Abstract
In vivo, RNA polymerases (RNAPs) do not transcribe naked DNA but do transcribe protein-associated DNA. Studies with the model enzyme T7 RNAP have shown that, in eukaryotic cells or in vitro, nucleosomes can inhibit both transcription initiation and elongation. We examine here whether the presence of HU, one of the major histone-like proteins in Escherichia coli cells (the genuine milieu for T7 RNAP) affects its activity. An engineered lac operon fused to the T7 late promoter was introduced into the chromosome of T7 RNAP-producing strains that either overexpress HU or lack it. The flows of RNAP that enter and exit this operon were compared with regard to the content of HU. We found that the fraction of T7 RNAP molecules that do not reach the end of the lac operon (ca. 15%) is the same whether the host cells overexpressed HU or lacked it: thus, the enzyme either freely displaces HU or transcribes through it. However, in these cells, the transcript yield was increased when HU is overexpressed and decreased in the hup mutants, presumably reflecting changes in DNA supercoiling. Thus, in contrast to eukaryotic nucleosomes, HU does not impair T7 RNAP activity but has a stimulatory effect. Finally, our results suggest that HU can also influence mRNA stability in vivo.
Bacteriophage T7 RNA polymerase (RNAP), the prototype of T-odd phage-encoded RNAPs, has been the subject of considerable interest over the last 15 years. In vitro, this monomeric enzyme (99 kDa), which is much simpler than cellular RNAPs, can initiate transcription without the help of an additional factor(s) from a specific promoter (PT7) consisting of a highly conserved 21-nucleotide sequence. Its crystal structure has been determined both in isolation (56) and within the initiation complex (10). Virtually any DNA sequence can be efficiently transcribed when fused downstream of PT7, a property which has proven invaluable for RNA studies. In addition, T7 RNAP can be expressed in a variety of hosts, including Escherichia coli, and this feature has been widely exploited for overexpressing genes of interest in these hosts (17, 57, 58).
Because of its simplicity, T7 RNAP is also attractive for investigating fundamental aspects of transcription. In particular, it has been used as a model for studying the effect of histones on this process. In vitro, in a reconstituted system, the presence of a nucleosome core within the promoter region strongly inhibits initiation, whereas nucleosomes are less efficient in inhibiting elongation: readthrough transcription still occurs, but pausing and/or premature termination are enhanced (30, 43). More recently, it has been shown that incorporation of histone H1 into nucleosomes results in a much more drastic inhibition of both initiation and elongation (42). In vivo it was reported that transcription by T7 RNAP is inhibited by nucleosomes in Drosophila cells (38); similarly, in mammalian nuclei, the presence of a nearby enhancer can favor transcription initiation by T7 RNAP, but subsequent elongation is impaired (24).
The natural environment of T7 RNAP in vivo is not the eukaryotic nucleus but rather the prokaryotic “nucleoid.” In E. coli the activity of T7 RNAP may be affected by obstructing proteins, depending upon their location and the tightness of their binding (19, 46). Thus, when bound at the very beginning of the transcribed sequence, the lac repressor causes T7 RNAP to terminate prematurely, whereas it has no effect when bound further downstream. This property, which reflects the sharp increase in the processivity of T7 RNAP after it has transcribed the first few nucleotides, has been used to control PT7 activity in vivo (15, 18, 34). However, it is not known whether the abundant histone-like proteins present in E. coli, such as protein HU, would affect T7 RNAP activity in a similar way.
HU is a basic, thermostable protein composed of two homologous subunits of 9 kDa which exists predominantly in E. coli as a heterodimer (αβ) during the stationary phase (11, 50, 52). HU, one of the most abundant DNA-binding proteins associated with the E. coli nucleoid (51, 60), is well conserved in bacteria, in eukaryotic organelles, and in some viruses (21, 41). Its interactions with DNA have been studied extensively. In vitro, HU binds with a relatively low affinity to linear DNA fragments with a density of one dimer per 9 bp regardless of the sequence or length (6), but it binds more avidly to supercoiled than to relaxed DNA (55). More recently, it was shown that HU binds much more tightly to specific DNA structures such as nicked or junction DNA, single-stranded or double-stranded DNA forks, or a 3′ overhang (25, 26). Finally, like the histones, HU introduces negative supercoils in vitro into a relaxed circular DNA in the presence of topoisomerase I and condenses DNA in pseudo-nucleosome-like particles (53). Whereas in eukaryotic chromatin essentially all of the supercoiling is constrained by proteins, in E. coli cells only half of the supercoiling is constrained (5, 8). Given its abundance and its in vitro properties, HU is a plausible candidate for this role (53). This hypothesis was supported by the fact that HU was shown in vivo to cross talk with the activity of topoisomerase I (4).
