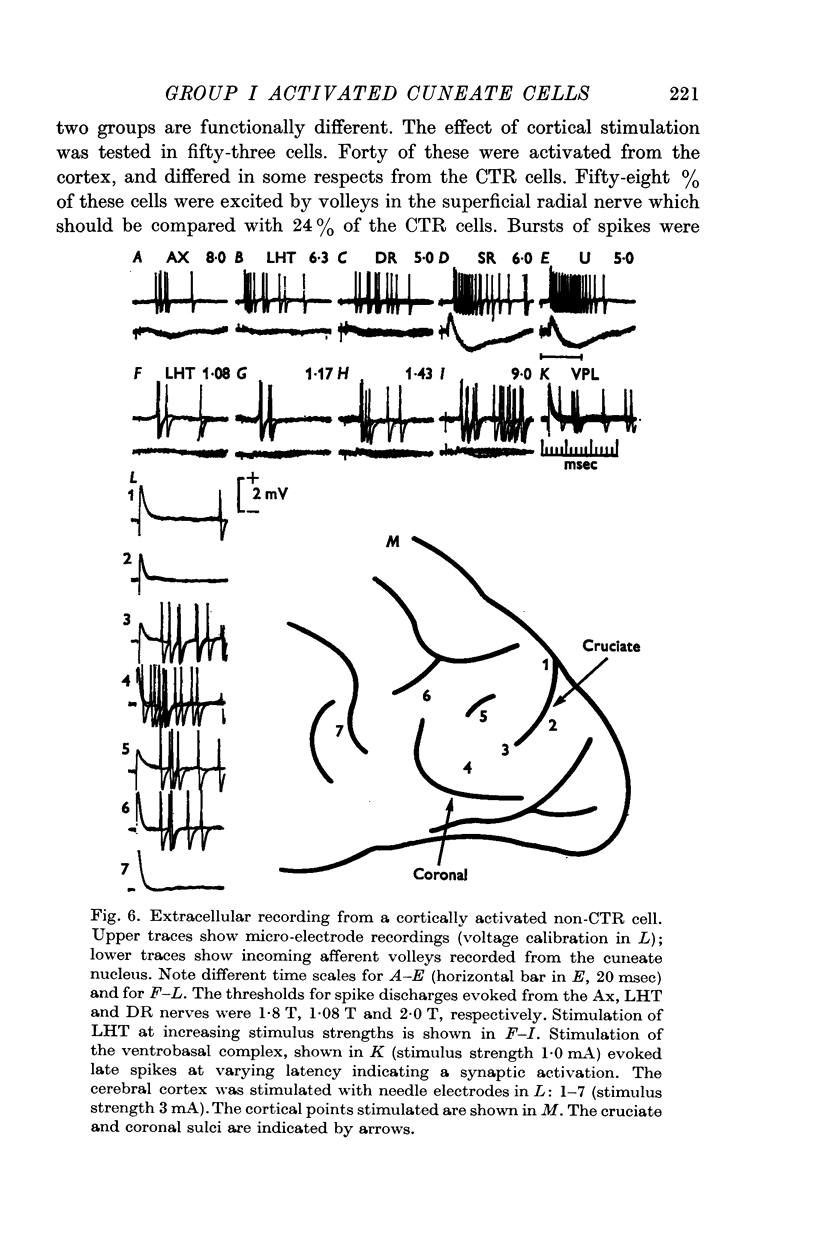
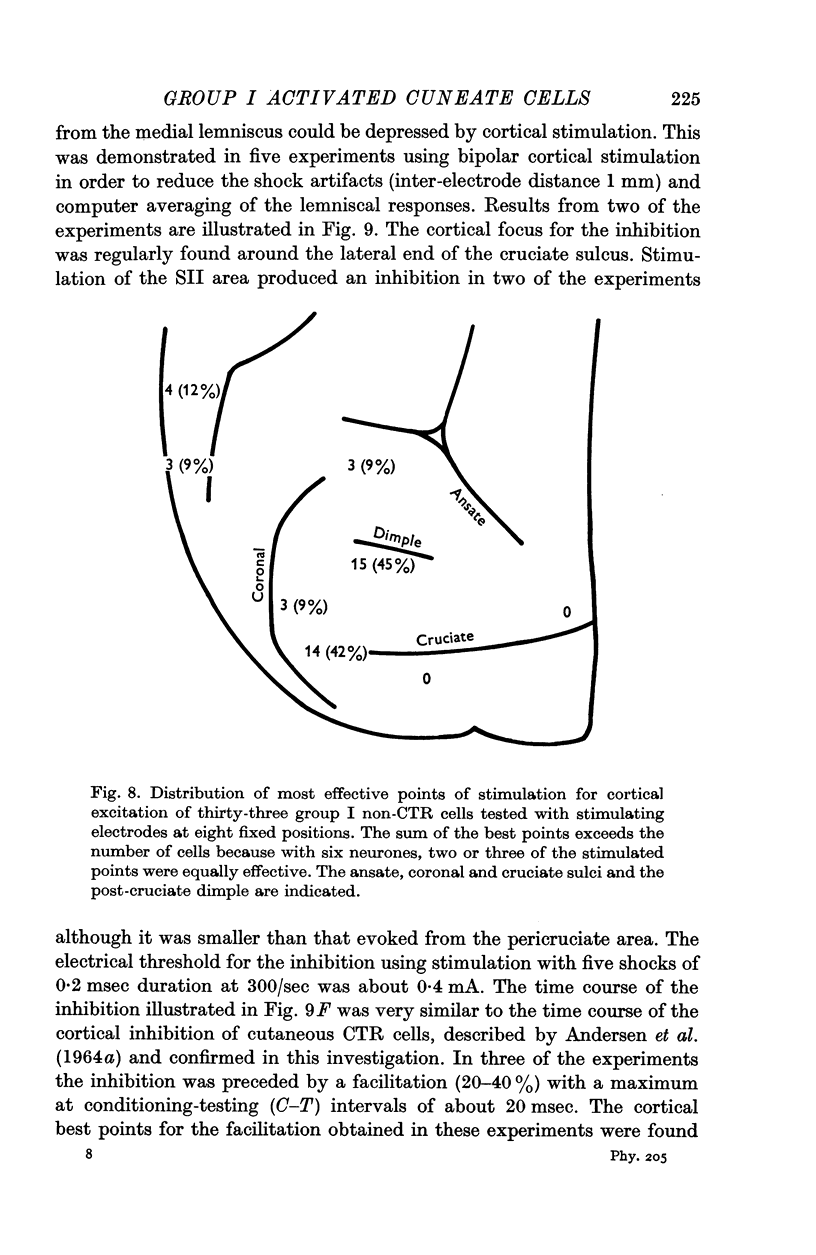
5. A majority of the group I activated cells not antidromically activated from the ventrobasal complex (`non-relay cells') were excited by cortical stimulation. Excitation from cutaneous afferents and group II muscle afferents was frequently found among these cells.
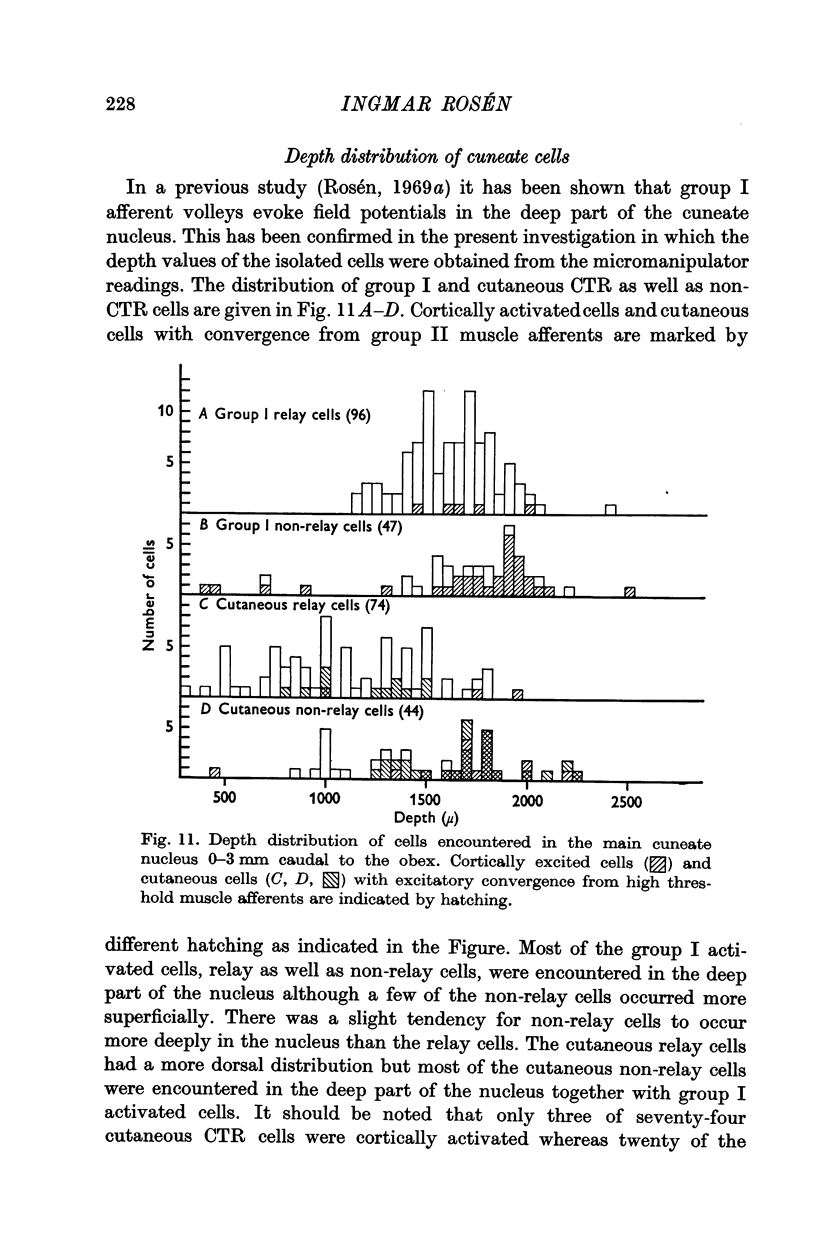
6. The group I activated cells were found almost exclusively in the ventral part of the nucleus.
7. The pattern of convergence found in eleven group I activated cells in the dorsal horn of the spinal cord from C 2 to C 4 is described.
Full text
PDF

















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., OSHIMA T., SCHMIDT R. F. MECHANISMS OF SYNAPTIC TRANSMISSION IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Nov;27:1096–1116. doi: 10.1152/jn.1964.27.6.1096. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., ECCLES J. C., SCHMIDT R. F., YOKOTA T. DEPOLARIZATION OF PRESYNAPTIC FIBERS IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Jan;27:92–106. doi: 10.1152/jn.1964.27.1.92. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., ECCLES J. C., SCHMIDT R. F., YOKOTA T. SLOW POTENTIAL WAVES PRODUCED IN THE CUNEATE NUCLEUS BY CUTANEOUS VOLLEYS AND BY CORTICAL STIMULATION. J Neurophysiol. 1964 Jan;27:78–91. doi: 10.1152/jn.1964.27.1.78. [DOI] [PubMed] [Google Scholar]
- Andersson S. A., Landgren S., Wolsk D. The thalamic relay and cortical projection of group I muscle afferents from the forelimb of the cat. J Physiol. 1966 Apr;183(3):576–591. doi: 10.1113/jphysiol.1966.sp007885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMBERS W. W., LIU C. N., McCOUCH G. P. Inhibition of the dorsal column nuclei. Exp Neurol. 1963 Jan;7:13–23. doi: 10.1016/0014-4886(63)90090-6. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C. Synaptic action during and after repetitive stimulation. J Physiol. 1960 Feb;150:374–398. doi: 10.1113/jphysiol.1960.sp006393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carli G., Diete-Spiff K., Pompeiano O. Presynaptic and postsynaptic inhibition of transmission of somatic afferent volleys through the cuneate nucleus during sleep. Arch Ital Biol. 1967 Mar;105(1):52–82. [PubMed] [Google Scholar]
