Abstract
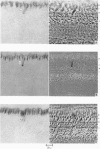
1. Intracellular recordings have been made from single photoreceptors in the retina of the turtle. Histological sections of the retina made after injection of dye through the recording electrode reveal dye in the inner segments of single cones.
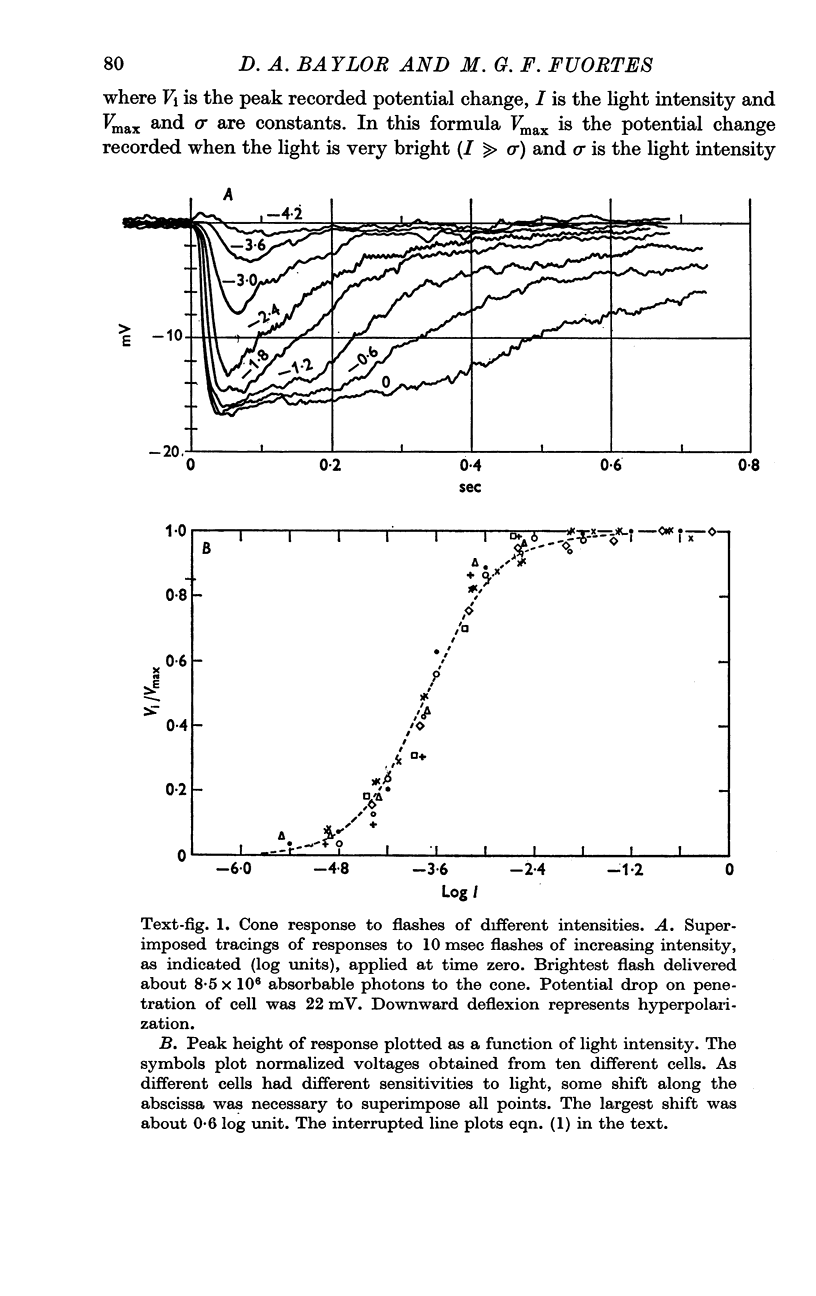
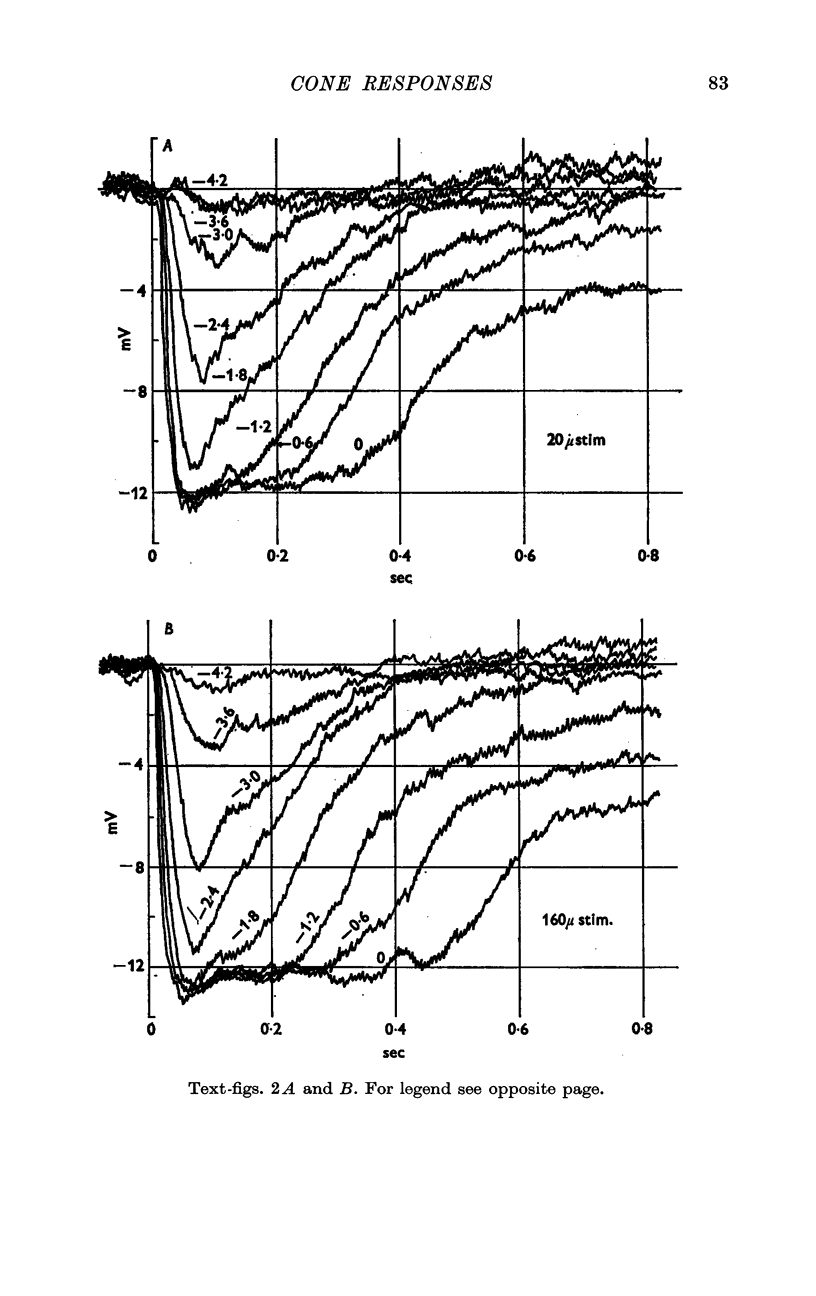
2. Following a brief flash of light the cone undergoes a hyperpolarization which is graded with the intensity of the flash.
