Abstract
1. The influx of 32P, applied externally as orthophosphate, into the axoplasm of squid giant axons has been studied.
2. An average orthophosphate influx of 20·9 f-mole/cm2.sec is obtained if the 32P found in the axoplasm is assumed to be indicative of orthophosphate which has crossed the axolemma.
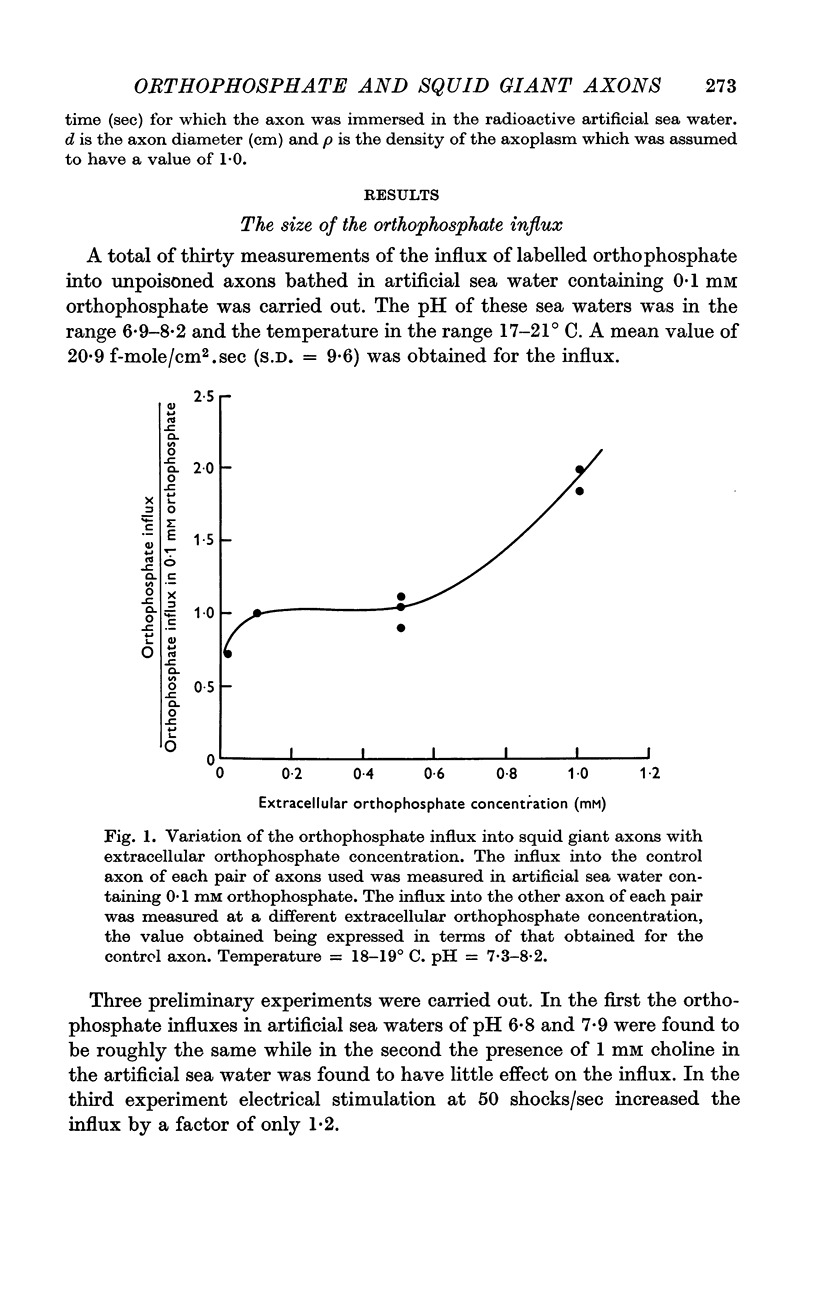
3. The influx does not show very much dependence on external orthophosphate concentration in the range 0·02-0·5 mM.
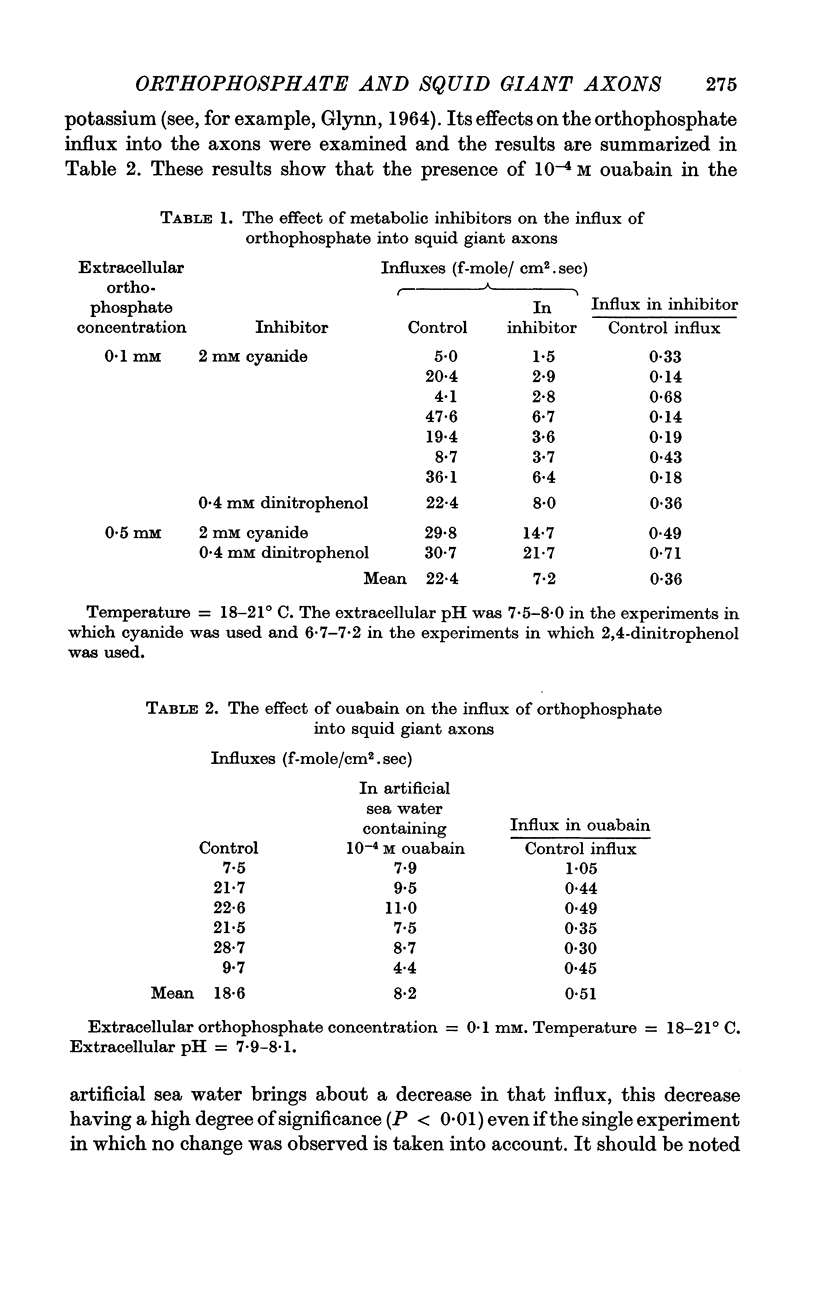