To assess the effect of HU on the activity of T7 RNAP in E. coli, we have exploited the techniques available for measuring accurately the level of transcription through the E. coli chromosome (35) and for manipulating the concentration of HU in vivo (54). We report here that the presence of putative HU obstructers has no detectable effect on transcription elongation. Whereas HU does not affect the processivity of elongating T7 RNAP, it clearly stimulates the transcript yield. This stimulation is also observed in vitro when the template is supercoiled but not when it is linear, which seems to link this stimulation to the effect of HU on DNA supercoiling. The molecular mechanisms underlying these effects, which differ from those observed with histones, are discussed below.
MATERIALS AND METHODS
Strains and plasmids.
ENS134 (35) is a derivative of the T7 RNAP-producing strain BL21(DE3) (57); its relevant features are summarized in Fig. 1. Strain ENS305 (this work) differs from ENS0305 (33) by the presence of a chromosome-borne T7 RNAP gene; it was constructed similarly, except that MO20 (35) was the recipient strain in the final transduction.
FIG. 1.
Schematic representation of the strains used here. ENS134 and ENS305 are Lac− derivatives of strain BL21(DE3), which harbors the T7 gene 1 encoding T7 RNAP (thin closed box). The engineered lac operon from ENS134 consists of the T7 late promoter (PT7), the lac leader sequence (open box), the lacZ coding sequence followed by the first third of the lacY sequence (hatched boxes), a tRNA gene used here as a transcriptional reporter (black box), and two tandemly arranged terminators (Ter). Strain ENS305 is identical to ENS134 except for a deletion (dotted lines) that brings the reporter just downsteam of the lac leader. In both strains, the hupA and hupB genes encoding protein HU can either be wild type or transposon interrupted (by Tn9 and Tn5, respectively). Cells also eventually carry multicopy plasmids harboring either the hupA or hupB genes (phup).
Plasmid pMW1 was described previously in (28). Plasmid pJW1 was obtained by inserting the 1.2-kb EcoRI-HindIII fragment from pK01 (27) containing hupA into pBR322 (J. Williams and J. Rouviere-Yaniv, unpublished data). Interruption of the hupA and hupB genes in ENS134 and ENS305 with chloramphenicol or kanamycin resistance cassettes, respectively, was achieved by P1 transduction with the corresponding C600 derivatives as donor strains (22).
For Northern and Western analysis, cells were grown at 37°C to an optical density at 600 nm of 0.5 in M9 medium (40) supplemented with thiamine (1 μg/ml), Casamino Acids (0.2%), glycerol (0.2%), IPTG (isopropyl-β-d-thiogalactopyranoside; 1 mM), and eventually ampicillin (100 μg/ml) for plasmid maintenance.
Northern analysis.
The extraction of RNA, its separation on agarose or acrylamide gels, and its blotting onto a nylon membrane were as described elsewhere, as is the probing of tRNAArg5 and 5S rRNA with complementary 32P-labeled oligonucleotides (33, 35). The lacZ mRNA was probed with a uniformly 32P-labeled 1.8-kb HincII fragment internal to the lacZ gene (33). Radioactive signals were quantified with a BAS 1000 Imager (Fuji).
Western analysis.
Heterodimeric HU was purified according to a previously described method (47). For analysis of HU expression, cells (30-ml culture) were centrifuged, washed, and kept at −20°C before use. Pellets were resuspended in 400 μl of extraction buffer (20 mM Tris HCl, pH 7.5; 400 mM NaCl; 1 mM EDTA), heated for 10 min at 100°C, and centrifuged. The protein content of the supernatant, which contains heat-resistant proteins, including HU, was determined with the Bio-Rad Protein Assay Reagent.
Samples (0.4 μg) were electrophoresed on a 10 to 25% gradient of sodium dodecyl sulfate-polyacrylamide gels (52) and blotted onto nitrocellulose (0.45 mm; Millipore HA) by using a Carboglas transblot apparatus (Schleicher & Schuell). All steps were carried out at room temperature. The membrane was incubated with phosphate-buffered saline containing 5% skimmed milk and then with anti-HU polyclonal antibody (diluted 1/1,000). After being washed, the membrane was incubated with an anti-rabbit immunoglobulin G (Fc)-peroxidase conjugate (at 1/1,000) and washed again; these latter steps were done in phosphate-buffered saline containing 0.8% skimmed milk and 0.1% Tween 20. The peroxidase activity was visualized with a solution of 3,3-diaminobenzidine (0.5 mg/ml)-30% H2O2 (1 ml/ml) in 100 mM Tris HCl (pH 7.6); the reaction was quenched with water. The relative intensities of each band were quantified by scanning with a PhosphorImager (Molecular Dynamics 410 System). The signal intensities are converted into amounts of protein by comparison with standard samples containing known quantities of purified HU. The concentration of pure HU was determined from the absorbance at 230 nm, assuming that a solution of pure HU at 1 mg/ml has an A230 of 2.3.
Calculation of mRNA stability.
Since their rate of synthesis is the same, the steady-state abundances of RNAs that derive from each other by sequential processing are proportional to their respective half-lives. This relationship holds in particular for the lac mRNA and reporter tRNA (Fig. 1). Since the latter is stable, its “half-life” equals the cell doubling time (3); therefore lac mRNA half-life = doubling time × the ratio of the mRNA and tRNA concentrations (Table 1).
