Abstract
1. Membrane currents and contractile responses have been measured in ventricular myocardial preparations under voltage clamp conditions.
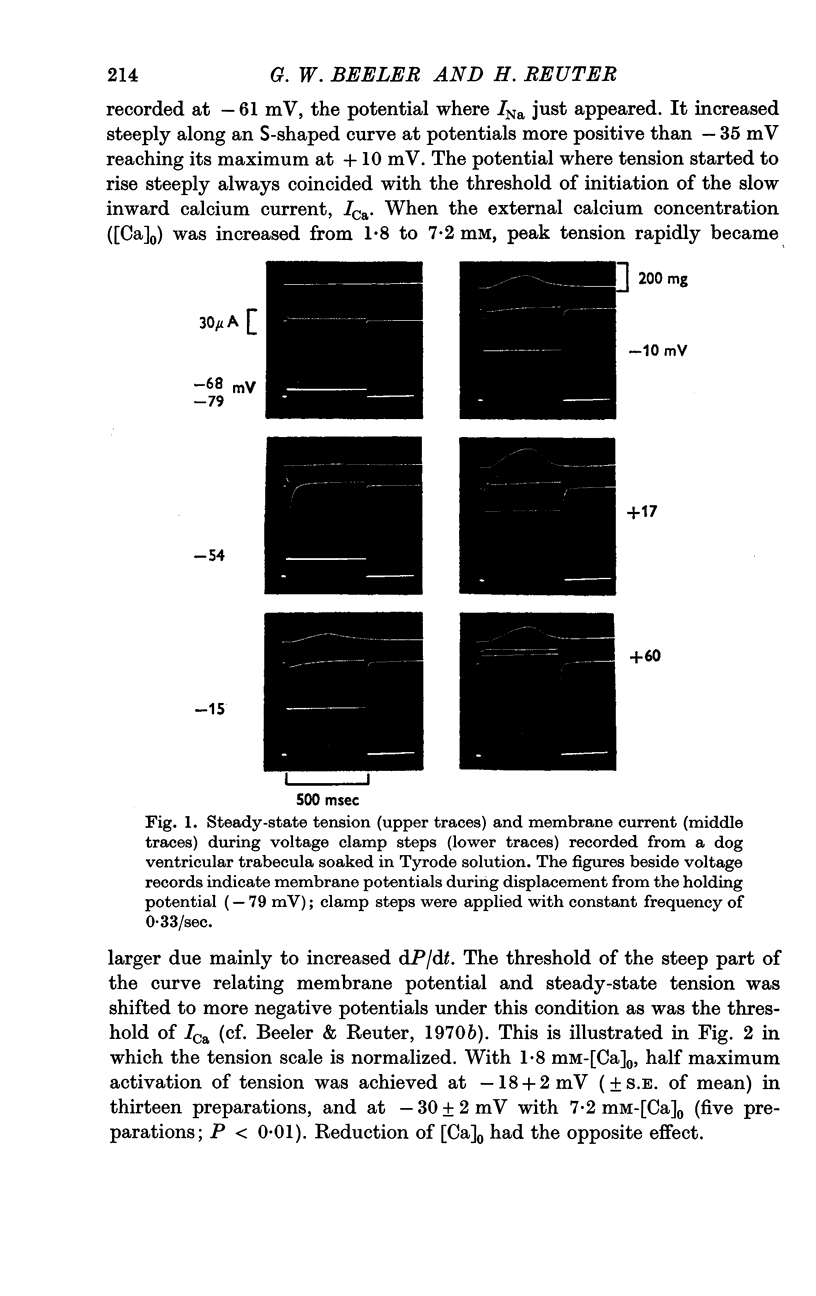
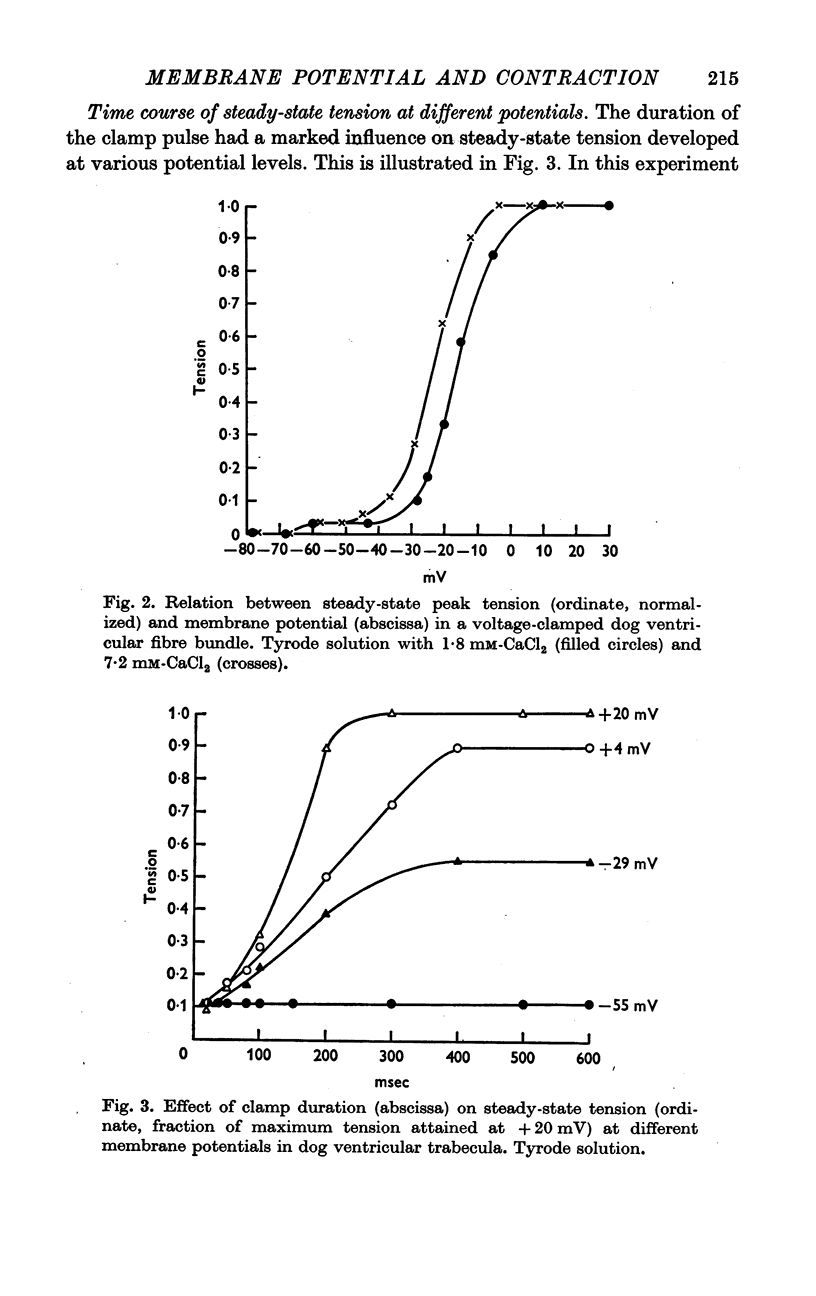
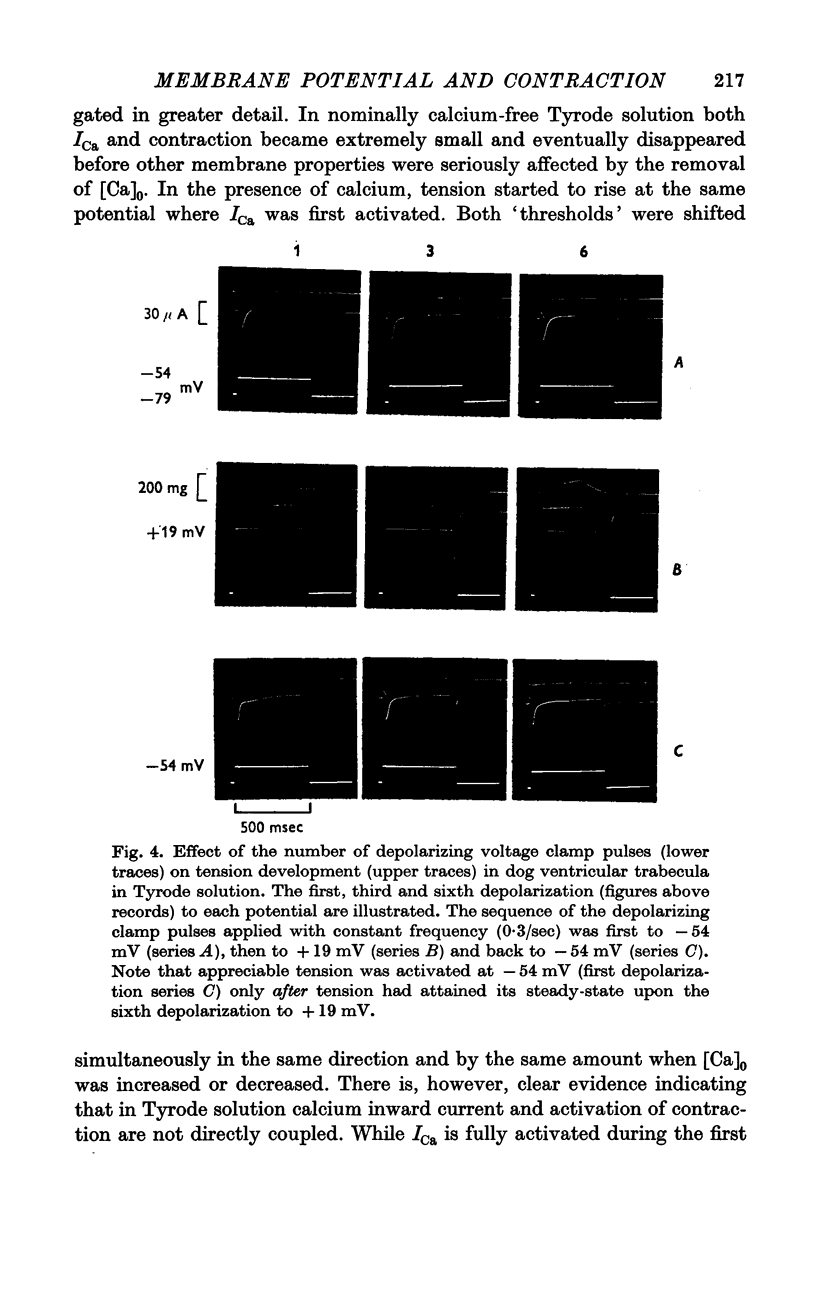
2. In Tyrode solution, steady-state contraction was obtained only after 5-8 depolarizations to a given potential level. The threshold of steady-state tension was identical to the potential where the calcium inward current, ICa, was activated (about -35 mV). Both thresholds were shifted in the same direction along the voltage axis and by the same amount by changing [Ca]o or [Na]o. Maximum tension was obtained at inside positive potentials.
