Abstract
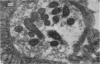
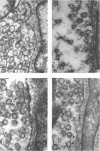
1. Electron micrographs of nerve terminals in rat phrenic nerve—diaphragm preparations have been studied. This has been done before and after prolonged nerve stimulation. The effectiveness of nerve stimulation has been monitored by intracellular micro-electrode recordings from the muscle cells.
2. Characteristic changes in the form and distribution of the nerve terminal mitochondria were noted after nerve stimulation.
3. Synaptic vesicle numbers in the region of nerve terminal less than 1800 Å from the synaptic cleft were significantly greater in tissue taken 2 and 3 min after nerve stimulation, than in unstimulated preparations.
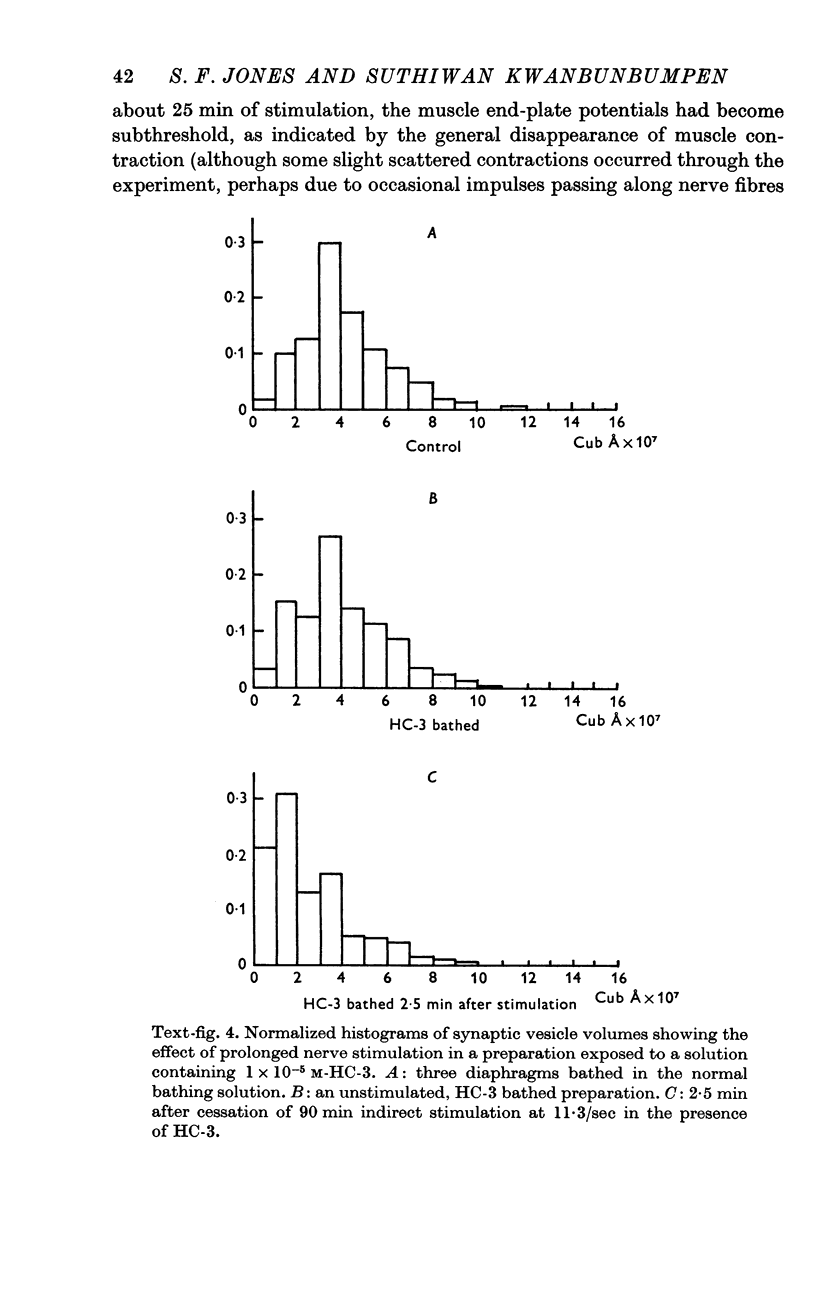
4. The long and short diameters of the synaptic vesicle profiles less than 1800 Å from the synaptic cleft were measured. Analysis of the distribution of the diameters indicated synaptic vesicles to be basically spherical structures. Estimates of synaptic vesicle volume were made from the measurements. Synaptic vesicle volume was significantly reduced in tissue taken 2 and 4 min following nerve stimulation.