TABLE 1.
Transcription efficiency and mRNA stability
| Strain (doubling time [h]) | ENS134 (long construct)
|
ENS305 (short construct)
|
|||
|---|---|---|---|---|---|
| tRNAa (mean concn ± SD) | lacZ mRNA a,b (mean concn ± SD) | mRNA stabilityc | tRNAa (mean concn ± SD) | tRNA (long/short) | |
| Wild type (0.8) | 100d | 100d | 100d | 117 ± 1 | 0.85 ± 0.01 |
| hupA mutant (0.8) | 72 ± 10 | 90 ± 10 | 125 | NDe | ND |
| hupB mutant (0.8) | 76 ± 8 | 90 ± 10 | 118 | ND | ND |
| hupA hupB mutant (1.1) | 59 ± 9 | 70 ± 5 | 163 | 87 ± 5 | 0.68 ± 0.13 |
| Wild type, pBR322 (0.8) | 100d | 100d | 100d | 123 ± 4 | 0.81 ± 0.03 |
| Wild type, phupA (1.2) | 163 ± 12 | 100 ± 10 | 92 | 170 ± 30 | 0.96 ± 0.3 |
| Wild type, phupB (1.0) | 168 ± 30 | 125 ± 12 | 93 | 140 ± 8 | 1.0 ± 0.25 |
tRNA and mRNA concentrations (average of three determinations) are measured with respect to 5S rRNA (see the text).
Summed abundance of the 4.3- and 3.2-kb species (see Fig. 4).
Defined as mRNA × doubling time/tRNA; see Materials and Methods.
Values for wild-type ENS134 cells are arbitrarily set to 100.
ND, not determined.
RESULTS
Effect of HU upon transcription of lacZ gene by T7 RNAP in vivo.
To evaluate the effect of HU upon T7 RNAP transcription in vivo, we started from the E. coli B strain ENS134 (35), a T7 RNAP-producing strain in which T7 RNAP is used to transcribe a chromosomal copy of the lacZ gene. Briefly, the endogenous lacZ gene has been inactivated, and an engineered version of the lac operon from the transcription start point to the middle of the lacY gene has been inserted on the chromosome after fusion to the PT7 promoter. This operon is followed by a modified copy of the E. coli tRNAArg5 gene and then by two transcription terminators (Fig. 1). The tRNA product remains stable after the decay of the transcript so that its accumulation directly reflects the level of transcription in the region where it is inserted, i.e., it serves as the stable transcriptional reporter (35).
Two procedures were used to manipulate the cellular HU content in ENS134. To reduce this content, we inactivated either one or both hup genes by replacing them by the transposon-interrupted alleles hupA::Cm and hupB::Km (17), resulting in hupA, hupB, and hupAB mutants. To increase the cellular HU content, we used multicopy plasmids bearing either the hupA or hupB genes under the control of their own promoters. These pBR322 derivatives, pJW1 and pMW1 (see Materials and Methods), are here named phupA and phupB. The cellular content of HU in these different situations was measured by Western blotting (Fig. 2A). As expected, the expression of HU was either reduced or abolished altogether, respectively, when one or both hup genes were inactivated. Conversely, this expression was increased 2- to 2.5-fold in the presence of plasmids phupA or phupB (Fig. 2A), as already observed in a K-12 background (54). This comparatively modest level of overproduction reflects the auto- and coregulation of HU (32, 54). It is also noteworthy that both the absence of HU and its overproduction resulted in a similar, moderate increase in cell doubling time (Table 1).
FIG. 2.
(A) Western blot showing the abundance of the HU protein in ENS134 derivatives (see Fig. 1) carrying a variable set of hupA or hupB genes, as indicated above each lane. (B) Northern blot showing the abundance of the reporter tRNA and 5S rRNA (arrow) in the same cultures used for panel A.
To test whether variations in HU content affect transcription from PT7, total RNA samples extracted from the same cultures as described above were electrophoresed on agarose or polyacrylamide gels and blotted onto a nylon membrane. In the experiment presented in Fig. 2B, the blots were probed for tRNAArg5, as well as for 5S rRNA, the latter probing being used for normalization purpose (point-to-point total RNA loading). Remarkably, the higher the HU content, the higher the expression of the tRNA relative to 5S rRNA (Fig. 2B), the increase being three- to fourfold from the strain lacking HU to the strains overexpressing it from either phupA or phupB (Table 1). A similar increase was observed in the experiment presented in Fig. 3 (ENS134 lanes). Since the expressions of the tRNA and 5S rRNA reflect the rate of transcription through the PT7 lac operon and through the rRNA gene, respectively, we conclude that the presence of HU stimulates transcription of PT7 controlled lac operon compared to that of the rRNA operons. To confirm the effect of HU on the activity of T7 RNAP, we measured in vitro the transcription yield in function of increasing amounts of HU with two different DNA substrates. When the template was supercoiled, a global rise in the yields of all transcripts was observed, whereas with a linear template such an increase was undetectable (data not shown).
