Abstract
1. In the isolated colonic mucosa of Bufo arenarum, under special circumstances, there is a variable fraction of the short-circuit current (0-38%) that is unaccounted for by either the Na or the Cl and bicarbonate transmembrane net fluxes.
2. The hypothesis that a special kind of bicarbonate transport may account for the non-Na component of the short-circuit current was investigated. According to this, bicarbonate ions formed within the membrane await transport towards the mucosal solution within a compartment that does not undergo isotopic exchange with the serosal bathing solution. This kind of transport may be detected by a lowering of mucosal specific activity of bicarbonate but would not be revealed by the classic method of comparing the difference between the unidirectional fluxes with the short-circuit current.
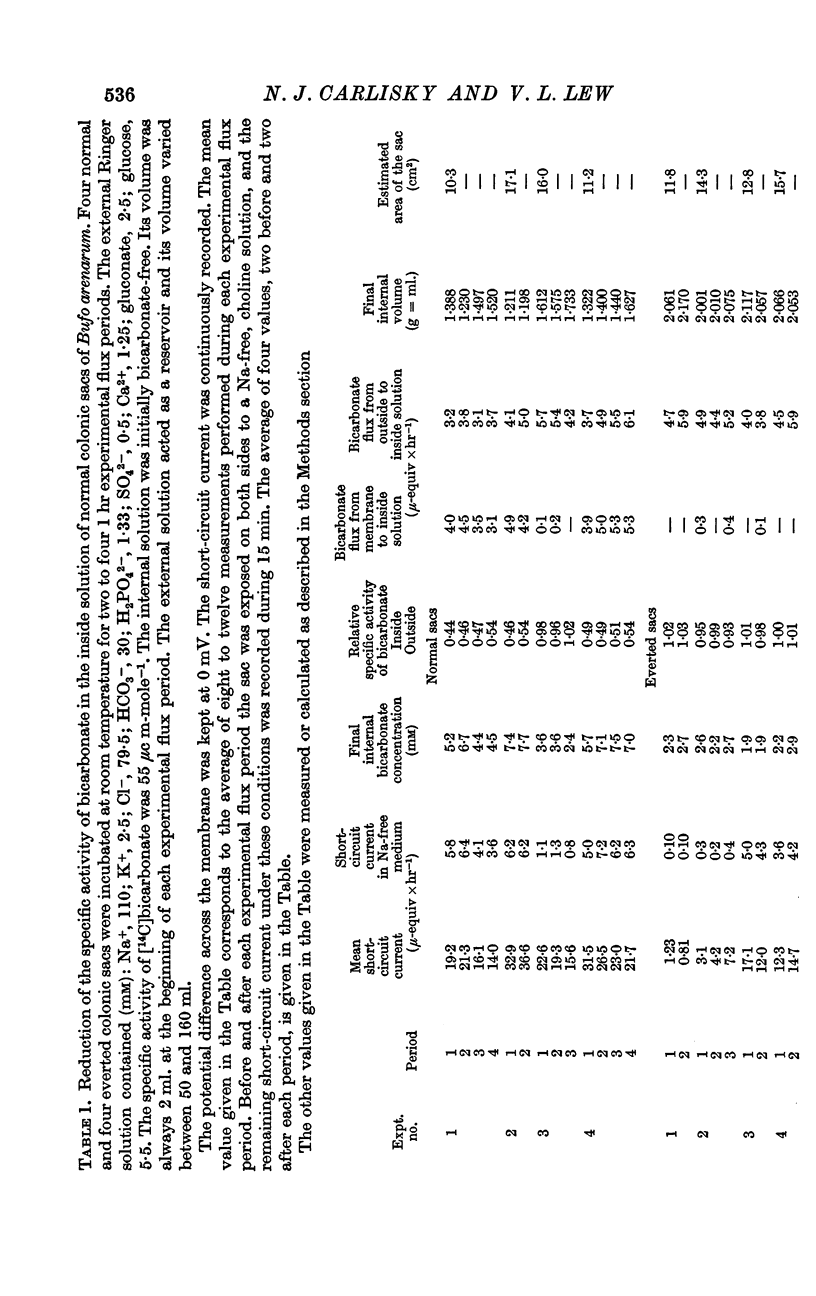
3. The specific activity of bicarbonate was determined in the inside solution (initially bicarbonate-free) of ten normal and four everted colonic sacs incubated in an external medium (reservoir) containing a constant specific activity of bicarbonate. Comparison between membrane-to-internal solution bicarbonate flux and non-Na component of the short-circuit current was carried out in two different ways: (a) by measuring the remaining short-circuit current in Na-free medium and (b) by determining simultaneously the Na net flux.
4. Whatever the value of the short-circuit current and its non-Na component, there is no reduction of the specific activity of the bicarbonate appearing in the inside solution of the everted colonic sacs.