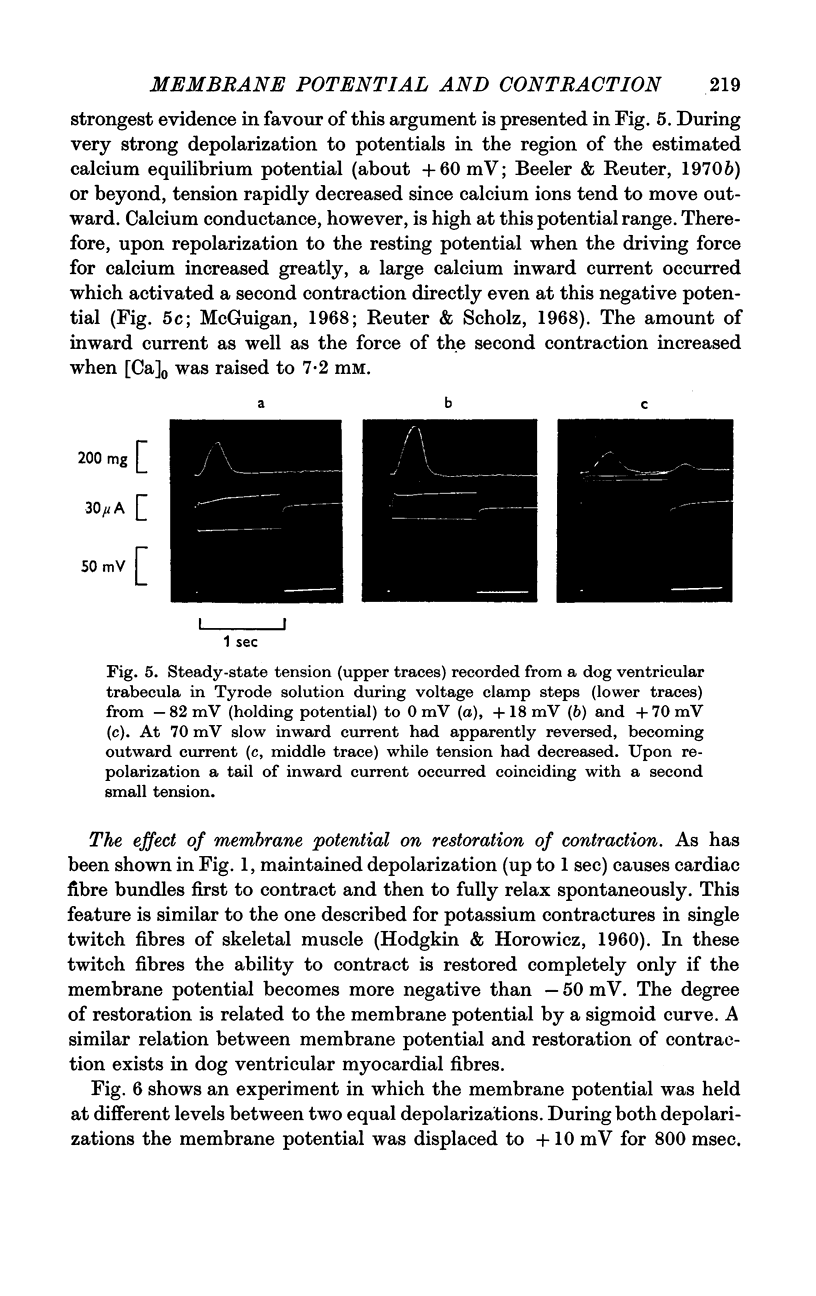
3. The time courses of steady-state tension and of activation of ICa were different by more than one order of magnitude in Tyrode solution. But in order to achieve any appreciable steady-state tension, ICa had to flow during several identical depolarizations. Tension decreased again at potentials above ECa. This suggests that calcium inward current must flow in order to fill intracellular calcium stores from which calcium can be released by an unknown mechanism.