5. If hemicholinium, a compound which inhibits acetylcholine synthesis, was present during the period of nerve stimulation, much greater reductions in synaptic vesicle volume occurred. Synaptic vesicle numbers in the region of nerve terminal less than 1800 Å from the synaptic cleft were also reduced, compared with unstimulated control preparations.
6. These results are regarded as support for the hypothesis that the synaptic vesicles in nerve terminals at the mammalian neuromuscular junction represent stores of the transmitter substance, acetylcholine.
Full text
PDF






















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akcasu A., Salafsky B. Migration of sodium ion along stimulated frog sciatic nerve. Exp Neurol. 1967 May;18(1):49–55. doi: 10.1016/0014-4886(67)90087-8. [DOI] [PubMed] [Google Scholar]
- BIRKS R. I., MACINTOSH F. C., SASTRY P. B. Pharmacological inhibition of acetylcholine synthesis. Nature. 1956 Nov 24;178(4543):1181–1181. doi: 10.1038/1781181a0. [DOI] [PubMed] [Google Scholar]
- BIRKS R., HUXLEY H. E., KATZ B. The fine structure of the neuromuscular junction of the frog. J Physiol. 1960 Jan;150:134–144. doi: 10.1113/jphysiol.1960.sp006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton A. A., Barton M. The relation of nerve fibers to Mycobacterium leprae. I. The phagocytic activity of cells in damaged nerve. Int J Lepr Other Mycobact Dis. 1967 Jul-Sep;35(3):382–387. [PubMed] [Google Scholar]
- DE ROBERTIS E. D., BENNETT H. S. Some features of the submicroscopic morphology of synapses in frog and earthworm. J Biophys Biochem Cytol. 1955 Jan;1(1):47–58. doi: 10.1083/jcb.1.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE ROBERTIS E., FERREIRA A. V. Submicroscopic changes of the nerve endings in the adrenal medulla after stimulation of the splanchnic nerve. J Biophys Biochem Cytol. 1957 Jul 25;3(4):611–614. doi: 10.1083/jcb.3.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE ROBERTIS E., RODRIGUEZ DE LORES ARNAIZ G., SALGANICOFF L., PELLEGRINO DE IRALDI A., ZIEHER L. M. Isolation of synaptic vesicles and structural organization of the acetycholine system within brain nerve endings. J Neurochem. 1963 Apr;10:225–235. doi: 10.1111/j.1471-4159.1963.tb05038.x. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Biophysical aspects of neuro-muscular transmission. Prog Biophys Biophys Chem. 1956;6:121–170. [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Local activity at a depolarized nerve-muscle junction. J Physiol. 1955 May 27;128(2):396–411. doi: 10.1113/jphysiol.1955.sp005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E. Ultrastructure and cytochemistry of the synaptic region. The macromolecular components involved in nerve transmission are being studied. Science. 1967 May 19;156(3777):907–914. doi: 10.1126/science.156.3777.907. [DOI] [PubMed] [Google Scholar]
- ELMQVIST D., QUASTEL D. M. PRESYNAPTIC ACTION OF HEMICHOLINIUM AT THE NEUROMUSCULAR JUNCTION. J Physiol. 1965 Apr;177:463–482. doi: 10.1113/jphysiol.1965.sp007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- GRAY E. G. The granule cells, mossy synapses and Purkinje spine synapses of the cerebellum: light and electron microscope observations. J Anat. 1961 Jul;95:345–356. [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Kwanbunbumpen S. Evidence for the vesicle hypothesis. J Physiol. 1968 Feb;194(2):407–420. doi: 10.1113/jphysiol.1968.sp008415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. F., Kwanbunbumpen S. Some effects of nerve stimulation andhemicholinium on quantal transmitter release at the mammalian neuromuscular junction. J Physiol. 1970 Mar;207(1):51–61. doi: 10.1113/jphysiol.1970.sp009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Failure of neuromuscular propagation in rats. J Physiol. 1958 Mar 11;140(3):440–461. [PMC free article] [PubMed] [Google Scholar]