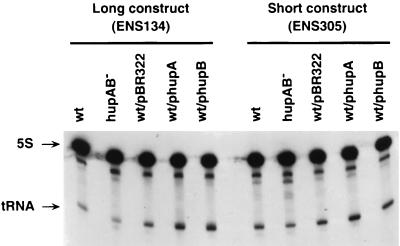
FIG. 3.
Comparison of tRNA expression in derivatives of ENS134 (“long construct”) or ENS305 (“short construct”) carrying a variable set of hupA or hupB genes. RNA separation, blotting, and probing were as in Fig. 2B except that polyacrylamide-urea gels were used in place of agarose gels. All symbols are as described in Fig. 2.
HU does not impair T7 RNAP elongation in vivo.
To test whether the presence or absence of HU affects the processivity of elongating T7 RNAP in vivo, we used strain ENS305, which is isogenic with ENS134 except for the deletion of the lacZ-lacY′ operon (“short transcript” versus “long transcript”; see Fig. 1). As a result of this deletion, the tRNA gene now immediately flanks the PT7 promoter (it extends from nucleotide 37 downward with respect to the transcription start) instead of being located 3.8 kb away. The ratio of the tRNA expression in the two different locations is a measure of the processivity of T7 RNAP over the lengthy lacZ-lacY′ operon (Fig. 1). We then compared the expressions of the tRNA transcriptional reporter (normalized to the 5S rRNA) in the two strains, either mutated for HU or carrying overproducing plasmids.
In hup+ cells harboring the natural content of HU, the expression of the reporter tRNA dropped by ca. 15% in going from the promoter-proximal to the promoter-distal location (ENS305 versus ENS134 lanes in Fig. 3; see also Table 1). This figure, which matches previous estimates, means that 15% of the T7 RNAP molecules that enter the lacZ-lacY′ operon fall off before reaching the end (33). Strikingly, this percentage did not vary significantly when HU was either overexpressed (i.e., in the presence of phupA or phupB) or totally absent (Table 1). Therefore, the presence of obstructing HU molecules over the transcribed template does not disfavor (nor indeed favor) the elongation of T7 RNAP across the template.
HU can destabilize mRNAs.
We next examined the effect of HU upon the steady-state level of the lac mRNA in ENS134 cells. To this end, the same membrane used to assess the effect of HU upon tRNA expression (Fig. 2B) was reprobed with a lacZ internal probe. As seen in Fig. 4, this probing revealed the full-length operon transcript (4.3 kb) and a 3.2-kb species corresponding to the processed lacZ mRNA (23), together with incomplete molecules. Interestingly, the comparison of Fig. 2B and 4 (see Table 1 for quantification of the data) shows that, when the HU content is raised (and regardless of the phup plasmid used for HU overexpression), the level of the 4.3- and 3.2-kb species does not follow the tRNA level but instead remains nearly constant. This observation suggests that, aside from stimulating the transcription of the lacZ gene compared to that of the rRNA operons, HU also destabilizes the lacZ mRNA so that it does not accumulate in proportion to the transcription rate. This inference was confirmed by a quantitative treatment of the data (mRNA stability column in Table 1; see Materials and Methods for details).
FIG. 4.
The same blot used in Fig. 2B was stripped and reprobed with a lacZ internal probe. The locations of the full-length operon transcript (4.3 kb) and the processed lacZ mRNA (3.2 kb) are indicated with arrows.
DISCUSSION
HU does not affect elongating T7 RNAP.
As shown in Fig. 3 and Table 1, the presence (or absence) of HU in E. coli cells does not significantly affect the ability of the elongating T7 RNAP to cross the lac operon. Like other RNAPs, T7 RNAP undergoes a sharp increase in processivity after completion of the transcription of the first few nucleotides (the “initially transcribed sequence”) (33). Because of the position and size of the reporter tRNA, our in vivo approach can only record processivity variations downstream of the initially transcribed sequence. Therefore, these experiments show that, after it has switched to the processive mode, T7 RNAP is insensitive to the presence of obstructing HU molecules in vivo. The same result has been observed with proteins such as the lac repressor which bind tightly to specific DNA sites (18, 34). Presumably, HU dimers, which bind double-stranded DNA with low and uniform affinity (6, 47), are easily ejected by the elongating T7 RNAP and therefore cannot hamper its movement, even though many of them are likely encountered over the lengthy lac operon. This view is in accordance with the hypothesis that HU can jump onto and off of linear or supercoiled DNA (14). A somewhat different result is observed with core histones: in vitro, their presence results in enhanced pausing or premature termination, although readthrough transcription still occurs (30, 43). Presumably, this difference reflects the fact that histones bind DNA much more avidly than does HU (53).
HU stimulates the overall transcript yield from PT7.
