Abstract
The transcriptional activator Rob consists of an N-terminal domain (NTD) of 120 amino acids responsible for DNA binding and promoter activation and a C-terminal domain (CTD) of 169 amino acids of unknown function. Although several thousand molecules of Rob are normally present per Escherichia coli cell, they activate promoters of the rob regulon poorly. We report here that in cells treated with either 2,2"- or 4,4"-dipyridyl (the latter is not a metal chelator), Rob-mediated transcription of various rob regulon promoters was increased substantially. A small, growth-phase-dependent effect of dipyridyl on the rob promoter was observed. However, dipyridyl enhanced Rob's activity even when rob was regulated by a heterologous (lac) promoter showing that the action of dipyridyl is mainly posttranscriptional. Mutants lacking from 30 to 166 of the C-terminal amino acids of Rob had basal levels of activity similar to that of wild-type cells, but dipyridyl treatment did not enhance this activity. Thus, the CTD is not an inhibitor of Rob but is required for activation of Rob by dipyridyl. In contrast to its relatively low activity in vivo, Rob binding to cognate DNA and activation of transcription in vitro is similar to that of MarA, which has a homologous NTD but no CTD. In vitro nuclear magnetic resonance studies demonstrated that 2,2"-dipyridyl binds to Rob but not to the CTD-truncated Rob or to MarA, suggesting that the effect of dipyridyl on Rob is direct. Thus, it appears that Rob can be converted from a low activity state to a high-activity state by a CTD-mediated mechanism in vivo or by purification in vitro.
Rob is an abundant 289-amino-acid protein originally discovered by virtue of its binding to DNA containing the “right side” of the origin of replication (oriC) in Escherichia coli (28). Subsequently, it was found that Rob, which has an N-terminal domain (NTD) of 120 amino acids that is highly homologous to the small transcriptional activators MarA and SoxS, is also a transcriptional activator when overexpressed and has DNA-binding, bending, and promoter specificities in vitro that are similar to those of MarA and SoxS (3, 11, 16). For convenience, the dozen or more promoters activated by these proteins are collectively referred to here as the mar/sox/rob regulon even though there are considerable differences among these activators in their abilities to activate particular promoters (see references 3 and 20 and references therein). In spite of these differences, the overexpression of MarA, SoxS, or Rob confers resistance to multiple antibiotics, superoxides, and organic solvents (1, 2, 3, 23, 35).
While the regulation of marA and soxS is well understood (1, 7), little is known about how rob is regulated. Transcription of a rob::lacZ reporter gene was found to increase severalfold during growth from early log phase to stationary phase, and this was partly dependent on rpoS (12). A similar rpoS dependency was observed for glucose-limited or phosphate-limited growth in which rob::lacZ transcription increased ∼5-fold (12). Western blot analysis indicated ca. 10,000 molecules of Rob (also called CbpB) per log-phase cell (comparable to the 5,000 molecules per cell estimated from protein purification in reference 28) and a higher concentration of Rob in the smaller stationary-phase cell (30). Thus, Rob is a highly abundant DNA-binding protein throughout the growth cycle.
In spite of this, basal levels of Rob do not seem to be effective in stimulating transcription. Null mutants of rob appear to have a normal phenotype under a variety of different growth conditions (12, 28). However, they are somewhat more sensitive to n-hexane than the wild type, presumably because they express lower levels of the mar/sox/rob regulon acrAB-encoded efflux pump than do wild-type cells (35). Recently, small reductions in transcription from the mar/sox/rob regulon promoters inaA, mar, and micF were found in rob null mutants, but other regulon promoters were not affected (20, 34). This indicates that, despite the very high basal amounts of Rob per cell, it is not an effective transcriptional activator. However, when rob is overexpressed from a strong promoter on a multicopy plasmid, many of the regulon genes are activated (3). Furthermore, on a molar basis, purified Rob activates the transcription of many regulon promoters in vitro about as well as MarA and half as well as SoxS (11).
The structure of a Rob:micF cocrystal shows that the DNA-binding NTD of Rob is very similar to that of MarA, whereas the C-terminal domain (CTD) resembles GalT (13, 24). The NTD is sufficient for DNA binding and transcriptional activation, but the CTD function is not known (3). The Rob CTD is also related by amino acid sequence to the CTDs of other AraC-type regulators, Caf1R and AfrR (3). Interestingly, the crystal structures show that Rob binds the DNA by inserting only one recognition helix into the major groove, whereas MarA inserts two helices into adjacent sections of the major groove (13, 24).
To explore the question of whether Rob activity in vivo can be increased, the effects of various chemicals on a regulon reporter inaA::lacZ transcriptional fusion in a mar sox rob+ strain were assayed. 2,2"-Dipyridyl and 4,4"-dipyridyl were found to increase the expression of inaA >6-fold, primarily by a posttranslational enhancement of Rob activity which requires the CTD of Rob.
MATERIALS AND METHODS
Bacterial strains, growth, and assay of β-galactosidase.
All strains were derivatives of Escherichia coli K-12 (Table 1) (see reference 25 for parental strains and genetic methods). Overnight cultures of bacteria, grown in Luria-Bertani (LB) broth (pH 7.5) at 37°C were diluted at least 1,000-fold into fresh medium and cultivated with shaking at 200 rpm until their absorbance at 600 nm (A600) reached ∼0.07. Samples (0.75 ml) were diluted with equal volumes of prewarmed LB broth containing the tester compound and then aerated for 1 h. The cultures were chilled and assayed for β-galactosidase by using the CHCl3-sodium dodecyl sulfate method. The specific activity is expressed in Miller units as described previously (21). To measure the kinetics of inaA activation, 10 ml of log-phase cells was added to 10 ml of prewarmed and aerated LB broth with dipyridyl (Sigma Chemical Co., St. Louis, Mo.) in a 250-ml flask, and 0.75-ml samples were removed at the indicated intervals to 0.75 ml of iced Z buffer (21) and assayed for β-galactosidase. When necessary, the cells were pelleted by centrifugation and resuspended in Z buffer prior to assay. All assays were performed at least twice in duplicate and agreed to within 15%.
TABLE 1.
List of selected strains useda
| Strain, plasmid, or phage | Parent strain | Relevant genotype and/or characteristics | Source or reference |
|---|---|---|---|
| Bacterial strains | |||
| DJ901 | GC4468 | soxS::kan | 25 |
| M542 | GC4468 | λRS45:rob2::lacZ kan | This study |
| M564 | RK5173 | inaA::lacZ | This study |
| M565 | RK12466 | inaA::lacZ | This study |
| M597 | N8461 | pTA108 | This study |
| M598 | N8461 | pTA108:marA | This study |
| M599 | N8461 | pTA108:soxS | This study |
| M600 | N8461 | pTA108:rob | This study |
| M794 | BL21 (λDE3) | pET15b:rob | This study |
| M808 | N8461 | pTA108:robΔ(370-867) | This study |
| M848 | N8461 | pTA108:robΔ(778-867) | This study |
| M851 | N8461 | pTA108:robΔ(643-867) | This study |
| M853 | N8461 | pTA108:robΔ(529-867) | This study |
| M854 | N8461 | pTA108:robΔ(451-867) | This study |
| M871 | N7840 | marΔ sox-8::cat | 32; this study |
| N7840 | GC4468 | marΔ Cams | 25 |
| N7962 | N7840 | inaA::lacZ | 25 |
| N7969 | DJ901 | soxS::kan marΔ inaA::lacZ | 25 |
| N8461 | GC4468 | marΔ sox-8::cat rob::kan | This study |
| N8695 | N7840 | mar::lacZ | 20 |
| N9082 | N7840 | fpr::lacZ | 20 |
| N9083 | N7840 | fumC::lacZ | 20 |
| N9084 | N7840 | micF::lacZ | 20 |
| N9085 | N7840 | nfo::lacZ | 20 |
| N9086 | N7840 | sodA::lacZ | 20 |
| N9087 | N7840 | zwf::lacZ | 20 |
| RK5173 | MC4100 | 9 | |
| RK12466 | MC4100 | tonB::kan | 9 |
| Plasmids and phage | |||
| pRS551 | pBR322 derivative; AmpR | 27 | |
| pTA108 | pSC101 derivative; lac promoter; AmpR | 31 | |
| λRS45 | λimm21; KanR | 27 | |
| P1 cat clr-100 | Used for transductions | 25 |
See Materials and Methods for additional strains used.