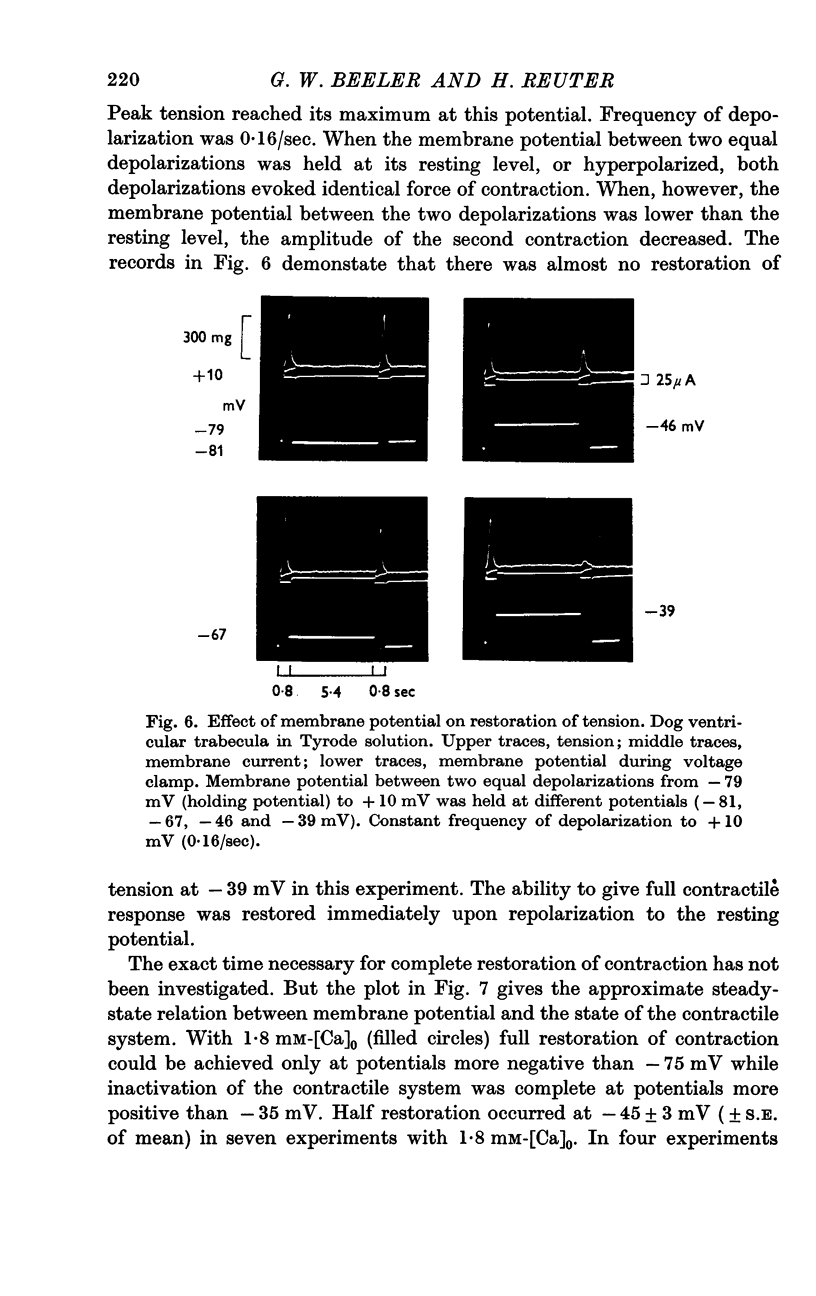
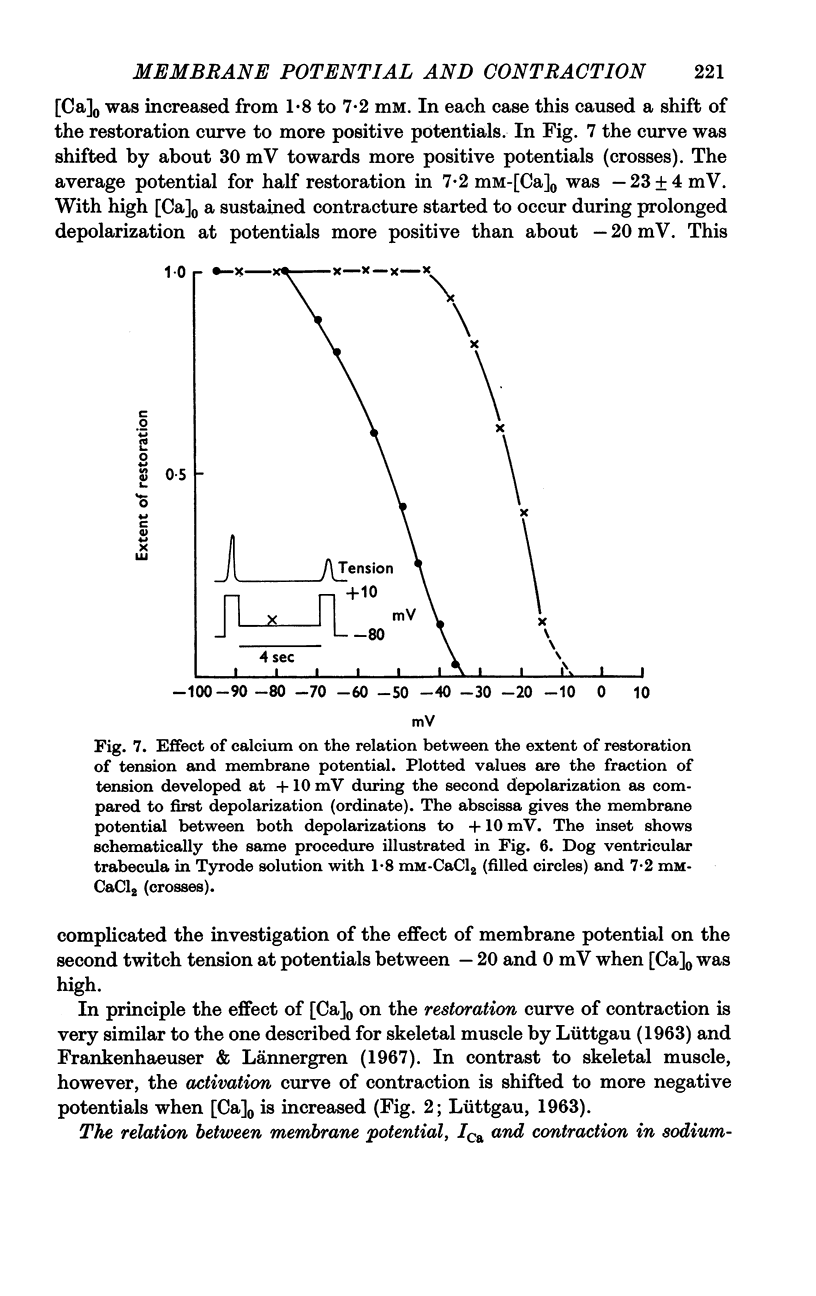
4. The ability of a fibre bundle to contract again after a preceding twitch is greatly dependent on the membrane potential between two equal depolarizations. In Tyrode solutions with 1·8 and 7·2 mM-CaCl2 half restoration of this ability occurred at -45 ± 3 mV (± S.E. of mean) and -23 ± 4 mV, respectively.
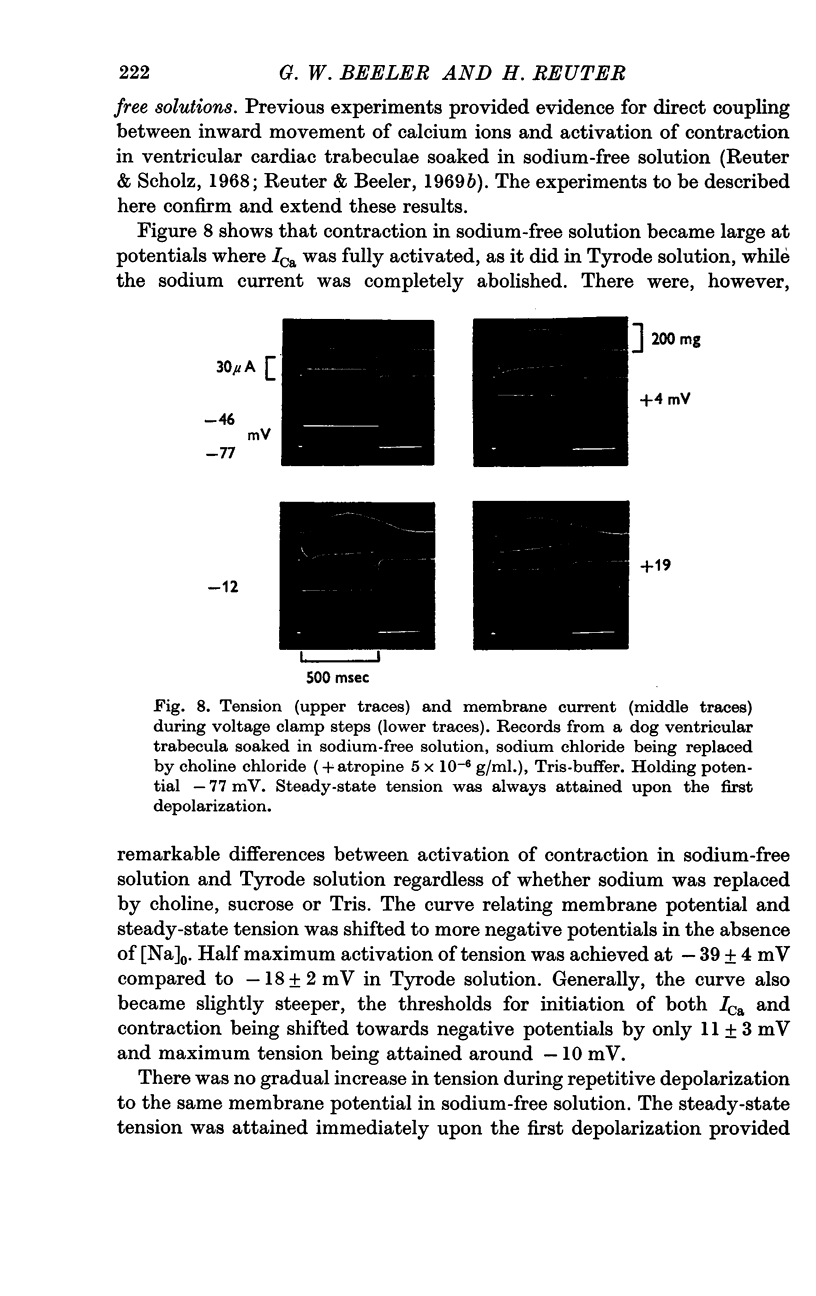
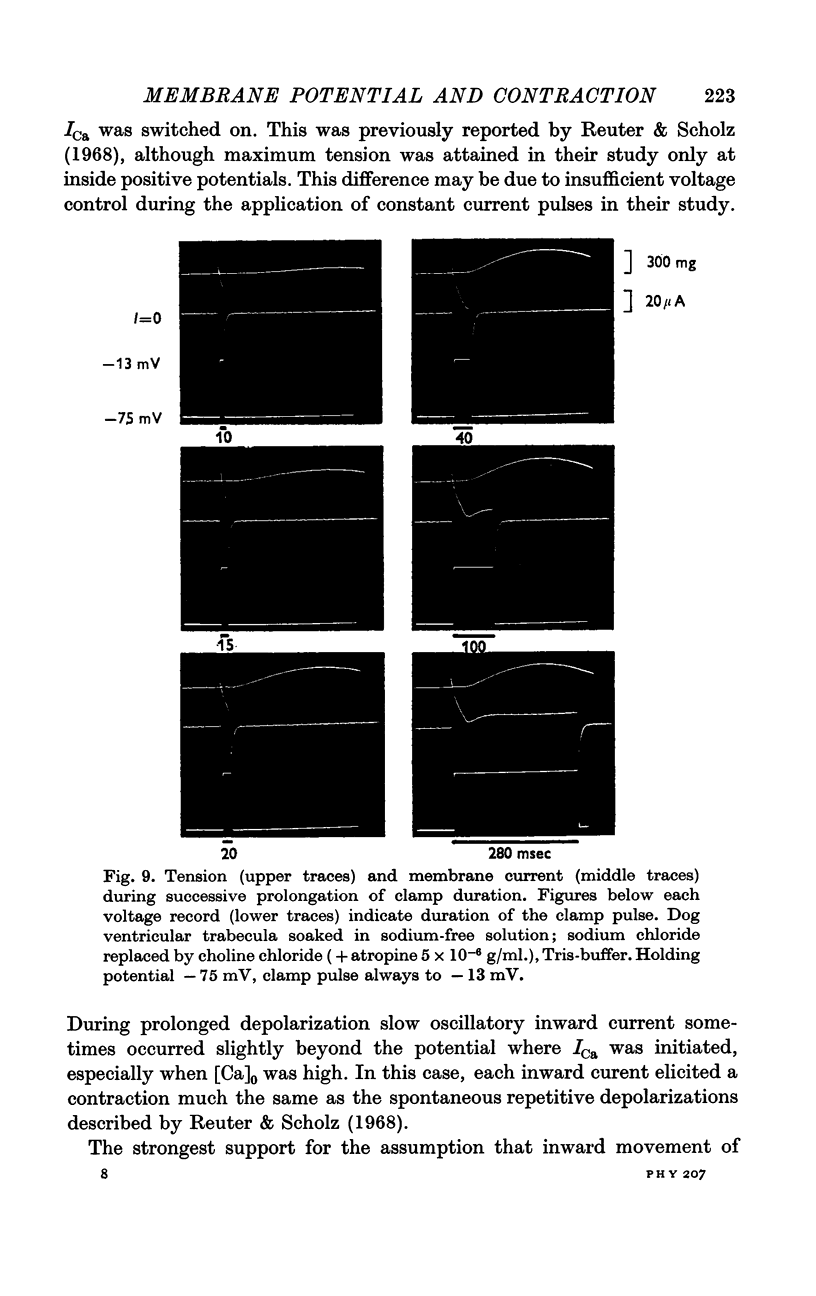
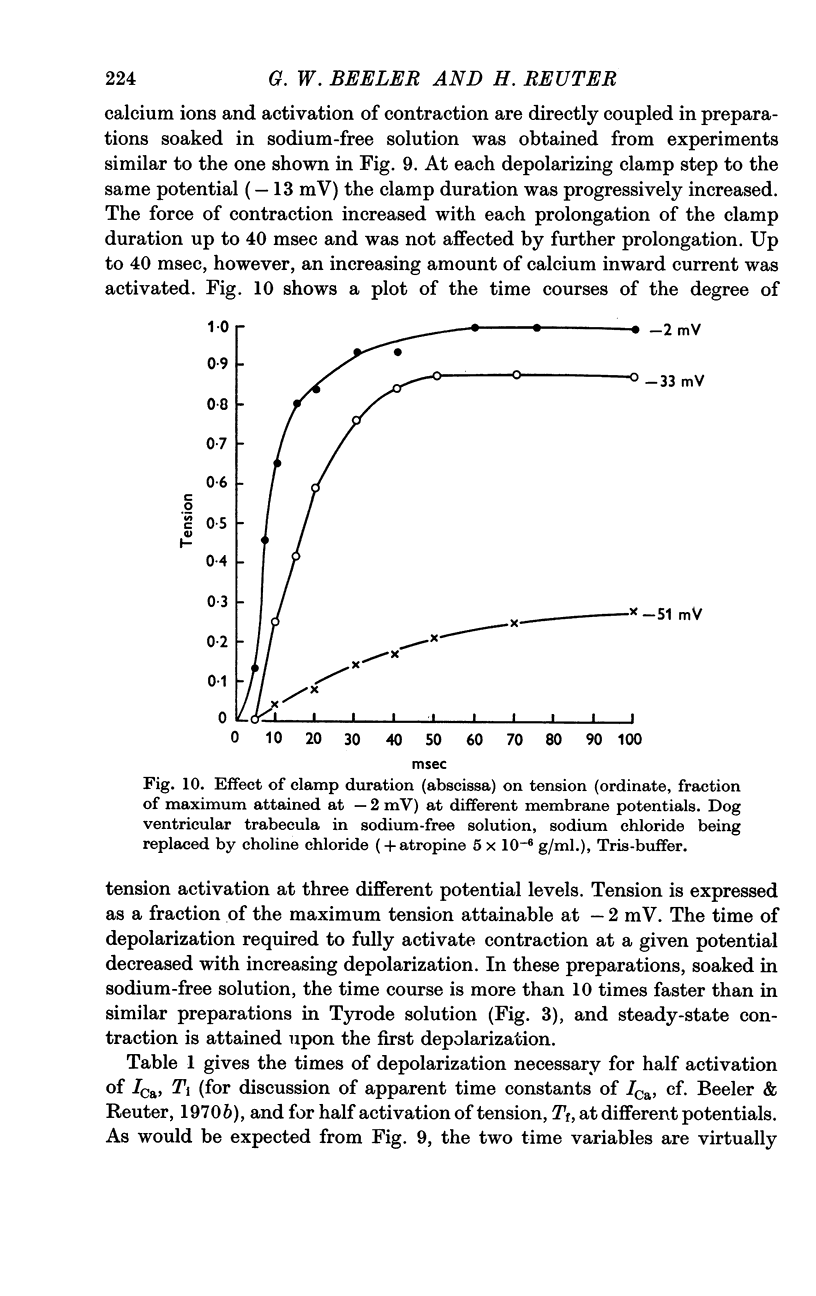
5. In sodium-free bathing solutions, steady-state tension was attained upon the first depolarization provided ICa was activated. Furthermore, at different potentials, the time courses of activation of tension and of activation of ICa were identical, i.e. tension reached its maximum when ICa was fully activated. This suggests that in sodium-free solutions the flow of calcium ions into the fibre directly activates contraction.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoni H., Jacob R., Kaufmann R. Mechanische Reaktionen des Frosch- und Säugetiermyokards bei Veränderung der Aktionspotential-Dauer durch konstante Gleichstromimpulse. Pflugers Arch. 1969;306(1):33–57. doi: 10.1007/BF00586610. [DOI] [PubMed] [Google Scholar]