Although it has no effect on elongation, the presence of HU increases the transcript yield from T7 RNAP (Fig. 2B and 3). Since this yield is evaluated relative to that of rRNA, this result could in principle reflect a decrease in rRNA synthesis by E. coli RNAP rather than a stimulation of lac mRNA synthesis by T7 RNAP. However, the growth rate of cells that either overexpress HU or lack it altogether is the same, suggesting that they synthesize rRNA at the same rate (7). We therefore infer that HU stimulates the activity of T7 RNAP in vivo. Since elongation is not affected, this stimulation must occur at the initiation level. Consistent with this view, we have found that, in vitro, pure HU can also stimulate transcription initiation by T7 RNAP on supercoiled templates. Again, HU differs markedly from core histones in this respect, since the latter markedly depress transcription initiation by T7 RNAP (30, 43).
At present, we can only speculate about the mechanism whereby HU increases transcription initiation in vivo (or in vitro when the template is supercoiled). The most likely possibility is that the stimulation reflects changes in the DNA topology. In vitro, HU constrains supercoiling by wrapping DNA and therefore reduces free superhelicity if the substrate is supercoiled (53). In vivo, variations in HU content also result in superhelicity changes, although these changes are largely offset by either an increase in topoisomerase I activity or by compensatory mutations in DNA gyrase (4, 36). We hypothesize that HU stimulates the activity of PT7 in vivo by constraining negative DNA supercoiling, thereby decreasing free superhelicity. Consistently, it has been observed that in vitro the transcript yield from PT7 is almost twofold higher when the template is relaxed rather than supercoiled (48). This positive effect of HU would more than compensate for the hindrance to initiation due to the presence of HU dimers in the promoter region. In contrast, with the more tightly bound histones, the hindrance effect would predominate.
That HU affects transcription in vivo is not unprecedented. It was first isolated as a factor enhancing the in vitro transcription of bacteriophage lambda genes by E. coli RNAP (50). Subsequent work has shown that HU can modulate the activity of individual promoters, including the hup promoters (32, 54). Moreover, HU was shown to modulate the interaction of several regulators with their specific sites on DNA. Thus, HU stimulates the binding of lac repressor and CAP to the lac promoter (16), it displaces the LexA repressor from its specific binding sites on the SOS gene promoters (49), and it modulates the repression of the glpD gene (61). HU was also shown to contribute to Gal repression (1) and to Mu transpososome assembly (31).
Regarding this stimulatory effect on transcription, it should be recalled that HU is considered a functional conserved prokaryotic counterpart of a mitochondrial DNA binding protein involved in the control of replication and transcription. In fact, this protein, called HM or ABF2 in yeast and mTFA in higher organisms, is often considered a transcription factor (12, 44). When investigating the possibility that HU, a highly conserved protein present in most bacteria but also in plant chloroplasts, is also present in mitochondria, we isolated the HM protein from yeast mitochondria. HM, like HU (but more efficiently than HU), could introduce negative supercoiling into a relaxed DNA in the presence of a topoisomerase I activity (9). However, HM (mTFA and ABF2) did not show any sequence homology with the HU family but displays, as shown more recently, a high homology with the HMG family of proteins (13, 29). Nevertheless, the functional link between HU and HM was confirmed by several groups which have shown that a deficiency in the yeast protein (HM or ABF2) was complemented not only by its human counterpart mTFA (45) but also by the E. coli HU protein (39). Interestingly, in this context, T7 RNAP is closely related to mitochondrial RNAP (37).
HU can destabilize mRNAs in vivo.
During the course of this work, we unexpectedly observed that the stability of the lac transcript synthesized in ENS134 cells is inversely correlated with the cellular HU content (Table 1). This property is unrelated to the fact that this transcript is synthesized by T7 RNAP: we have previously described a strain (ENS133) that is identical to ENS134 except for the replacement of PT7 by the genuine lac promoter (35). Interestingly, the stability of the lac transcripts from either ENS133 or ENS134 is similarly affected by variations in the cellular HU content (P. Morales, unpublished results). This increase in HU content might affect mRNA stability indirectly by stimulating the synthesis of RNases (e.g., RNase E) that are involved in degradation. Alternatively, HU could act more directly by binding to mRNAs. Indeed, HU was initially characterized as a heat-stable, acid-soluble E. coli protein that binds equally well to double-stranded DNA, single-stranded DNA, or RNA cellulose columns, resembling eukaryotic histones in this respect (6, 53). Interestingly, protein Hfq, another chromosomal protein which binds to both RNA and DNA, has recently attracted attention as an mRNA-destabilizing factor (20, 59). In this respect, it is interesting to recall that HU, like Hfq, has been recently found to stimulate the translation of RpoS, the sigma factor specific for the stationary phase and stress (2).
Acknowledgments
We are grateful to Olivier Pellegrini for providing purified HU protein and to J. Guillerez, P. J. Lopez, J. Oberto, V. Pinson, and L. Kaguni for discussions.