Dipyridyl-insensitive rob mutants were selected by spreading 200 μl of an overnight culture of strain M600 on LB plates supplemented with 6 mM 4,4"-dipyridyl and incubating the plates at 37°C for 3 to 5 days. Cells that had lost the plasmid (AmpS) or had mutations in the NTD of Rob made pink colonies, whereas those with mutations in the CTD of Rob made red colonies on MacConkey-lactose plates (Difco, Detroit, Mich.) after overnight incubation at 37°C.
DNA manipulations.
A rob promoter::lacZ transcriptional fusion was constructed by amplifying rob from the chromosome of N7969 by PCR with the primers 813 (5"-CCATTTTTATGAATTCCACGAGCAATTAGTTCGTCACGG-3"; the EcoRI site is underlined) and 814 (CCTTGGATCCAGATTAAAAGGTCGCGAATAATGCCGGCCTGATCC; the BamHI site is underlined), respectively. The 250-bp fragment was digested with EcoRI and BamHI and cloned in similarly cut plasmid pRS551 (27), thereby fusing the 192-bp upstream of the rob initiation codon and the first 38 bp of the coding sequence to lacZ. This transcriptional fusion, called rob2::lacZ, was transferred to λRS45, and single-copy lysogens were isolated (27).
Plasmid pTA108 is an AmpR, low-copy-number plasmid derived from pSC101 and contains the lac operator-promoter region of plasmid pUC8 (31). Derivatives of pTA108 with the lac promoter controlling the marA, soxS, or rob structural genes were constructed as follows. The marA and soxS coding sequences were amplified by PCR from the pRGM9817-based plasmids, pRGM9818 (marA) and pJLR70 (soxS) (20), with primer 864 (GAAGCTTAACTATGCGGCATCAGAGCACGGATCC; the HindIII site is underlined) and either primer 865 (AGGAATTCGATGTCCAGACGCAATACTGACGC; the EcoRI site is underlined) or primer 866 (AGGAATTCCCATCAGAAAATTATTCAGGATCTTATCGCATGG), respectively. These fragments were digested with EcoRI and HindIII and ligated to similarly cut plasmid pTA108. The rob coding sequence was amplified from the pRGM9817:rob plasmid, pRGMM489 (see below), with primers 867 (ACCAATTGTCAGGCCGGCATTATTCGCGACC; the underlined MfeI site precedes the sixth base pair of the rob coding sequence) and primer 864 (see above). The fragment was digested with MfeI and HindIII and ligated to EcoRI- and HindIII-cut plasmid pTA108, resulting in plasmid pRGMM649 (and replacement of the pTA108 EcoRI site GAATTC with GAATTG). Accordingly, transcription and translation from the lac promoter of pRGMM649 results in a modified Rob protein with the first five amino acids derived from lacZ (Met-Ile-Thr-Asn-Cys) substituted for the first two amino acids of Rob (Met-Asp). The lac promoter in the pTA108 plasmids is not repressed in these strains since they have no lacI gene. However, the promoter is not very active in the early logarithmic phase or in cells grown in LB broth supplemented with 0.4% glucose due to the absence of cyclic AMP (data not shown).
pRGMM489 plasmid (pRGM9817:rob) was constructed by amplification of the rob coding sequence from strain N7969 by PCR with primers 821 (AACATATGGATCAGGCCGGCATTATTCGCGACC; the NdeI site [underlined] includes the first ATG codon of rob) and 822 (AAGGATCCTTAACGACGGATCGGAATCAGCAGTTCACAGCG; the BamHI site adjacent to ochre codon is underlined). The fragment was cut with NdeI and BamHI and ligated to similarly cut and phosphatase-treated plasmid pRGM9817. For overexpression and purification of Rob, pRGMM489 was cut with NdeI and BamHI and ligated to similarly cut and phosphatase-treated plasmid pET15b (Novagen, Madison, Wis.) to make plasmid pRGMM794. Strain M794 is strain BL21(λDE3) transformed with this plasmid.
Deletions of the 3" end of rob were constructed by PCR amplification of pRGMM489 with, as the 5" primer in each case, primer 871 (GGCTTTACACTTTATGCTTCCGGCTCG) corresponding to bp 145 to 171 of the pTA108 sequence, which lies 65 bp upstream of the site where rob is inserted in pRGMM489. The 3" primers used to make the CTD-truncated rob plasmids in the N8461-derived strains M854, M853, M851, and M848 were, respectively, primers 983 (TTTAAGCTTACTCCAGCGAACAGGAGTAGCTCTGGG; rob nucleotides [nt] 450 to 427, underlined), 984 (TTTAAGCTTACGGCGGAATGGTCGGCGCGTTGCCGAG; rob nt 528 to 502, underlined), 985 (TTTAAGCTTACCCCGTCAGTACATAGCCATCTGCC; rob nt 642 to 618, underlined), and 986 (TTTAAGCTTACTGACCTTTACGGCGCGTCAGG; rob nt 777 to 756, underlined). The underlined segments correspond to the new positions of the 3" ends of the rob coding sequence with the TTA creating an ochre codon and the AAGCTT creating a HindIII site. The resulting fragments of 518, 596, 710, and 845 bp were cut with EcoRI and HindIII to yield fragments of 86, 164, 278, and 413 bp, respectively, and were ligated to similarly cut and phosphatase-treated plasmid pRGMM489, thereby substituting the truncated fragments for the wild-type rob sequence downstream of the EcoRI site. To make the plasmid present in the N8461 derivative, strain M808, the pTA108:rob plasmid was deleted back from the 3" portion of the rob coding sequence to the EcoRI site at bp 364 and an ochre codon inserted as follows. Plasmid pRGMM489 was treated with EcoRI, HindIII, and phosphatase and ligated to a linker containing an ochre codon made by annealing oligomers 881 (AATTCTAAGGATCCA) and 882 (AGCTTGGATCCTTAG) that had been treated with polynucleotide kinase. DNA oligomers were prepared by using an ABI DNA/RNA Synthesizer, and the sequences were confirmed for all constructs by using an ABI Prism 310 Genetic Analyzer.
Electrophoretic mobility assays were performed as described previously (18). EcoRI-to-BamHI fragments containing the minimal promoters (20) of the fumC (−56 to +6), fpr (−66 to +3), and mar (−72 to +3) promoters were 32P end labeled and used as probes with purified Rob protein at final concentrations of 50, 100, and 200 nM (without or with equal amounts of RNAP) in 50 mM Tris (pH 7.5)-20% glycerol-0.1 M NaCl-1 μg of poly(I-C)/ml with or without 5 mM 2,2"-dipyridyl. The samples were subjected to electrophoresis at 90 V on 6% polyacrylamide gels made up in 0.5× Tris-borate-EDTA with or without 5 mM 2,2"-dipyridyl and in electrolyte of the same composition.