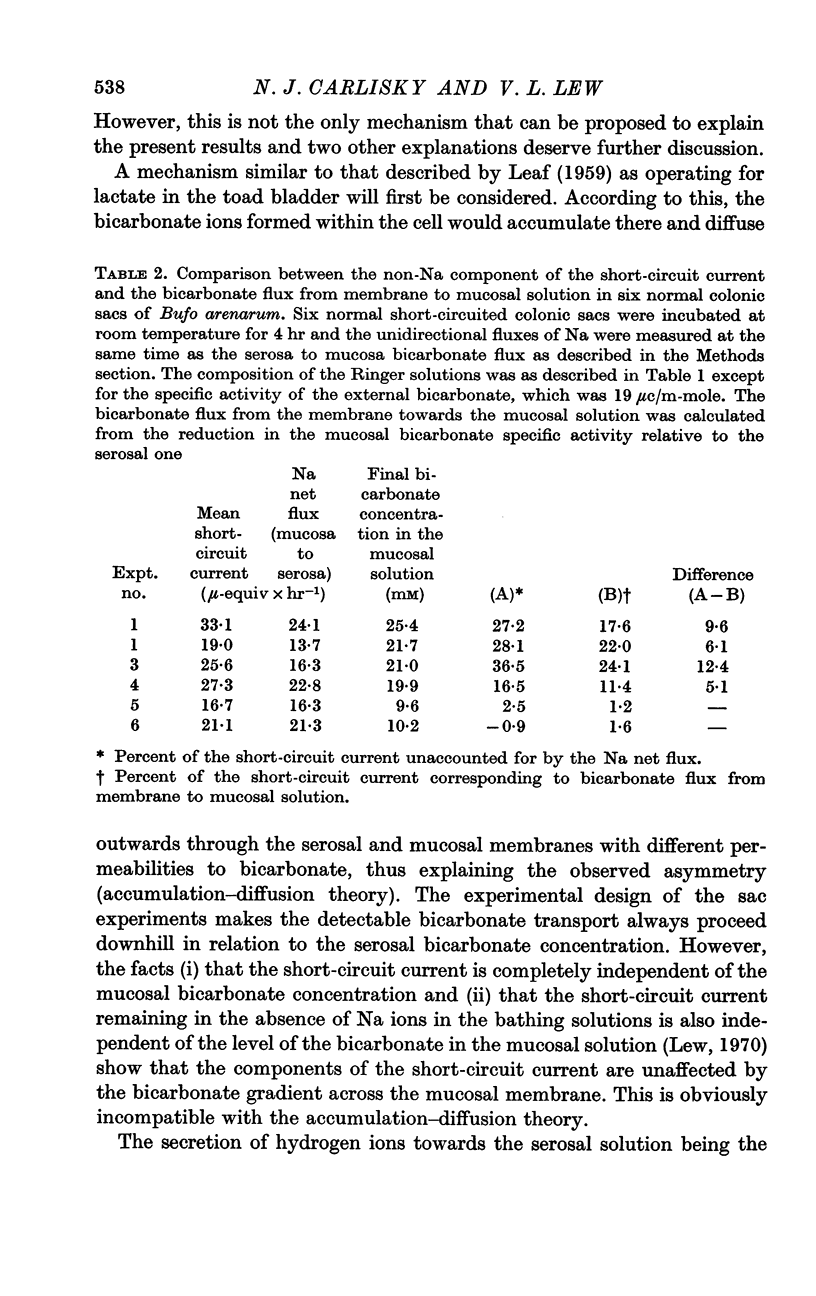
5. In the normal sacs there is a reduction of the specific activity of bicarbonate which accounts for a membrane-to-mucosa bicarbonate flux which parallels the variations of the non-Na component of the short-circuit current although quantitatively representing only 68-87% of it.
6. There is no systematic decrease in the rate of reduction of the mucosal specific activity of bicarbonate in successive experimental flux periods; this excludes a slow equilibration of the intracellular bicarbonate with serosal bicarbonate.
7. Other possible explanations of the present results are discussed, as well as the availability and hydration rate of metabolic CO2 necessary to account for this kind of bicarbonate transport.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry R. J., Smyth D. H., Wright E. M. Short-circuit current and solute transfer by rat jejunum. J Physiol. 1965 Nov;181(2):410–431. doi: 10.1113/jphysiol.1965.sp007770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPERSTEIN I. L., HOGBEN C. A. Ionic transfer across the isolated frog large intestine. J Gen Physiol. 1959 Jan 20;42(3):461–473. doi: 10.1085/jgp.42.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edsall J. T. The carbonic anhydrases of erythrocytes. Harvey Lect. 1966;62:191–230. [PubMed] [Google Scholar]
- LEAF A. The mechanism of the asymmetrical distribution of endogenous lactate about the isolated toad bladder. J Cell Comp Physiol. 1959 Aug;54:103–108. doi: 10.1002/jcp.1030540111. [DOI] [PubMed] [Google Scholar]
- Lew V. L. Short-circuit current and ionic fluxes in the isolated colonic mucosa of Bufo arenarum. J Physiol. 1970 Mar;206(3):509–528. doi: 10.1113/jphysiol.1970.sp009028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffly R. H., Coggins C. H. Carbon dioxide production and sodium transport by the toad bladder. Nature. 1965 Apr 10;206(980):197–198. doi: 10.1038/206197a0. [DOI] [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]