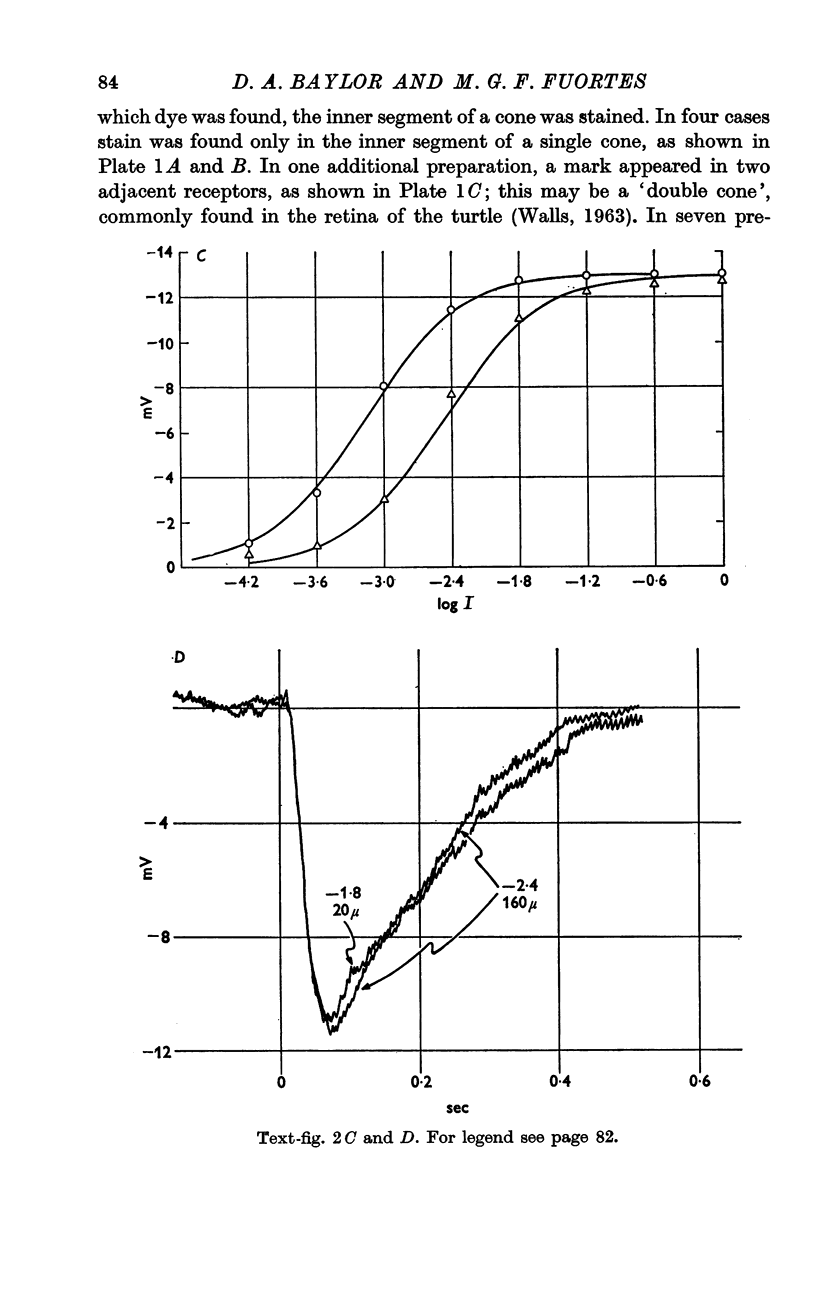
3. The excitatory receptive field of a receptor is probably as small as the cross-section of a single cone, but accurate measurements are rendered difficult by scattering of light within the retina.