Purification of MarA, Rob, and truncated Rob.
MarA, Rob, and truncated Rob (amino acids 124 to 289 absent) were purified as His-tagged proteins from strains N8224, M794, and M796, respectively, as described previously (10, 19), and the His tags were removed with thrombin. The truncated Rob was purified from strain M796 by using the scheme for MarA since it was highly insoluble like MarA. To construct M796, plasmid pRGMM794 was digested with EcoRI, BamHI, and calf alkaline phosphatase and purified on a Promega PCR column (Madison, Wis.). The digested plasmid was ligated to a fragment consisting of the two kinase-treated oligonucleotides: AATTCTAAG (TTC regenerates the Phe-123 codon and TAA creates the terminator codon) and GATCCTTAG and cloned into the BL21 ompT strain from Stratagene (La Jolla, Calif.).
NMR studies.
Uniformly 15N-labeled proteins were produced by growing cells in M9 minimal medium containing 15N-labeled ammonium chloride as the sole source of nitrogen. Purification of the proteins was carried out as for the unlabeled material. Each of the proteins, MarA, Rob, and the 166-residue CTD-truncated Rob were complexed with a 26-bp double-stranded DNA containing the sequence for the mar-binding site so that they would be soluble at the required concentrations. The complexes were dialyzed against a buffer containing 20 mM sodium phosphate, 20 μM EDTA, and 0.02% sodium azide (pH 6.5) and then concentrated to ∼0.5 mM for the nuclear magnetic resonance (NMR) studies. 2,2"-Dipyridyl was added to each of the samples to a final concentration of 5 mM. 1H-15N HSQC spectra (5) were collected for each of the complexes in the presence or absence of 2,2"-dipyridyl. All NMR data were acquired on Bruker DMX-600 or DMX-500 MHz spectrometers at 35°C. NMR data were processed by using the NMRPipe suite of programs (6).
RESULTS
Both 2,2"- and 4,4"-dipyridyl activate inaA via rob.
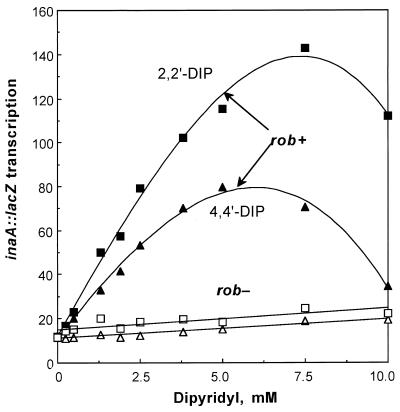
Overexpression of Rob transcriptionally activates a number of genes of the mar/sox/rob regulon including inaA (3). To identify compounds that induce the overexpression or activation of Rob, various compounds were tested for their ability to stimulate β-galactosidase synthesis in a rob+ mar sox strain (M871) bearing an inaA::lacZ transcriptional fusion. The inaA::lacZ fusion was used as a reporter since it has a low basal level of activity but is highly activated in response to Rob (3, 25, 34). Among these compounds, 2,2"-dipyridyl and 4,4"-dipyridyl increased the levels of β-galactosidase by 19- and 13-fold, respectively (Table 2). 2,2"-Dipyridyl is a potent chelator of iron and has been used at 100 to 200 μM to deplete culture media of iron (see, for example, reference 33). However, the optimal activation of inaA was obtained at much higher concentrations than that needed for chelation (Fig. 1). The 4,4"-dipyridyl isomer, which is not a metal chelator, also activated inaA but to a lesser extent (Fig. 1). To determine whether rob is required for the inaA activation, the effects of both 2,2"-dipyridyl and 4,4"-dipyridyl were assayed on strain N8461 (rob::kan mar sox inaA::lacZ), a rob mutant version of M871 (Fig. 1). Neither 2,2"-dipyridyl nor 4,4"-dipyridyl substantially activated inaA in this strain, showing that Rob is required for the effect. Similarly, inaA was not activated by dipyridyl in rob::kan strains carrying wild-type marA and/or soxS (data not shown).
TABLE 2.
Effects of various compounds on inaA::lacZ activity in rob+ strainsa
| Addition | Concn | β-galactosidase activity (Miller units) | Ratio relative to untreated controlb |
|---|---|---|---|
| None (M871)c | 5.0 | 1.0 | |
| Biphenyl + ethanol | 194 μM | 10.6 | 2.1 |
| 3% | |||
| 2,2"-Dipyridyl | 5 mM | 97.7 | 19.4 |
| 4,4"-Dipyridyl | 5 mM | 63.8 | 12.7 |
| 2,4-Dinitrophenol | 0.5 mM | 15.1 | 3.0 |
| 2,4-Dinitrophenol | 1.0 mM | 26.4 | 5.3 |
| Ethanol | 3% | 6.1 | 1.2 |
| Ethanol | 25% | 10.1 | 2.0 |
| 8-Hydroxyquinoline + ethanol | 5 mM | 7.0 | 1.4 |
| 25% | |||
| Naphthalene + ethanol | 0.5 mM | 11.9 | 2.4 |
| 0.5% | |||
| Paraquat | 50 μM | 10.0 | 2.0 |
| Pyridine | 5 mM | 5.6 | 1.1 |
| Pyridine | 10 mM | 6.1 | 1.2 |
| Pyridoxal | 5 mM | 9.9 | 2.0 |
| Pyridoxine | 5 mM | 6.8 | 1.3 |
| Na salicylate | 5 mM | 13.4 | 2.7 |
| None (N7969)d | 10.8 | 1.0 | |
| Desferroxamine | 5 mM | 14.9 | 1.4 |
| 2,2"-Dipyridyl | 5 mM | 84.0 | 7.8 |
| EDDAf | 5 mM | 10.6 | 1.0 |
| EGTA | 5 mM | 13.3 | 1.2 |
| Na salicylate | 10 mM | 23.9 | 2.2 |
| Na salicylate | 20 mM | 42.5 | 3.9 |
| Na salicylate | 40 mM | 60.2 | 5.6 |
| None (N7969)d | 6.0 | 1.0 | |
| CCCP | 10 μM | 5.2 | 0.9 |
| CCCP | 50 μM | 6.7 | 1.1 |
| 2,4-Dinitrophenol | 0.5 mM | 17.1 | 2.8 |
| 2,4-Dinitrophenol | 1.0 mM | 22.0 | 3.7 |
| EDTA | 0.1 mM | 6.3 | 1.1 |
| EDTA | 1.0 mM | 8.9 | 1.5 |
| EDTA | 10 mM | 2.6 | 0.4 |
| Ferric citrate | 7 mM | 8.6 | 1.4 |
| NaN3 | 1.0 mM | 6.4 | 1.1 |
| NaN3 | 10 mM | 6.0 | 1.0 |
| NaN3 | 100 mM | 5.1 | 0.8 |
| Na citrate | 7 mM | 6.8 | 1.1 |
| NaF | 1.0 mM | 6.1 | 1.0 |
| NaF | 10 mM | 6.5 | 1.1 |
| NaF | 100 mM | 6.9 | 1.1 |
| None (M564)e | 33.0 | 1.0 | |
| 2,2"-Dipyridyl | 5 mM | 151.9 | 4.6 |
| FeSO4 | Saturated | 32.0 | 1.0 |
| 8-Hydroxyquinoline + ethanol | 5 mM | 13.9 | 0.4 |
| 25% | |||
| Nonidet P-40 | 0.5% | 23.0 | 0.7 |
| Pyridine | 5 mM | 36.3 | 1.1 |
| Na acetate | 50 mM | 19.2 | 0.6 |
| None (M565)e | 35.6 | 1.0 | |
| 2,2"-Dipyridyl | 5 mM | 156.6 | 4.4 |
| FeSO4 | Saturated | 28.4 | 0.8 |
| 8-Hydroxyquinoline + ethanol | 5 mM | 12.6 | 0.4 |
| 25% | |||
| Nonidet P-40 | 0.5% | 17.1 | 0.5 |
| Pyridine | 5 mM | 32.9 | 0.9 |
| Na acetate | 50 mM | 19.5 | 0.5 |
The indicated log-phase inaA::lacZ cells were incubated with the indicated compounds in LB broth for 1 h at 37°C and assayed for β-galactosidase activity.