- Carli G., Diete-Spiff K., Pompeiano O. Vestibular influences during sleep. V. Vestibular control on somatic afferent transmission in the cuneate nucleus during desynchronized sleep. Arch Ital Biol. 1967 Mar;105(1):83–103. [PubMed] [Google Scholar]
- DARIAN-SMITH I., PHILLIPS G., RYAN R. D. FUNCTIONAL ORGANIZATION IN THE TRIGEMINAL MAIN SENSORY AND ROSTRAL SPINAL NUCLEI OF THE CAT. J Physiol. 1963 Aug;168:129–146. doi: 10.1113/jphysiol.1963.sp007182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON G. D., PODACHIN V. P., SCHATZ S. W. Facilitation of cortical responses by competing stimuli. J Physiol. 1963 Apr;166:363–381. doi: 10.1113/jphysiol.1963.sp007109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darian-Smith I., Yokota T. Corticofugal effects on different neuron types within the cat's brain stem activated by tactile stimulation of the face. J Neurophysiol. 1966 Mar;29(2):185–206. doi: 10.1152/jn.1966.29.2.185. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., OSCARSSON O., WILLIS W. D. Synaptic action of group I and II afferent fibres of muscle on the cells of the dorsal spinocerebellar tract. J Physiol. 1961 Oct;158:517–543. doi: 10.1113/jphysiol.1961.sp006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol. 1958 Dec 4;144(2):271–298. doi: 10.1113/jphysiol.1958.sp006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIAQUINTO S., POMPEIANO O., SWETT J. E. EEG AND BEHAVIORAL EFFECTS OF FORE-AND HINDLIMB MUSCULAR AFFERENT VOLLEYS IN UNRESTRAINED CATS. Arch Ital Biol. 1963 Apr 2;101:133–148. [PubMed] [Google Scholar]
- GORDON G., JUKES M. G. DESCENDING INFLUENCES ON THE EXTEROCEPTIVE ORGANIZATIONS OF THE CAT'S GRACILE NUCLEUS. J Physiol. 1964 Sep;173:291–319. doi: 10.1113/jphysiol.1964.sp007457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUZMAN-FLORES C., BUENDIA N., ANDERSON C., LINDSLEY D. B. Cortical and reticular influences upon evoked responses in dorsal column nuclei. Exp Neurol. 1962 Jan;5:37–46. doi: 10.1016/0014-4886(62)90068-7. [DOI] [PubMed] [Google Scholar]