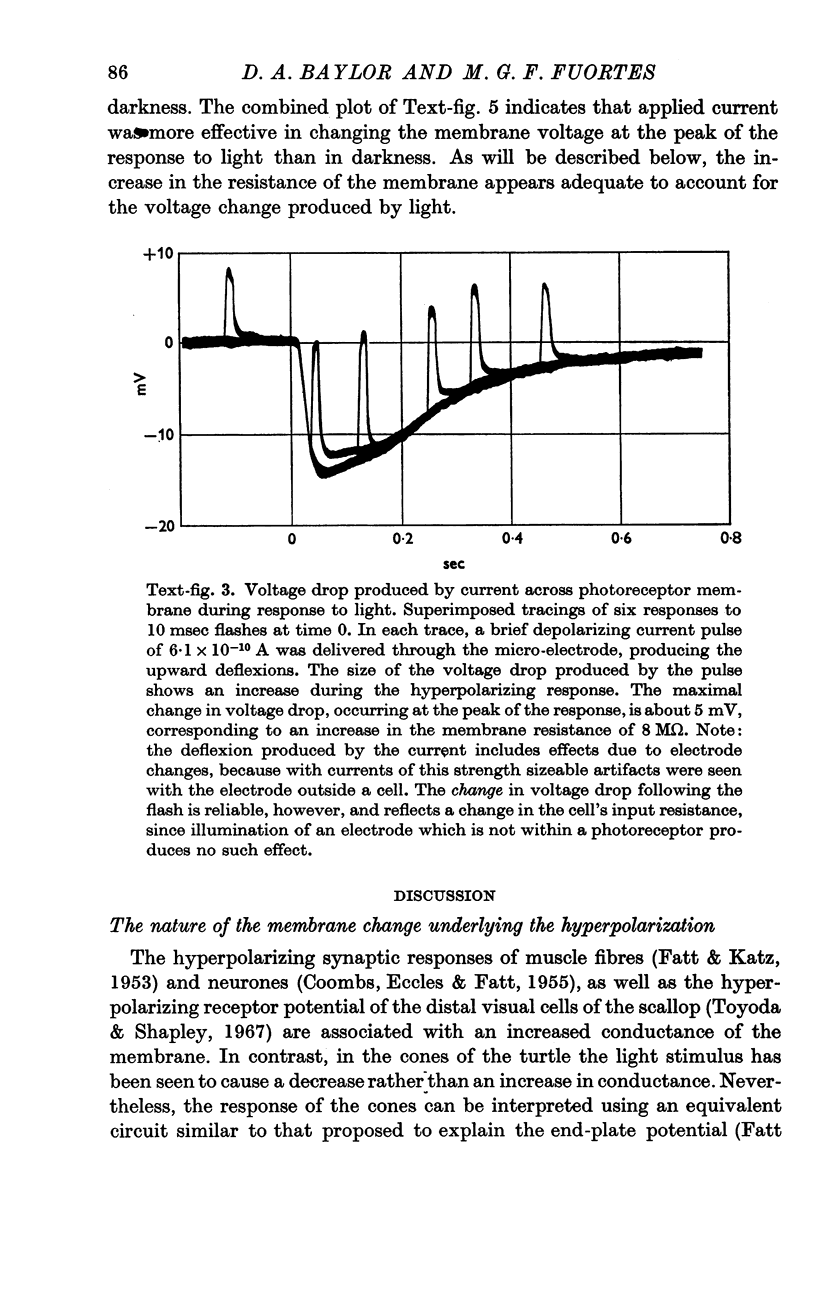
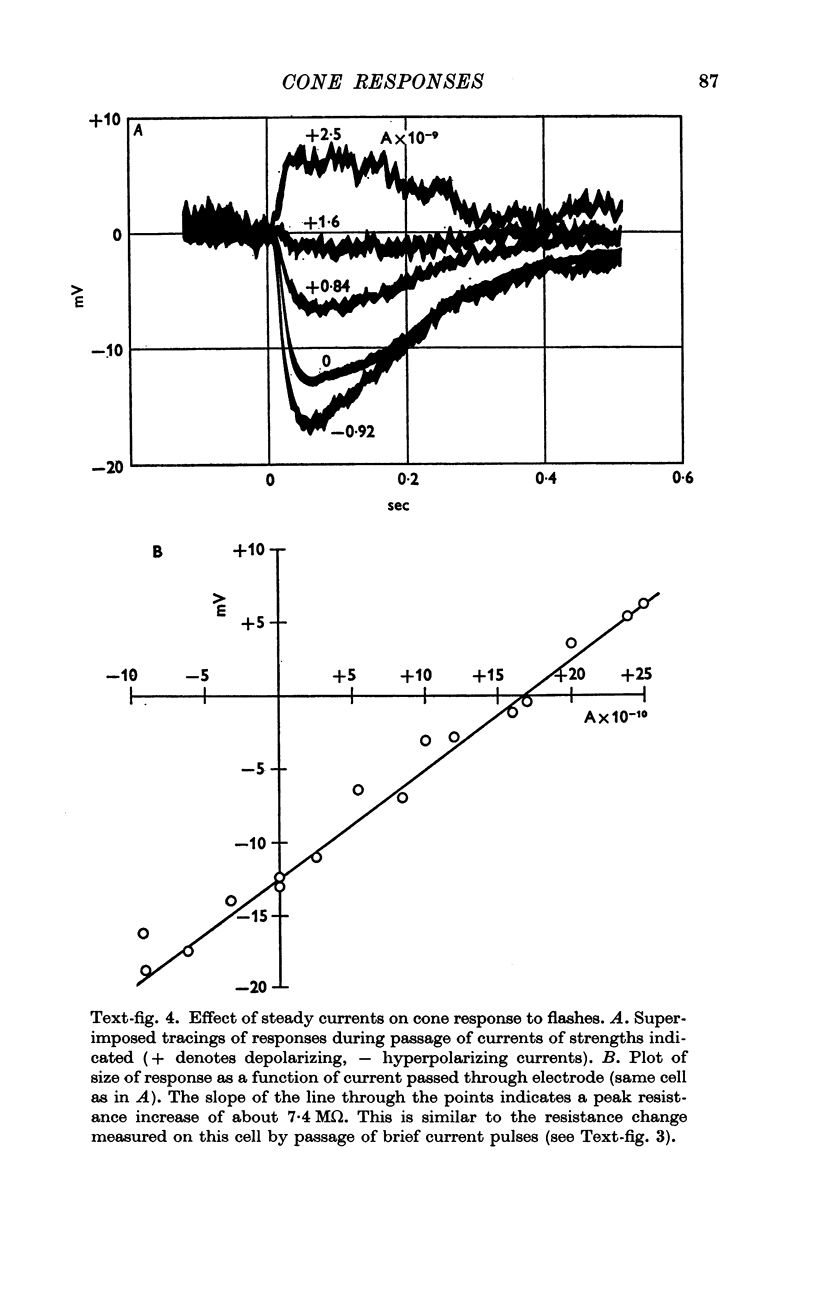
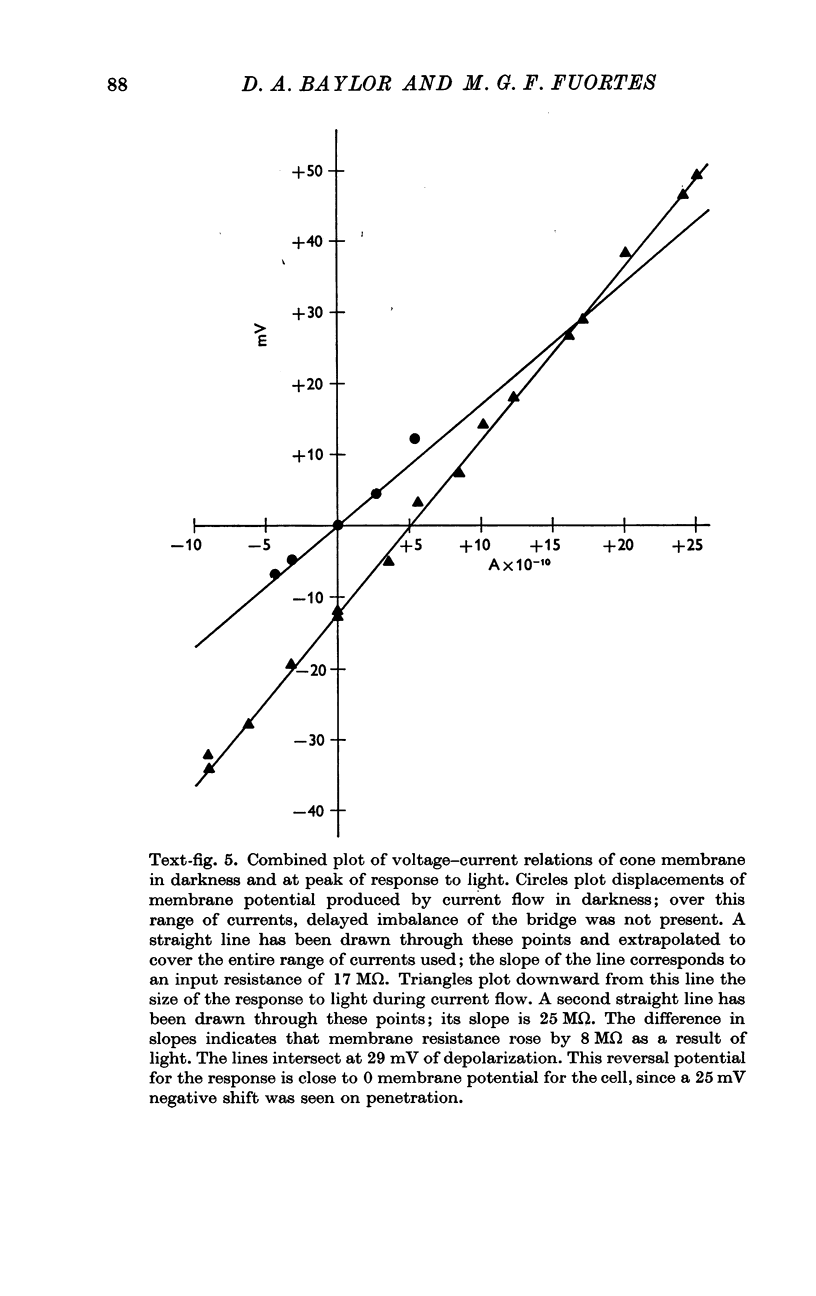
4. The voltage drop produced by a current injected into the cell is increased during the response to light. Steady hyperpolarizing currents increase the size of the response to light; depolarizing currents of increasing strength reduce and then reverse the response.