Ratios of ≥3.0 are indicated in boldface.
The mar sox strain M871 was used.
The mar sox strain N7969 was used.
Strains M564 and M565 (mar+ sox+) have higher basal levels of inaA::lacZ expression due to mar expression (25, 34). In addition, M565 has a tonB::kan mutation.
EDDA, ethylenediamine-N,N"-diacetic acid.
FIG. 1.
Effects of different concentrations of dipyridyl on transcriptional activation of inaA::lacZ. The mar sox inaA::lacZ strain M871 (solid symbols) and its rob mutant derivative N8461 (open symbols) were treated with the indicated concentrations of 2,2"-dipyridyl (squares) or 4,4"-dipyridyl (triangles) for 1 h and assayed for β-galactosidase (Miller units).
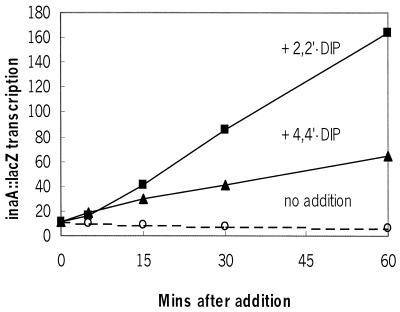
The effects of both dipyridyl isomers were fairly rapid (Fig. 2). Within 10 min of addition of 5 mM dipyridyl, inaA::lacZ activity was elevated and increased linearly for at least 60 min. This increase occurred even though growth was inhibited. The cellular A600 value showed that the growth of log-phase cultures treated with 5 mM 2,2"-dipyridyl was severely inhibited: growth slowed within 20 min and increased by <4-fold after overnight incubation. The growth of the 4,4"-dipyridyl-treated cells was less affected: it also slowed within 20 min but increased 20-fold after overnight incubation. Untreated cells grew with a 30-min doubling time and increased 35-fold overnight. rob mutants were similarly inhibited by 2,2"- and 4,4"-dipyridyl. Thus, in addition to their effects on Rob activity, both 2,2"- and 4,4"-dipyridyl have rob-independent inhibitory effects on growth.
FIG. 2.
Kinetics of acccumulation of inaA::lacZ upon treatment of cells with 0 (○) or 5 mM 2,2"-dipyridyl (▪) or 4,4"-dipyridyl (▴). Samples of the mar sox inaA::lacZ M871 cells treated for the indicated times were rapidly chilled on ice and then assayed for β-galactosidase (Miller units).
Metal chelation does not activate inaA.
Since 2,2"-dipyridyl is a powerful chelator of Fe, the possibility was explored that part of the Rob-mediated activation of inaA might be due to removal of Fe from the culture. Accordingly, various rob+ inaA::lacZ strains were treated with other chelators, including citrate, EDTA, EDDA, EGTA, and 8-hydroxyquinoline. None were effective in activating inaA transcription over the range of concentrations tested (Table 2). Other compounds with some structural resemblance to dipyridyl, such as pyridine, biphenyl, naphthalene, pyridoxal, and pyridoxine had little or no effect on inaA expression (Table 2). Inhibitors of cellular energy generation, such as CCCP (carbonyl cyanide m-chlorophenylhydrazone), sodium azide, and sodium flouride, were also ineffective, as were a number of other compounds tested. Interestingly, 2,4-dinitrophenol and high concentrations of sodium salicylate, both of which derepress the mar operon (4), were somewhat effective in activating inaA::lacZ in Δmar strains M871 and N7969. mar-Independent effects of salicylate have been noted previously (4, 25) and are not further explored here.
TonB is an important component of the Fe uptake systems (for a review, see reference 22). Strain M565, a mar sox rob inaA::lacZ strain that is defective in Fe uptake because of a tonB::kan null mutation (15), and its tonB+ parent, strain M564, were treated with dipyridyl. No significant effect of the tonB mutation was seen (Table 2). Furthermore, neither mutations in fnr, arcA, or fur nor the anaerobic growth of strain N7969 significantly affected the activation of inaA::lacZ by 2,2"-dipyridyl (data not shown). Finally, the addition of various divalent metals to the cultures did not affect inaA transcription (data not shown). Thus, metal chelation is not a relevant aspect of the activation of inaA by dipyridyl.
The 2,2"-dipyridyl-iron complex does not activate inaA.
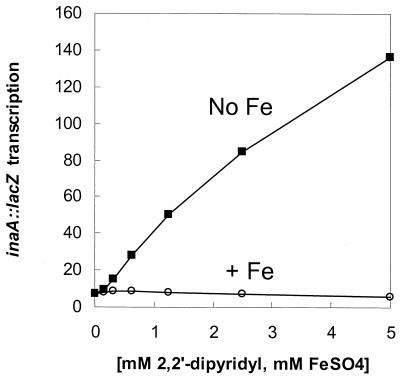
Nevertheless, since 2,2"-dipyridyl (but not 4,4"-dipyridyl) forms coordination complexes with iron, we asked whether the presence of iron would affect the activity of 2,2"-dipyridyl. Interestingly, the addition of equimolar FeSO4 to 2,2"-dipyridyl completely abolished the activation of inaA (Fig. 3). This suggests that, by binding iron, the configuration of 2,2"-dipyridyl is so altered that it is rendered inactive or unable to gain entrance to its cellular target.
FIG. 3.
Effect of Fe on activation of inaA::lacZ by 2,2"-dipyridyl. The mar sox rob+ inaA::lacZ strain N7969 was treated with the indicated concentrations of 2,2"-dipyridyl (▪) or 2,2"-dipyridyl plus equimolar FeSO4 (○) for 1 h in LB broth and assayed for β-galactosidase (Miller units).
To test whether 2,2"- and 4,4"-dipyridyl activate Rob by independent mechanisms, we investigated the effects of the combined isomers on inaA transcription. A synergistic effect would suggest that the isomers have independent modes of action. Strain N7969 (inaA::lacZ rob+) was treated with either a 2.5 mM concentration of both isomers or with a 2.5 mM concentration of only one of the isomers. The β-galactosidase activity in the culture treated with a 2.5 mM concentration of both isomers (97 Miller units) was approximately equal to the total of the separately treated cultures (93 Miller units). This absence of synergy suggests that the two isomers activate Rob via the same mechanism.
Activation of other mar/sox/rob regulon promoters.