- Gelfan S., Carter S. Muscle sense in man. Exp Neurol. 1967 Aug;18(4):469–473. doi: 10.1016/0014-4886(67)90064-7. [DOI] [PubMed] [Google Scholar]
- Gordon G., Miller R. Identification of cortical cells projecting to the dorsal column nuclei of the cat. Q J Exp Physiol Cogn Med Sci. 1969 Jan;54(1):85–98. doi: 10.1113/expphysiol.1969.sp002009. [DOI] [PubMed] [Google Scholar]
- HASSLER R., MUHS-CLEMENT K. ARCHITEKTONISCHER AUFBAU DES SENSOMOTORISCHEN UND PARIETALEN CORTEX DE KATZE. J Hirnforsch. 1964;7:377–420. [PubMed] [Google Scholar]
- HOLMQVIST B., LUNDBERG A., OSCARSSON O. Functional organization of the dorsal spino-cerebellar tract in the cat. V. Further experiments on convergence of excitatory and inhibitory actions. Acta Physiol Scand. 1956 Dec 29;38(1):76–90. doi: 10.1111/j.1748-1716.1957.tb00174.x. [DOI] [PubMed] [Google Scholar]
- HOLMQVIST B., OSCARSSON O., ROSEN I. Functional organization of the cuneocrebellar tract in the cat. Acta Physiol Scand. 1963 Jun-Jul;58:216–235. doi: 10.1111/j.1748-1716.1963.tb02643.x. [DOI] [PubMed] [Google Scholar]
- Hongo T., Okada Y. Cortically evoked pre- and postsynaptic inhibition of impulse transmission to the dorsal spinocerebellar tract. Exp Brain Res. 1967;3(2):163–177. doi: 10.1007/BF00233260. [DOI] [PubMed] [Google Scholar]
- JABBUR S. J., TOWE A. L. Cortical excitation of neurons in dorsal column nuclei of cat, including an analysis of pathways. J Neurophysiol. 1961 Sep;24:499–509. doi: 10.1152/jn.1961.24.5.499. [DOI] [PubMed] [Google Scholar]
- Jänig W., Schmidt R. F., Zimmermann M. Two specific feedback pathways to the central afferent terminals of phasic and tonic mechanoreceptors. Exp Brain Res. 1968;6(2):116–129. doi: 10.1007/BF00239166. [DOI] [PubMed] [Google Scholar]
- Kuno M., Miyahara J. T. Factors responsible for multiple discharge of neurons in Clarke's column. J Neurophysiol. 1968 Jul;31(4):624–638. doi: 10.1152/jn.1968.31.4.624. [DOI] [PubMed] [Google Scholar]