P.M. was supported by postdoctoral fellowships from the Ministère de Affaires Etrangères and from the Fondation pour la Recherche Médicale. This work was funded by the Association de la Recherche contre le Cancer (ARC 5376 and 5474), the Action “Interface Chimie-Physique-Biologie” du Ministère de l'Enseignement Supérieur et de la Recherche (AAC-SV-1995) and the Action Concertée CNRS-ARC 1995 (“Interactions Protéine-protéine”), and by MNERT grants “Recherche Fondamentale en Microbiologie, Maladies Infectieuses et Parasitaires” to J.R.-Y. and M.D.
REFERENCES
- 1.Aki, T., and S. Adhya. 1997. Repressor induced site-specific binding of HU for transcriptional regulation. EMBO J. 16:3666-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balandina, A., and J. Rouviere-Yaniv. 2001. The Escherichia coli histone-like protein HU regulates rpoS translation. Mol. Microbiol. 39:1069-1079. [DOI] [PubMed] [Google Scholar]
- 3.Belasco, J. G., and G. Brawerman. 1993. Experimental approaches to the study of mRNA decay, p. 475-493. In J. G. Belasco and G. Brawerman (ed.), Control of messenger RNA stability. Academic Press, Inc., San Diego, Calif.
- 4.Bensaid, A., A. Almeida, K. Drlica, and J. Rouviere-Yaniv. 1996. Cross-talk between topoisomerase I and HU in Escherichia coli. J. Mol. Biol. 256:292-300. [DOI] [PubMed] [Google Scholar]
- 5.Bliska, J., and N. Cozarelli. 1987. Use of site-specific recombination as a probe of DNA structure and metabolism in vivo. J. Mol. Biol. 94:205-218. [DOI] [PubMed] [Google Scholar]
- 6.Bonnefoy, E., and J. Rouviere-Yaniv. 1991. HU and IHF, two homologous histone-like proteins of Escherichia coli, form different protein-DNA complexes with short DNA fragments. EMBO J. 10:687-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bremer, H., and P. P. Dennis. 1987. Modulation of chemical composition and other parameters of the cell by growth rate, p. 1527-1542. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, vol. 2. ASM Press, Washington, D.C. [Google Scholar]
- 8.Broyles, S., and D. E. Pettijohn. 1986. Interaction of the E. coli HU protein with DNA: evidence for formation of nucleosome-like structures with altered DNA helical pitch. J. Mol. Biol. 187:47-60. [DOI] [PubMed] [Google Scholar]
- 9.Caron, F., C. Jacq, and J. Rouviere-Yaniv. 1979. Characterization of a histone-like protein extracted from yeast mitochondria. Proc. Natl. Acad. Sci. USA 76:4265-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheetham, G. M., and T. A. Steitz. 1999. Structure of a transcribing T7 RNA polymerase initiation complex. Science 286:2305-2309. [DOI] [PubMed] [Google Scholar]
- 11.Claret, L., and J. Rouviere-Yaniv. 1997. Variation in HU composition during growth of Escherichia coli: the heterodimer is required for long term survival. J. Mol. Biol. 273:93-104. [DOI] [PubMed] [Google Scholar]
- 12.Diffey, J. F., and B. Stillman. 1991. A close relative of the nuclear, chromosomal high-mobility group protein HMG1 in yeast mitochondria. Proc. Natl. Acad. Sci. USA 88:7864-7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diffey, J. F., and B. Stillman. 1992. DNA binding properties of an HMG1-related protein from yeast mitochondria. J. Biol. Chem. 267:3368-3374. [PubMed] [Google Scholar]
- 14.Drlica, K., and J. Rouviere-Yaniv. 1987. Histonelike proteins of bacteria. Microbiol. Rev. 51:301-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubendorff, J. W., and W. F. Studier. 1991. Controlling basal expression in an inductible T7 expression system by blocking the target T7 promoter with lac repressor. J. Mol. Biol. 219:45-59. [DOI] [PubMed] [Google Scholar]
- 16.Flashner, Y., and J. D. Gralla. 1988. DNA dynamic flexibility and protein recognition: differential stimulation by bacterial histone-like protein HU. Cell 54:713-721. [DOI] [PubMed] [Google Scholar]
- 17.Fuerst, T. R., and B. Moss. 1989. Structure and stability of mRNA synthesized by vaccinia virus-encoded bacteriophage T7 RNA polymerase in mammalian cells. Importance of the 5′ untranslated leader. J. Mol. Biol. 206:333-348. [DOI] [PubMed] [Google Scholar]
- 18.Giordano, T. J., U. Deuschle, H. Bujard, and W. T. McAllister. 1989. Regulation of coliphage T3 and T7 RNA polymerases by the lac repressor-operator system. Gene 84:209-219. [DOI] [PubMed] [Google Scholar]