If the effect of dipyridyl is to increase the expression or activity of Rob, it should also activate other promoters of the mar/sox/rob regulon, just as the overexpression of Rob on a multicopy plasmid does. This was tested by using appropriate regulon promoter-lacZ transcriptional fusions (Table 3). Both 2,2"- and 4,4"-dipyridyl were found to increase the expression of the different regulon promoters, albeit to different extents. The effects were substantial for the fumC, inaA, and micF promoters (8- to 19-fold increases), moderate for mar and fpr, and modest (2,2"-dipyridyl) or insignificant (4,4"-dipyridyl) for nfo, sodA, and zwf. A similar profile of promoter-specific activation by Rob has been seen when rob is overexpressed from a plasmid (in the absence of dipyridyl) (3, 20).
TABLE 3.
Activation of rob regulon promoters by treatment with dipyridyla
| Strain (promoter-lacZ fusion) | β-Galactosidase activity (Miller units) with:
|
Ratiob | β-Galactosidase activity (Miller units) with 4,4"-dipyridyl | Ratiob | |
|---|---|---|---|---|---|
| No added dipyridyl | 5 mM 2,2"-dipyridyl | ||||
| N9082 (fpr) | 32 | 159 | 4.9 | 69 | 2.2 |
| N9083 (fumC) | 22 | 417 | 18.8 | 248 | 11.2 |
| N7962 (inaA) | 9.8 | 125 | 12.7 | 83 | 8.5 |
| N8695 (mar) | 344 | 1,117 | 3.2 | 912 | 2.7 |
| N9084 (micF) | 39 | 293 | 7.5 | 337 | 8.6 |
| N9085 (nfo) | 107 | 232 | 2.2 | 139 | 1.3 |
| N9086 (sodA) | 518 | 1,378 | 2.7 | 705 | 1.4 |
| N9087 (zwf) | 103 | 229 | 2.2 | 129 | 1.2 |
Derivatives of strain N7840 (Δmar) with the indicated single-copy promoter-lacZ transcriptional fusions were treated with 5 mM 2,2"- or 4,4"-dipyridyl for 1 h at 37°C and assayed for β-galactosidase.
That is, the ratio of β-galactosidase activities in the cells treated with dipyridyl to that in the untreated cells.
Enhancement of Rob activity by a posttranscriptional mechanism.
To determine whether the transcription of rob itself is activated by 2,2"-dipyridyl, a strain with a rob2::lacZ transcriptional fusion was tested. As found previously (12), growth into the stationary phase increased the transcription of rob ∼3-fold (Table 4). Treatment with 2,2"-dipyridyl in the early log phase reproducibly induced rob transcription by 1.5- to 2-fold, but this effect disappeared as cells entered the late log phase. In contrast, the basal level of inaA transcription was not affected significantly by growth phase but was increased by treatment with 2,2"-dipyridyl even in stationary phase. This suggested that 2,2"-dipyridyl enhances Rob activity primarily by a nontranscriptional mechanism.
TABLE 4.
2,2"-Dipyridyl affects the transcription of inaA and rob promoters differently depending on the growth phase of the cellsa
| Strain (promoter-lacZ fusion) | 2,2"-Dipyridyl concn (mM) or ratiob | β-Galactosidase activity (Miller units) at various growth phases
|
||
|---|---|---|---|---|
| Early log | Mid log | Stationary | ||
| M542 (rob) | 0 | 240 | 469 | 732 |
| 5 | 396 | 507 | 741 | |
| Ratio | 1.7 | 1.1 | 1.0 | |
| M871 (inaA) | 0 | 16 | 16 | 20 |
| 5 | 89 | 131 | 119 | |
| Ratio | 5.6 | 8.2 | 6.0 | |
Cells were grown from small inoculae in LB broth to an A600 of 0.07 (early log phase) or 0.5 (mid-log phase) or overnight to an A600 of 2.5 (stationary phase). The samples were then diluted with an equal volume of prewarmed 10 mM 2,2"-dipyridyl in LB broth. After aeration for 1 h at 37°C, the β-galactosidase activity was assayed.
That is, the ratio of activities in the cells treated with dipyridyl to that in the untreated cells.
To test this possibility, the marA, soxS, and rob coding sequences were inserted downstream of the lac promoter in the low-copy-number plasmid pTA108 (31) so that lac and not rob provided the promoter and translational signals. These plasmids were introduced into the rob sox mar mutant strain N8461, and the effect of 2,2"-dipyridyl on inaA transcription was monitored (Table 5). In the absence of 2,2"-dipyridyl, the basal levels of inaA found in the low-copy marA, soxS, or rob plasmid-bearing strains were three- to fourfold greater than in the control strain M597. In the presence of 2,2"-dipyridyl, inaA expression was further increased 18-fold when the plasmid carried rob but not when the plasmid carried marA or soxS. A similar effect was found when these strains were treated with 4,4"-dipyridyl (data not shown). Thus, the effect of dipyridyl is specific for rob and occurs primarily at a posttranscriptional level.
TABLE 5.
Effects of 2,2"-dipyridyl on Rob-mediated activation of inaA::lacZ when Rob is expressed from the heterologous lac promotera
| Strain | Activator controlled by the lac promoter | β-Galactosidase activity (Miller units) with 2,2"-dipyridyl at:
|
Ratiob | |
|---|---|---|---|---|
| 0 mM | 5 mM | |||
| M597 | None | 10 | 16 | 1.6 |
| M598 | MarA | 45 | 23 | 0.51 |
| M599 | SoxS | 30 | 32 | 1.1 |
| M600 | Rob | 33 | 587 | 18.0 |
The mar sox rob mutant strains carrying the indicated plasmids were grown to early log phase, treated with 0 or 5 mM 2,2"-dipyridyl for 1 h at 37°C, and assayed for β-galactosidase activity.
That is, the ratio of activities in the cells treated with 2,2"-dipyridyl to that in the untreated cells.
Isolation of Rob CTD mutants that do not respond to dipyridyl treatment.
Overexpression of marA, soxS, or rob can be deleterious to the growth of the cell (3; R. G. Martin and J. L. Rosner, unpublished data). We observed that the growth of the pTA108:rob strain (M600) was severely inhibited when streaked on LB agar plates containing 6 mM 4,4"-dipyridyl compared to the strain containing only the pTA108 vector (M597) or the single-copy rob chromosomal gene (M871). We reasoned that rob mutants that were not activated by 4,4"-dipyridyl should survive these conditions. Indeed, faster-growing spontaneous mutants were readily selected on such plates after several days of incubation at 37°C. When purified, three types of dipyridyl-insensitive clones were found: 19 of 37 had lost the pTA108:rob plasmid and made pink colonies on MacConkey-lactose plates; 8 of 37 retained the plasmid and made pink colonies on MacConkey-lactose plates but expressed inaA::lacZ at the very low basal levels typical of strains containing no functional rob gene (β-galactosidase activity of inaA::lacZ of <10 Miller units); and 10 of 37 retained the plasmid, made red colonies on MacConkey-lactose plates, and showed slightly higher levels of inaA expression (10 to 40 Miller units) but showed no increase in β-galactosidase after treatment with 2,2"-dipyridyl or 4,4"-dipyridyl (ratio of Miller units of treated to untreated cultures of 1.3 ± 0.3). The rob genes in three isolates from the latter group were sequenced. Two were found to have the 6-kb transposon Tn1000 (γδ) (17) inserted in the portion of rob that encodes the C-terminal domain: in one (rob-9) between the rob structural gene from nt 590 to 591 and in the other (rob-28) between nt 607 to 608. The third mutant (rob-13) contained an in-frame deletion of the rob structural gene from nt 560 to 772 and therefore encodes a protein deleted of 71 amino acids within the CTD. Two 1-bp mutants were later identified that have Rob activity but did not respond to dipyridyl: C658T and C709T, which change the Gln-220 and Gln-237 codons, respectively, to UAG amber codons. While we do not have direct evidence that the rob mRNA from these amber mutants is normal, it seems unlikely that it is the rob mRNA that is responding to the dipyridyl treatment. We tentatively conclude that dipyridyl has a posttranslational effect on the Rob CTD.