- LAPORTE Y., LUNDBERG A., OSCARSSON O. Functional organization of the dorsal spino-cerebellar tract in the cat. II. Single fibre recording in Flechsig's fasciculus on electrical stimulation of various peripheral nerves. Acta Physiol Scand. 1956 Mar 24;36(1-2):188–203. doi: 10.1111/j.1748-1716.1956.tb01317.x. [DOI] [PubMed] [Google Scholar]
- LEVITT M., CARRERAS M., LIU C. N., CHAMBERS W. W. PYRAMIDAL AND EXTRAPYRAMIDAL MODULATION OF SOMATOSENSORY ACTIVITY IN GRACILE AND CUNEATE NUCLEI. Arch Ital Biol. 1964 Apr 18;102:197–229. [PubMed] [Google Scholar]
- LIVINGSTON A., PHILLIPS C. G. Maps and thresholds for the sensorimotor cortex of the cat. Q J Exp Physiol Cogn Med Sci. 1957 Apr;42(2):190–205. doi: 10.1113/expphysiol.1957.sp001250. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., OSCARSSON O. Functional organization of the dorsal spinocerebellar tract in the cat. IV. Synaptic connections of afferents from Golgi tendon organs and muscle spindles. Acta Physiol Scand. 1956 Dec 29;38(1):53–75. doi: 10.1111/j.1748-1716.1957.tb00173.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., WINSBURY G. Selective adequate activation of large afferents from muscle spindles and Golgi tendon organs. Acta Physiol Scand. 1960 Jul 15;49:155–164. doi: 10.1111/j.1748-1716.1960.tb01939.x. [DOI] [PubMed] [Google Scholar]
- Landgren S., Silfvenius H., Wolsk D. Somato-sensory paths to the second cortical projection area of the group I muscle afferents. J Physiol. 1967 Aug;191(3):543–559. doi: 10.1113/jphysiol.1967.sp008267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merton P. A. Human position sense and sense of effort. Symp Soc Exp Biol. 1964;18:387–400. [PubMed] [Google Scholar]
- OSCARSSON O. FUNCTIONAL ORGANIZATION OF THE SPINO- AND CUNEOCEREBELLAR TRACTS. Physiol Rev. 1965 Jul;45:495–522. doi: 10.1152/physrev.1965.45.3.495. [DOI] [PubMed] [Google Scholar]