- BRADY A. J. EXCITATION AND EXCITATION-CONTRACTION COUPLING IN CARDIAC MUSCLE. Annu Rev Physiol. 1964;26:341–356. doi: 10.1146/annurev.ph.26.030164.002013. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. Membrane calcium current in ventricular myocardial fibres. J Physiol. 1970 Mar;207(1):191–209. doi: 10.1113/jphysiol.1970.sp009056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. Voltage clamp experiments on ventricular myocarial fibres. J Physiol. 1970 Mar;207(1):165–190. doi: 10.1113/jphysiol.1970.sp009055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady A. J. Active state in cardiac muscle. Physiol Rev. 1968 Jul;48(3):570–600. doi: 10.1152/physrev.1968.48.3.570. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Fozzard H. A., Hellman D. C. Relationship between membrane voltage and tension in voltage-clamped cardiac purkinje fibres. Nature. 1968 May 11;218(5141):588–589. doi: 10.1038/218588a0. [DOI] [PubMed] [Google Scholar]
- Frankenhaeuser B., Lännergren J. The effect of calcium on the mechanical response of single twitch muscle fibres of Xenopus laevis. Acta Physiol Scand. 1967 Mar;69(3):242–254. doi: 10.1111/j.1748-1716.1967.tb03518.x. [DOI] [PubMed] [Google Scholar]