The role of the CTD was further examined by deletion analysis. The 3" end of the rob coding sequence (bp 867) was deleted back to bp 777, 642, 528, 450, or 369, and a TAA ochre codon was added, thereby creating a new 3" terminus of translation. These constructs were placed downstream of the lac promoter of pTA108 (as in the original pTA108:rob plasmid), and the resulting plasmids transformed into the rob sox mar inaA::lacZ strain N8461. The 3" deletions of rob (encoding proteins with C-terminal truncations of 30, 75, 113, 139, and 166 amino acids, respectively) did not significantly lower inaA::lacZ activity in the absence of dipyridyl compared to strain M600, whose plasmid carries wild-type rob (Table 6). Thus, the full-length Rob CTD is not needed for basal Rob activity. Two strains, M851 and M854, had ca. 50% higher basal levels than strain M600. These strains are similar in inaA::lacZ activity to rob133, which encodes the first 123 amino acids of Rob plus 10 amino acids from the vector (3). Thus, while we do not know whether these truncations influence the stability of the protein, it seems likely that the CTD of Rob neither promotes nor inhibits the basal-level activity of Rob to a great extent. Nevertheless, even when the CTD of Rob was truncated by only 30 amino acids, the posttranslational response (measured by inaA::lacZ activity) to 2,2"- or 4,4"-dipyridyl was abolished. Thus, determinants near the carboxy terminus of Rob are necessary for activation by dipyridyl.
TABLE 6.
Effects of carboxyl-terminus deletions on activation of Rob by dipyridyla
| Strain | rob coding sequences present (bp) | β-Galactosidase activity (Miller units):
|
Ratiob | β-Galactosidase activity (Miller units) with 5 mM 4,4"-dipyridyl | Ratiob | |
|---|---|---|---|---|---|---|
| In untreated samples | With 5 mM 2,2"-dipyridyl | |||||
| M597 | None | 7.8 | 18 | 2.3 | 13 | 1.6 |
| M600 | 1-867 (wild type) | 21 | 392 | 18.3 | 149 | 6.9 |
| M848 | 1-777 | 16 | 19 | 1.2 | 21 | 1.3 |
| M851 | 1-642 | 29 | 23 | 0.80 | 25 | 0.87 |
| M853 | 1-528 | 20 | 15 | 0.74 | 18 | 0.92 |
| M854 | 1-450 | 30 | 32 | 1.1 | 19 | 0.74 |
| M808 | 1-369 | 17 | 18 | 1.1 | 24 | 1.4 |
The mar sox rob mutant strains carrying the indicated plasmids were grown to early log phase, treated with 0 or 5 mM 2,2"- or 4,4"-dipyridyl for 1 h at 37°C, and assayed for β-galactosidase activity.
That is, the ratio of activities in the cells treated with 2,2"- or 4,4"-dipyridyl to that in the untreated cells.
Binding of 2,2"-dipyridyl to Rob.
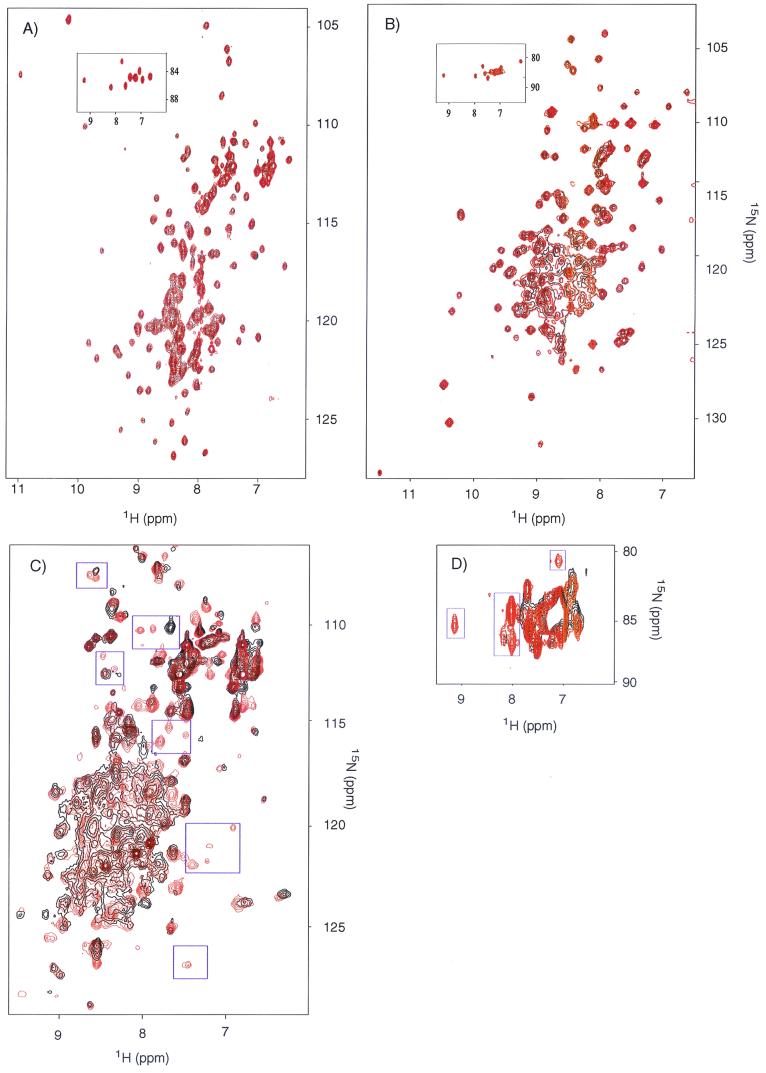
Binding of 2,2"-dipyridyl to Rob was assessed by NMR spectroscopy. The resonance positions for each amide group are indicative of the particular chemical, conformational, or electronic environments of the associated proton and nitrogen nuclei. Slight changes in the environment induced by ligand binding, hydrogen bonding, or conformational changes manifest themselves by differences in chemical shifts and provide a useful tool for mapping binding sites in a protein (8, 26). Uniformly 15N-labeled full-length Rob, CTD-truncated Rob, and MarA were purified and complexed with a mar DNA-binding site to maintain the proteins in solution. 1H-15N HSQC spectra for the MarA-mar and the CTD-truncated Rob-mar complexes in the presence or absence of excess 2,2"-dipyridyl were determined (Fig. 4A and B). No chemical shift differences in the backbone amide, side chain amino, or arginine side chain NɛH groups were observed. In contrast, similar spectra for the full-length Rob-mar complex (Fig. 4C and D) exhibited several significant shifts in resonance frequencies upon 2,2"-dipyridyl addition, both in the backbone amide and in the side chain region of the spectra. This demonstrates that 2,2"-dipyridyl binds to the Rob-mar complex by a mechanism involving the CTD and perturbs the structure of Rob.
FIG. 4.
An overlay of the 1H-15N HSQC spectra of mar DNA-15N-labeled MarA complex (A), mar DNA-15N-labeled CTD-truncated Rob complex (B), and mar DNA-15N-labeled full-length Rob complex (C). The spectra in black and red were acquired in the absence and presence of 5 mM 2,2"-dipyridyl, respectively. The NɛH protons of the arginines in the protein-DNA complexes are represented in the insets of panels A and B and in panel D. The blue boxes in panels C and D indicate some of the prominent differences in the spectra due to the presence of 2,2"-dipyridyl.