- 19.Guajardo, R., and R. Sousa. 1999. Characterization of the effects of Escherichia coli replication terminator protein (Tus) on transcription reveals dynamic nature of tus block to transcription complex progression. Nucleic Acids Res. 27:2814-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hajnsdorf, E., and P. Régnier. 2000. Host factor Hfq of Escherichia coli stimulates elongation of poly(A) tails by poly(A) polymerase I. Proc. Natl. Acad. Sci. USA 97:1501-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haselkorn, R., and J. Rouviere-Yaniv. 1976. Cyanobacterial DNA-binding protein related to Escherichia coli HU. Proc. Natl. Acad. Sci. USA 73:1917-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huisman, O., M. Faelen, D. Girard, A. Toussaint, and J. Rouviere-Yaniv. 1989. Multiple defects in Escherichia coli mutants lacking HU protein. J. Bacteriol. 171:3704-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iost, I., and M. Dreyfus. 1995. The stability of Escherichia coli lacZ mRNA depends upon the simultaneity of its synthesis and translation. EMBO J. 14:3252-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenuwein, T., W. C. Forrester, R.-G. Qiu, and R. Grosschedl. 1993. The immunoglobulin μ enhancer core establishes local factor access in nuclear chromatin independent of transcriptional stimulation. Genes Dev. 7:2016-2032. [DOI] [PubMed] [Google Scholar]
- 25.Kamashev, I., A. Balandina, and J. Rouviere-Yaniv. 1999. The binding motif recognized by HU on both nicked and cruciform DNA. EMBO J. 18:5434-5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamashev, D., and J. Rouviere-Yaniv. 2000. The histone-like protein binds specifically to DNA recombination and repair intermediates. EMBO J. 19:6527-6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kano, Y., K. Osato, M. Wada, and F. Imamoto. 1987. Cloning and sequencing of the HU2 gene of Escherichia coli. Mol. Gen. Genet. 209:408-410. [DOI] [PubMed] [Google Scholar]
- 28.Kano, Y., S. Yoshino, M. Wada, K. Yokoyama, M. Nobuhara, and F. Imamoto. 1985. Molecular cloning and nucleotide sequence of the HU1 gene of Escherichia coli. Mol. Gen. Genet. 201:360-362. [DOI] [PubMed] [Google Scholar]
- 29.Kao, L., T. Megraw, and C. B. Chae. 1993. Essential role of the HMG domain in the function of yeast mitochondrial histone HM: functional complementation of HM by the nuclear nonhistone protein NHP6A. Proc. Natl. Acad. Sci. USA 90:5598-5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirov, N., I. Tsaneva, E. Einbinder, and R. Tsanev. 1992. In vitro transcription through nucleosomes by T7 RNA polymerase. EMBO J. 11:1941-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobryn, K., B. D. Lavoie, and G. Chaconas. 1999. Supercoiling-dependent site-specific binding of HU to naked Mu DNA. J. Mol. Biol. 289:777-784. [DOI] [PubMed] [Google Scholar]
- 32.Kohno, K., M. Wada, Y. Kano, and F. Imamoto. 1990. Promoters and autogenous control of the Escherichia coli hupA and hupB genes. J. Mol. Biol. 213:27-36. [DOI] [PubMed] [Google Scholar]
- 33.Lopez, P. J., J. Guillerez, R. Sousa, and M. Dreyfus. 1997. The low processivity of T7 RNA polymerase over the initially transcribed sequence can limit productive initiation in vivo. J. Mol. Biol. 269:41-51. [DOI] [PubMed] [Google Scholar]
- 34.Lopez, P. J., J. Guillerez, R. Sousa, and M. Dreyfus. 1998. On the mechanism of inhibition of phage T7 RNA polymerase by lac repressor. J. Mol. Biol. 276:861-875. [DOI] [PubMed] [Google Scholar]
- 35.Lopez, P. J., I. Iost, and M. Dreyfus. 1994. The use of a tRNA as a transcriptional reporter: the T7 late promoter is extremely efficient in Escherichia coli but its transcripts are poorly expressed. Nucleic Acids Res. 22:1186-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malik, M., A. Bensaid, J. Rouviere-Yaniv, and K. Dlica. 1996. Histone-like protein HU and bacterial DNA topology: suppression of an HU deficiency by gyrase mutations. J. Mol. Biol. 256:66-76. [DOI] [PubMed] [Google Scholar]
- 37.Masters, B. S., L. L. Stohl, and D. A. Clayton. 1987. Yeast mitochondrial RNA polymerase is homologous to those encoded by bacteriophages T3 and T7. Cell 51:89-99. [DOI] [PubMed] [Google Scholar]
- 38.McCall, K., and W. Bender. 1996. Probes for chromatin accessibility in the Drosophila bithorax complex responds differently to polycomb-mediated repression. EMBO J. 15:569-580. [PMC free article] [PubMed] [Google Scholar]
- 39.Megraw, T. L., and C. B. Chae. 1993. Functional complementarity between the HMG1-like yeast mitochondrial histone HM and the bacterial histone-like protein HU. J. Biol. Chem. 268:12758-12763. [PubMed] [Google Scholar]