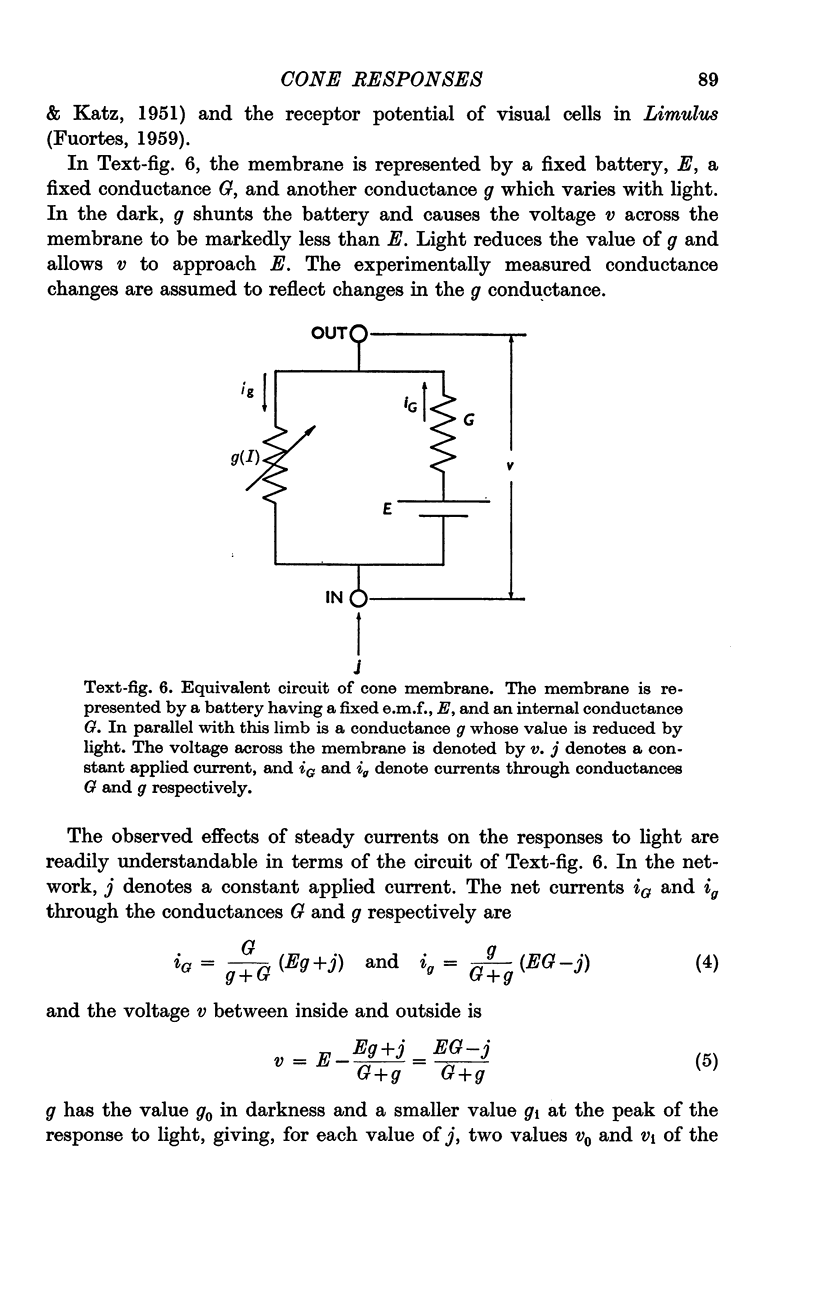
5. The results are consistent with the hypothesis that light activates the visual cell by decreasing the permeability of membrane channels which in darkness act as a shunt of the membrane.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN P. K., WALD G. VISUAL PIGMENTS IN SINGLE RODS AND CONES OF THE HUMAN RETINA. DIRECT MEASUREMENTS REVEAL MECHANISMS OF HUMAN NIGHT AND COLOR VISION. Science. 1964 Apr 3;144(3614):45–52. doi: 10.1126/science.144.3614.45. [DOI] [PubMed] [Google Scholar]
- Borsellino A., Fuortes M. G., Smith T. G. Visual responses in Limulus. Cold Spring Harb Symp Quant Biol. 1965;30:429–443. doi: 10.1101/sqb.1965.030.01.042. [DOI] [PubMed] [Google Scholar]
- Bortoff A. Localization of slow potential responses in the Necturus retina. Vision Res. 1964 Dec;4(11):627–635. doi: 10.1016/0042-6989(64)90048-3. [DOI] [PubMed] [Google Scholar]
- Bortoff A., Norton A. L. An electrical model of the vertebrate photoreceptor cell. Vision Res. 1967 Mar;7(3):253–263. doi: 10.1016/0042-6989(67)90089-2. [DOI] [PubMed] [Google Scholar]
- COOMBS J. S., ECCLES J. C., FATT P. The specific ionic conductances and the ionic movements across the motoneuronal membrane that produce the inhibitory post-synaptic potential. J Physiol. 1955 Nov 28;130(2):326–374. doi: 10.1113/jphysiol.1955.sp005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. The effect of inhibitory nerve impulses on a crustacean muscle fibre. J Physiol. 1953 Aug;121(2):374–389. doi: 10.1113/jphysiol.1953.sp004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANK K., FUORTES M. G. Stimulation of spinal motoneurones with intracellular electrodes. J Physiol. 