- Larramendi L. M., Fickenscher L., Lemkey-Johnston N. Synaptic vesicles of inhibitory and excitatory TERMINALS IN THE CEREBELLUM. Science. 1967 May 19;156(3777):967–969. doi: 10.1126/science.156.3777.967. [DOI] [PubMed] [Google Scholar]
- Marchbanks R. M. Exchangeability of radioactive acetylcholine with the bound acetylcholine of synaptosomes and synaptic vesicles. Biochem J. 1968 Jan;106(1):87–95. doi: 10.1042/bj1060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALAY S. L. The morphology of synapses in the central nervous system. Exp Cell Res. 1958;14(Suppl 5):275–293. [PubMed] [Google Scholar]
- Quastel D. M., Curtis D. R. A central action of hemicholinium. Nature. 1965 Oct 9;208(5006):192–194. doi: 10.1038/208192a0. [DOI] [PubMed] [Google Scholar]
- ROBERTSON J. D. The ultrastructure of a reptilian myoneural junction. J Biophys Biochem Cytol. 1956 Jul 25;2(4):381–394. doi: 10.1083/jcb.2.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROUILLER C. Physiological and pathological changes in mitochondrial morphology. Int Rev Cytol. 1960;9:227–292. doi: 10.1016/s0074-7696(08)62748-5. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Rasmussen G. L. Degeneration in the efferent nerve endings in the cochlea after axonal section. J Cell Biol. 1965 Jul;26(1):63–77. doi: 10.1083/jcb.26.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchizono K. Characteristics of excitatory and inhibitory synapses in the central nervous system of the cat. Nature. 1965 Aug 7;207(997):642–643. doi: 10.1038/207642a0. [DOI] [PubMed] [Google Scholar]
- VAN BREEMEN V. L., ANDERSON E., REGER J. F. An attempt to determine the origin of synaptic vesicles. Exp Cell Res. 1958;14(Suppl 5):153–167. [PubMed] [Google Scholar]
- WHITTAKER V. P., SHERIDAN M. N. THE MORPHOLOGY AND ACETYLCHOLINE CONTENT OF ISOLATED CEREBRAL CORTICAL SYNAPTIC VESICLES. J Neurochem. 1965 May;12:363–372. doi: 10.1111/j.1471-4159.1965.tb04237.x. [DOI] [PubMed] [Google Scholar]
- WHITTAKER V. P. The isolation and characterization of acetylcholine-containing particles from brain. Biochem J. 1959 Aug;72:694–706. doi: 10.1042/bj0720694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walberg F. The fine structure of the cuneate nucleus in normal cats and following interruption of afferent fibres. An electron microscopical study with particular reference to findings made in glees and nauta sections. Exp Brain Res. 1966;2(2):107–128. doi: 10.1007/BF00240401. [DOI] [PubMed] [Google Scholar]
- Weiss P., Pillai A. Convection and fate of mitochondria in nerve fibers: axonal flow as vehicle. Proc Natl Acad Sci U S A. 1965 Jul;54(1):48–56. doi: 10.1073/pnas.54.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker V. P., Michaelson I. A., Kirkland R. J. The separation of synaptic vesicles from nerve-ending particles ('synaptosomes'). Biochem J. 1964 Feb;90(2):293–303. doi: 10.1042/bj0900293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley N. G. The lattice spacing of crystalline catalase as an internal standard of length in electron microscopy. J Ultrastruct Res. 1968 Sep;24(5):454–464. doi: 10.1016/s0022-5320(68)80048-6. [DOI] [PubMed] [Google Scholar]