- Glitsch H. G., Reuter H., Scholz H. Influence of intracellular sodium concentration on calcium influx in isolated guinea pig auricles. Naunyn Schmiedebergs Arch Pharmakol. 1969;264(3):236–237. doi: 10.1007/BF02431433. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell B. R., Blinks J. R. Drugs and the mechanical properties of heart muscle. Annu Rev Pharmacol. 1968;8:113–130. doi: 10.1146/annurev.pa.08.040168.000553. [DOI] [PubMed] [Google Scholar]
- KAVALER F. Membrane depolarization as a cause of tension development in mammalian ventricular muscle. Am J Physiol. 1959 Nov;197:968–970. doi: 10.1152/ajplegacy.1959.197.5.968. [DOI] [PubMed] [Google Scholar]
- LUETTGAU H. C. THE ACTION OF CALCIUM IONS ON POTASSIUM CONTRACTURES OF SINGLE MUSCLE FIBRES. J Physiol. 1963 Oct;168:679–697. doi: 10.1113/jphysiol.1963.sp007215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer G. A. Ion fluxes in cardiac excitation and contraction and their relation to myocardial contractility. Physiol Rev. 1968 Oct;48(4):708–757. doi: 10.1152/physrev.1968.48.4.708. [DOI] [PubMed] [Google Scholar]
- Morad M., Trautwein W. The effect of the duration of the action potential on contraction in the mammalian heart muscle. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;299(1):66–82. doi: 10.1007/BF00362542. [DOI] [PubMed] [Google Scholar]