- OSCARSSON O., ROSEN I. PROJECTION TO CEREBRAL CORTEX OF LARGE MUSCLE-SPINDLE AFFERENTS IN FORELIMB NERVES OF THE CAT. J Physiol. 1963 Dec;169:924–945. doi: 10.1113/jphysiol.1963.sp007305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSCARSSON O., UDDENBERG N. PROPERTIES OF AFFERENT CONNECTIONS TO THE ROSTRAL SPINOCEREBELLAR TRACT IN THE CAT. Acta Physiol Scand. 1965 May-Jun;64:143–153. doi: 10.1111/j.1748-1716.1965.tb04163.x. [DOI] [PubMed] [Google Scholar]
- Oscarsson O., Rosén I. Short-latency projections to the cat's cerebral cortex from skin and muscle afferents in the contralateral forelimb. J Physiol. 1966 Jan;182(1):164–184. doi: 10.1113/jphysiol.1966.sp007816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarsson O., Rosén I., Sulg I. Organization of neurones in the cat cerebral cortex that are influenced from group I muscle afferents. J Physiol. 1966 Mar;183(1):189–210. doi: 10.1113/jphysiol.1966.sp007860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATTERFIELD J. H. Effect of sensorimotor cortical stimulation upon cuneate nuclear output through medial lemniscus in cat. J Nerv Ment Dis. 1962 Dec;135:507–512. doi: 10.1097/00005053-196212000-00004. [DOI] [PubMed] [Google Scholar]
- Swett J. E., Bourassa C. M. Comparison of sensory discrimination thresholds with muscle and cutaneous nerve volleys in the cat. J Neurophysiol. 1967 May;30(3):530–545. doi: 10.1152/jn.1967.30.3.530. [DOI] [PubMed] [Google Scholar]
- TOWE A. L., JABBUR S. J. Cortical inhibition of neurons in dorsal column nuclei of cat. J Neurophysiol. 1961 Sep;24:488–498. doi: 10.1152/jn.1961.24.5.488. [DOI] [PubMed] [Google Scholar]
- Uddenberg N. Functional organization of long, second-order afferents in the dorsal funiculus. Exp Brain Res. 1968;4(4):377–382. doi: 10.1007/BF00235702. [DOI] [PubMed] [Google Scholar]
- WALL P. D. Excitability changes in afferent fibre terminations and their relation to slow potentials. J Physiol. 1958 Jun 18;142(1):1–21. doi: 10.1113/jphysiol.1958.sp005997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINTER D. L. N. GRACILIS OF CAT. FUNCTIONAL ORGANIZATION AND CORTICOFUGAL EFFECTS. J Neurophysiol. 1965 Jan;28:48–70. doi: 10.1152/jn.1965.28.1.48. [DOI] [PubMed] [Google Scholar]