Rob binds more tightly to many of its cognate sites in vitro than does MarA or SoxS (13, 19), and yet basal levels of Rob activate the mar/sox/rob regulon promoters to a rather low extent in vivo (3, 20, 34). Importantly, single-round in vitro transcription assays showed that Rob is as active as MarA and half as active as SoxS on a molar basis with respect to the extent of transcriptional activation of six regulon promoters (11). To determine whether 2,2"-dipyridyl enhances the affinity of Rob for its binding sites in the promoters of mar regulon genes in vitro or whether it stabilizes Rob-DNA-RNAP ternary complexes, gel retardation assays were performed. The DNA fragments contained the cognate binding sites of the promoters for fumC, mar, or fpr. No significant change in complex formation by Rob with any of these DNAs was seen with 2,2"- dipyridyl at 5 mM (data not shown). Explanations for the lack of a dipyridyl effect in vitro are presented below.
DISCUSSION
Posttranslational activation by dipyridyl.
There are about 10,000 molecules of the transcriptional activator Rob in E. coli, and yet they have marginal effects on the cell (3, 12, 20, 28, 30, 35). Dramatic activation of the regulon promoters by Rob (e.g., a 10-fold effect on inaA::lacZ activity) is only achieved by vast overexpression of rob with heterologous promoters and multicopy plasmids (3, 14, 23). In contrast, substantial activation of the regulon promoters is achieved by induction of marRAB or soxRS leading to the production of an estimated 2,300 MarA or 350 SoxS molecules per cell, respectively (19; S. Ishita, K. L. Griffith, and R. E. Wolf, Jr., unpublished data). Thus, Rob has comparatively low activity in vivo. The present study shows that, by treating cells with dipyridyl, this low activity form of Rob is converted to a high-activity form. We tentatively conclude that this is a posttranslational event since (1) it occurs in the absence of the rob promoter and translational signals (2); it does not occur in rob nonsense mutants, which presumably synthesize otherwise wild-type rob mRNA, and (3) it does not occur when the CTD is truncated.
Activation requires the CTD of Rob.
In a crystal complex with DNA, the CTD of Rob was seen to lie on top of the NTD and to make no contact with the DNA (13). The smaller MarA and SoxS proteins do not have a CTD but are homologous to Rob's NTD. Therefore, we considered the possibility that the CTD is an inhibitor of the NTD of Rob and that dipyridyl treatment antagonizes the inhibition. If so, truncation of the CTD should relieve the inhibition and increase the activity of Rob to levels seen with dipyridyl treatment. A twofold increase in the activation of the inaA::lacZ mutant over that of wild type was reported previously for a Rob construct (rob133) in which 156 C-terminal amino acids were replaced with 10 amino acids of the vector (3). However, in our systematic study, no increase in inaA::lacZ expression due to Rob's activity was seen with complete or partial C-terminal truncations of 168, 113, or 30 amino acids, and a modest 1.5-fold increase in activity was seen for Rob with C-terminal truncations of 75 or 139 amino acids (Table 6). In contrast, 2,2"-dipyridyl had an 18-fold effect on inaA::lacZ expression in a strain encoding the wild-type Rob. Thus, if the Rob CTD inhibits the NTD's activity, it is only to a minor extent. This indicates that the normally low activity of Rob is a function of the NTD. Nevertheless, removing as few as 30 amino acids from the carboxyl terminus of Rob prevented activation by dipyridyl. The simplest interpretation is that dipyridyl treatment reconfigures Rob by an interaction involving the CTD, which then converts the NTD from a low-activity form to a high-activity form in vivo.
Is dipyridyl the direct effector of the Rob activation?
2,2"- and 4,4"-dipyridyl are hydrophobic compounds that are not known to be normal cellular or environmental constituents of E. coli. 2,2"-Dipyridyl has long been used to chelate environmental iron, and it has been assumed that little if any of it is taken up by the cells. Thus, the treatment of cells with millimolar concentrations are likely to produce cellular concentrations in the micromolar range or lower. Substantial evidence that the effects studied here are not due to metal chelation has been provided above. Whether the dipyridyls are direct effectors of Rob, whether they are first converted intracellularly into the direct effectors, or whether they stimulate the cell to produce the direct effector is not known. Evidence for a direct interaction of dipyridyl with Rob in vitro comes from NMR studies. 2,2"-Dipyridyl engendered striking changes in the backbone amide and side chain regions of the 1H-15N HSQC spectra with full-length Rob but not with either CTD-truncated Rob or MarA, which has no CTD (Fig. 4). In addition, the kinetics of induction of inaA transcription by dipyridyl in vivo are consistent with a rapid activation of Rob (Fig. 2). However, preliminary attempts to demonstrate that 2,2"-dipyridyl enhances either the binding of purified Rob to DNA or the activation of transcription in vitro have been unsuccessful (data not shown).
Three explanations for the lack of activation in vitro may be considered. (i) 2,2"-Dipyridyl is a direct effector of Rob, but the purified Rob used in vitro differs from the low activity form found in vivo. Purified Rob has been found to be as active as MarA and half as active as SoxS in stimulating transcription of six regulon promoters in vitro (11). Thus, the low activity of Rob in vivo may be due to the binding of an inhibitor or to the cellular sequestration of Rob (3). Indeed, immunostaining has shown that Rob proteins are clustered in a few discrete foci in the nucleoid (29). The interaction of dipyridyl with Rob in vivo would reduce its affinity for inhibitor or reverse the sequestration. However, if purification of Rob eliminates the inhibition or sequestration, as seems to be the case (11), dipyridyl would not show an effect in vitro. (ii) 2,2"-Dipyridyl is not a direct effector of Rob, and thus is not effective in vitro. The binding seen in the NMR data would then be ascribed to an interaction with the CTD that is not relevant to the activation mechanism. (iii) A negative in vitro result could mean that the conditions of the assay are not appropriate for detecting the effects of 2,2"-dipyridyl or that an additional factor necessary for activation is missing. Analysis of mutants with increased basal level activities of Rob and decreased response to dipyridyl may help distinguish between these possibilities.
Acknowledgments
We thank C. Ueguchi and R. Kadner for strains; T. Brodigan for help in DNA sequencing; C. Earhart, K. Hantke, R. Kadner, and I. Stojiljkovic for discussions of chelation by 2,2"-dipyridyl; and R. E. Wolf, Jr., for sharing unpublished results.