- NIEDERGERKE R. MOVEMENTS OF CA IN FROG HEART VENTRICLES AT REST AND DURING CONTRACTURES. J Physiol. 1963 Jul;167:515–550. doi: 10.1113/jphysiol.1963.sp007166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERGERKE R. Movements of Ca in beating ventricles of the frog heart. J Physiol. 1963 Jul;167:551–580. doi: 10.1113/jphysiol.1963.sp007167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkand R. K. Facilitation of heart muscle contraction and its dependence on external calcium and sodium. J Physiol. 1968 May;196(2):311–325. doi: 10.1113/jphysiol.1968.sp008509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter M. Der Einfluss der Natriumionen auf die Beziehung zwischen Frequenz und Kraft der Kontraktion des isolierten Meerschweinchenmyokards. Naunyn Schmiedebergs Arch Pharmakol Exp Pathol. 1966;254(3):261–286. [PubMed] [Google Scholar]
- Reuter H., Beeler G. W., Jr Calcium current and activation of contraction in ventricular myocardial fibers. Science. 1969 Jan 24;163(3865):399–401. doi: 10.1126/science.163.3865.399. [DOI] [PubMed] [Google Scholar]
- Reuter H., Beeler G. W., Jr Sodium current in ventricular myocardial fibers. Science. 1969 Jan 24;163(3865):397–399. doi: 10.1126/science.163.3865.397. [DOI] [PubMed] [Google Scholar]