- 40.Miller, J. H. 1972. Experiments in molecular biology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 41.Oberto, J., and J. Rouviere-Yaniv. 1996. Serratia marcescens contains a heterodimeric HU protein like Escherichia coli and Salmonella typhimurium. J. Bacteriol. 178:293-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Neill, T. E., G. Meersseman, S. Pennings, and E. M. Bradbury. 1995. Deposition of histone H1 onto reconstituted nucleosome arrays inhibits both initiation and elongation of transcripts by T7 RNA polymerase. Nucleic Acids Res. 23:1075-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Neill, T. E., M. Roberge, and E. M. Bradbury. 1992. Nucleosome arrays inhibit both initiation and elongation of transcripts by bacteriophage T7 RNA polymerase. J. Mol. Biol. 223:67-78. [DOI] [PubMed] [Google Scholar]
- 44.Parisi, M. A., and D. A. Clayton. 1991. Similarity of human mitochondrial transcription factor 1 to high mobility group proteins. Science 252:965-969. [DOI] [PubMed] [Google Scholar]
- 45.Parisi, M. A., B. Xu, and D. A. Clayton. 1993. A human mitochondrial activator can functionally replace a yeast mitochondrial HMG-box protein both in vivo and in vitro. Mol. Cell. Biol. 13:1951-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pavco, P. A., and D. A. Steege. 1991. Characterization of elongating T7 and SP6 RNA polymerases and their response to a roadblock generated by a site-specific DNA binding protein. Nucleic Acids Res. 19:4639-4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pinson, V., M. Takahashi, and J. Rouviere-Yaniv. 1999. Differential binding of the Escherichia coli HU, homodimeric forms and heterodimeric form to linear, gapped and cruciform DNA. J. Mol. Biol. 287:485-497. [DOI] [PubMed] [Google Scholar]
- 48.Portugal, J., and A. Rodriguez-Campos. 1996. T7 RNA polymerase cannot transcribe though a highly knotted DNA template. Nucleic Acids Res. 24:4890-4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Preobrazhenskaya, O., A. Boullard, F. Boubrik, M. Schnarr, and J. Rouviere-Yaniv. 1994. The protein HU can displace the LexA repressor from its DNA binding sites. Mol. Microbiol. 13:459-467. [DOI] [PubMed] [Google Scholar]
- 50.Rouviere-Yaniv, J., and F. Gros. 1975. Characterization of a novel, low-molecular weight DNA-binding protein from Escherichia coli. Proc. Natl. Acad. Sci. USA 22:3428-3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rouviere-Yaniv, J. 1978. Localization of the HU protein on the Escherichia coli. Cold Spring Harbor Symp. Quant. Biol. 42:439-447. [DOI] [PubMed] [Google Scholar]
- 52.Rouviere-Yaniv, J., and N. O. Kjeldgaard. 1979. Native Escherichia coli HU protein is a heterotypic dimer. FEBS Lett. 106:297-300. [DOI] [PubMed] [Google Scholar]
- 53.Rouviere-Yaniv, J., M. Yaniv, and J. E. Germond. 1979. Escherichia coli DNA-binding protein HU forms nucleosome-like structure with circular double-stranded DNA. Cell 17:265-274. [DOI] [PubMed] [Google Scholar]
- 54.Rouviere-Yaniv, J., E. Bonnefoy, O. Huisman, and A. Almeida. 1990. Regulation of HU protein synthesis in E. coli, p. 247-257. In K. Drlica and M. Riley (ed.), The bacterial chromosome. American Society for Microbiology, Washington, D.C.
- 55.Shindo, H., A. Furubayashi, M. Shimizu, M. Miyaka, and F. Imamoto. 1992. Preferential binding of E. coli histone-like protein HUa to negatively supercoiled DNA. Nucleic Acids Res. 20:1553-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sousa, R., Y. J. Chung, J. P. Rose, and B.-C. Wang. 1993. Crystal structure of bacteriophage T7 RNA polymerase at 3.3 Å resolution. Nature 364:593-599. [DOI] [PubMed] [Google Scholar]
- 57.Studier, F. W., A. H. Rosenberg, J. J. Dunn, and J. W. Dubendorff. 1990. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 185:60-89. [DOI] [PubMed] [Google Scholar]
- 58.Tabor, S., and C. C. Richardson. 1985. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc. Natl. Acad. Sci. USA 82:1074-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vytvytska, O., J. S. Jakobsen, G. Balcunaite, J. S. Andersen, M. Baccarini, and A. von Gabain. 1998. Host factor 1, Hfq, binds to Escherichia coli ompA mRNA in a growth-rate dependent fashion and regulates its stability. Proc. Natl. Acad. Sci. USA 95:14118-14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wery, M., C. L. Woldringh, and J. Rouviere-Yaniv. 2001. HU-GFP and DAPI co-localize on the Escherichia coli nucleoid. Biochimie 83:193-200. [DOI] [PubMed] [Google Scholar]
- 61.Yang, B., and T. J. Larson. 1996. Action at a distance for negative control of transcription of the glpD gene encoding sn-glycerol 3-phosphate dehydrogenase of Escherichia coli K-12. J. Bacteriol. 178:7090-7098. [DOI] [PMC free article] [PubMed] [Google Scholar]