REFERENCES
- 1.Alekshun, M. N., and S. B. Levy. 1997. Regulation of chromosomally mediated multiple antibiotic resistance: the mar regulon. Antimicrob. Agents Chemother. 41:2067-2075. [DOI] [PMC free article] [PubMed]
- 2.Aono, R., N. Tsukagoshi, and M. Yamamoto. 1998. Involvement of outer membrane protein TolC, a possible member of the mar-sox regulon, in maintenance and improvement of organic solvent tolerance of Escherichia coli K-12. J. Bacteriol. 180:938-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ariza, R. R., Z. Li, N. Ringstad, and B. Demple. 1995. Activation of multiple antibiotic resistance and binding of stress-inducible promoters by Escherichia coli Rob protein. J. Bacteriol. 177:1655-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen, S. P., S. B. Levy, J. Foulds, and J. L. Rosner. 1993. Salicylate induction of antibiotic resistance in Escherichia coli: activation of the mar operon and a mar-independent pathway. J. Bacteriol. 175:7856-7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clore, G. M., and A. M. Gronenborn. 1991. Two-, three-, and four-dimensional NMR methods for obtaining larger and more precise three-dimensional structures of proteins in solution. Annu. Rev. Biophys. Chem. 20:29-63. [DOI] [PubMed] [Google Scholar]
- 6.Delaglio, F., S. Grzesiek, G. W. Vuister, G. Zhu, J. Pfeifer, and A. Bax. 1995. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6:277-293. [DOI] [PubMed] [Google Scholar]
- 7.Demple, B. 1996. Redox signaling and gene control in the Escherichia coli soxRS oxidative stress regulon--a review. Gene 179:53-57. [DOI] [PubMed] [Google Scholar]
- 8.Hajduk, P. J., J. Dinges, G. F. Miknis, M. Merlock, T. Middleton, D. J. Kempf, D. A. Egan, K. A. Walter, T. S. Robins, S. B. Shuker, T. F. Holzman, and S. W. Fesik. 1997. NMR-based discovery of lead inhibitors that block DNA binding of the human papillomavirus E2 protein. J. Med. Chem. 40:3144-3150. [DOI] [PubMed] [Google Scholar]
- 9.Heller, K., B. J. Mann, and R. J. Kadner. 1985. Cloning and expression of the gene for the vitamin B12 receptor protein in the outer membrane of Escherichia coli. J. Bacteriol. 161:896-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jair, K. W., R. G. Martin, J. L. Rosner, N. Fujita, A. Ishihama, and R. E. Wolf, Jr. 1995. Purification and regulatory properties of MarA protein, a transcriptional activator of Escherichia coli multiple antibiotic and superoxide resistance promoters. J. Bacteriol. 177:7100-7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jair, K. W., X. Yu, K. Skarstad, B. Thöny, N. Fujita, A. Ishihama, and R. E. Wolf, Jr. 1996. Transcriptional activation of promoters of the superoxide and multiple antibiotic resistance regulons by Rob, a binding protein of the Escherichia coli origin of chromosomal replication. J. Bacteriol. 178:2507-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kakeda, M., C. Ueguchi, H. Yamada, and T. Mizuno. 1995. An Escherichia coli curved DNA-binding protein whose expression is affected by the stationary phase-specific sigma factor sigma S. Mol. Gen. Genet. 248:629-634. [DOI] [PubMed] [Google Scholar]
- 13.Kwon, H. J., M. H. Bennik, B. Demple, and T. Ellenberger. 2000. Crystal structure of the Escherichia coli Rob transcription factor in complex with DNA. Nat. Struct. Biol. 7:424-430. [DOI] [PubMed] [Google Scholar]
- 14.Lee, E. H., E. Collatz, I. Podglajen, and L. Gutmann. 1996. A rob-like gene of Enterobacter cloacae affecting porin synthesis and susceptibility to multiple antibiotics. Antimicrob. Agents Chemother. 40:2029-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsen, R. A., M. G. Thomas, G. E. Wood, and K. Postle. 1994. Partial suppression of an Escherichia coli TonB transmembrane domain mutation (delta V17) by a missense mutation in ExbB. Mol. Microbiol. 13:627-640. [DOI] [PubMed] [Google Scholar]
- 16.Li, Z., and B. Demple. 1996. Sequence specificity for DNA binding by Escherichia coli SoxS and Rob proteins. Mol. Microbiol. 20:937-945. [DOI] [PubMed] [Google Scholar]
- 17.Maekawa, T., and E. Ohtsubo. 1994. Identification of the region that determines the specificity of binding of the transposases encoded by Tn3 and gamma delta to the terminal inverted repeat sequences. Jpn. J. Genet. 69:269-285. [DOI] [PubMed] [Google Scholar]
- 18.Martin, R. G., and J. L. Rosner. 1995. Binding of purified multiple antibiotic-resistance repressor protein (MarR) to mar operator sequences. Proc. Natl. Acad. Sci. USA 92:5456-5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin, R. G., W. K. Gillette, N. I. Martin, and J. L. Rosner. 2002.. Complex formation between activator and RNA polymerase as the basis for transcriptional activation by MarA and SoxS in Escherichia coli. Mol. Microbiol. 43:355-370. [DOI] [PubMed]
- 20.Martin, R. G., W. K. Gillette, and J. L. Rosner. 2000. Promoter discrimination by the related transcriptional activators MarA and SoxS: differential regulation by differential binding. Mol. Microbiol. 35:623-634. [DOI] [PubMed] [Google Scholar]
- 21.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 22.Moeck, G. S., and J. W. Coulton. 1998. TonB-dependent iron acquisition: mechanisms of siderophore-mediated active transport. Mol. Microbiol. 28:675-681. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima, H., K. Kobayashi, Kobayashi, M., H. Asako, and R. Aono. 1995. Overexpression of the robA gene increases organic solvent tolerance and multiple antibiotic and heavy metal ion resistance in Escherichia coli. Appl. Environ. Microbiol. 61:2302-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhee, S., R. G. Martin, J. L. Rosner, and D. R. Davies. 1998. A novel DNA-binding motif in MarA: the first structure for an AraC family transcriptional activator. Proc. Natl. Acad. Sci. USA 95:10413-10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosner, J. L., and J. L. Slonczewski. 1994. Dual regulation of inaA by the multiple antibiotic resistance (mar) and superoxide (soxRS) stress response systems of Escherichia coli. J. Bacteriol. 176:6262-6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shuker, S. B., P. J. Hajduk, R. P. Meadows, and S. W. Fesik. 1996. Discovering high-affinity ligands for proteins: SAR by NMR. Science 274:1531-1534. [DOI] [PubMed] [Google Scholar]
- 27.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single copy and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 28.Skarstad, K., B. Thöny, D. S. Hwang, and A. Kornberg. 1993. A novel binding protein of the origin of the Escherichia coli chromosome. J. Biol. Chem. 268:5365-5370. [PubMed] [Google Scholar]
- 29.Talukder, A. A., S. Hiraga, and A. Ishihama. 2000. Two types of localization of the DNA-binding proteins within the Escherichia coli nucleoid. Genes Cells 5:613-626. [DOI] [PubMed] [Google Scholar]
- 30.Talukder, A. A., A. Iwata, A. Nishimura, S. Ueda, and A. Ishihama. 1999. Growth-phase-dependent variation in protein composition of the Escherichia coli nucleoid. J. Bacteriol. 181:6361-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trun, N. J., and T. J. Silhavy. 1987. Characterization and in vivo cloning of prlC, a suppressor of signal sequence mutations in Escherichia coli K12. Genetics 116:513-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsaneva, I. R., and B. Weiss. 1990. soxR, a locus governing a superoxide response regulon in Escherichia coli K-12. J. Bacteriol. 172:4197-4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsolis, R. M., A. J. Baumler, I. Stojiljkovic, and F. Heffron. 1995. Fur regulon of Salmonella typhimurium: identification of new iron-regulated genes. J. Bacteriol. 177:4628-4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Dyk, T. K., B. L. Ayers, R. W. Morgan, and R. A. Larossa. 1998. Constricted flux through the branched-chain amino acid biosynthetic enzyme acetolactate synthase triggers elevated expression of genes regulated by rpoS and internal acidification. J. Bacteriol. 180:785-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White, D. G., J. D. Goldman, B. Demple, and S. B. Levy. 1997. Role of the acrAB locus in organic solvent tolerance mediated by expression of marA. soxS, or robA in Escherichia coli. J. Bacteriol. 179:6122-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]