- Reuter H., Scholz H. Uber den Einfluss der extracellulären Ca-Konzentration auf Membranpotential und Kontraktion isolierter Herzpräparate bei graduierter Depolarisation. Pflugers Arch Gesamte Physiol Menschen Tiere. 1968;300(2):87–107. [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley N. A., Benson E. S. The ultrastructure of frog ventricular cardiac muscle and its relationship to mechanism of excitation-contraction coupling. J Cell Biol. 1968 Jul;38(1):99–114. doi: 10.1083/jcb.38.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDMANN S. Effect of increasing the calcium concentration during a single heart-beat. Experientia. 1959 Apr 15;15(4):128–128. doi: 10.1007/BF02165518. [DOI] [PubMed] [Google Scholar]
- WINEGRAD S., SHANES A. M. Calcium flux and contractility in guinea pig atria. J Gen Physiol. 1962 Jan;45:371–394. doi: 10.1085/jgp.45.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winegrad S. Intracellular calcium movements of frog skeletal muscle during recovery from tetanus. J Gen Physiol. 1968 Jan;51(1):65–83. doi: 10.1085/jgp.51.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E. H., Heppner R. L., Weidmann S. Inotropic effects of electric currents. I. Positive and negative effects of constant electric currents or current pulses applied during cardiac action potentials. II. Hypotheses: calcium movements, excitation-contraction coupling and inotropic effects. Circ Res. 1969 Mar;24(3):409–445. doi: 10.1161/01.res.24.3.409. [DOI] [PubMed] [Google Scholar]