1956 Nov 28;134(2):451–470. doi: 10.1113/jphysiol.1956.sp005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G., HODGKIN A. L. CHANGES IN TIME SCALE AND SENSITIVITY IN THE OMMATIDIA OF LIMULUS. J Physiol. 1964 Aug;172:239–263. doi: 10.1113/jphysiol.1964.sp007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G. Initiation of impulses in visual cells of Limulus. J Physiol. 1959 Oct;148:14–28. doi: 10.1113/jphysiol.1959.sp006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Hashimoto H. Recording site of the single cone response determined by an electrode marking technique. Vision Res. 1967 Nov;7(11):847–851. doi: 10.1016/0042-6989(67)90005-3. [DOI] [PubMed] [Google Scholar]
- MARKS W. B., DOBELLE W. H., MACNICHOL E. F., Jr VISUAL PIGMENTS OF SINGLE PRIMATE CONES. Science. 1964 Mar 13;143(3611):1181–1183. doi: 10.1126/science.143.3611.1181. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):536–555. doi: 10.1113/jphysiol.1966.sp008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature. 1969 Jul 12;223(5202):201–204. doi: 10.1038/223201a0. [DOI] [PubMed] [Google Scholar]
- Potter D. D., Furshpan E. J., Lennox E. S. Connections between cells of the developing squid as revealed by electrophysiological methods. Proc Natl Acad Sci U S A. 1966 Feb;55(2):328–336. doi: 10.1073/pnas.55.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Electrophysiological study of the mechanisms subserving color coding in the fish retina. Cold Spring Harb Symp Quant Biol. 1965;30:559–566. doi: 10.1101/sqb.1965.030.01.054. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Nosaki H., Tomita T. Light-induced resistance changes in single photoreceptors of Necturus and Gekko. Vision Res. 1969 Apr;9(4):453–463. doi: 10.1016/0042-6989(69)90134-5. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]