4. The influx is reduced by cyanide, 2,4-dinitrophenol, ouabain and by the absence of external potassium.
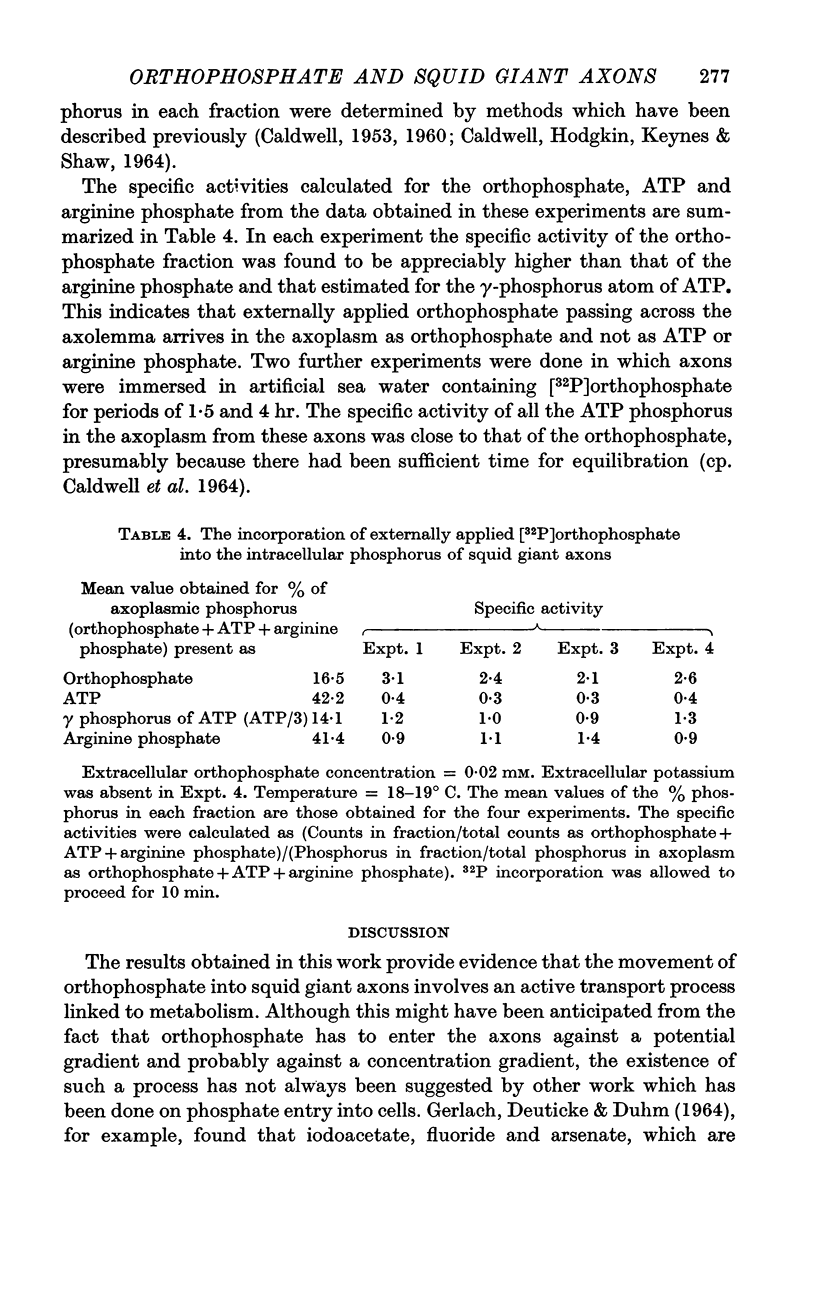
5. The 32P appears to arrive in the axoplasm as orthophosphate.
6. It is concluded that there is an inward movement of orthophosphate into the axons which is mediated by an active transport process and that this may have some connexion with the active transport of sodium and potassium.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the axoplasm of giant nerve fibres with artificial solutions. J Physiol. 1962 Nov;164:330–354. doi: 10.1113/jphysiol.1962.sp007025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER P. F. THE RELATIONSHIP BETWEEN PHOSPHORUS METABOLISM AND THE SODIUM PUMP IN INTACT CRAB NERVE. Biochim Biophys Acta. 1963 Sep 24;75:287–289. doi: 10.1016/0006-3002(63)90613-9. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Shaw T. I. A comparison of the phosphorus metabolism of intact squid nerve with that of the isolated axoplasm and sheath. J Physiol. 1965 Sep;180(2):424–438. doi: 10.1113/jphysiol.1965.sp007710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C., HODGKIN A. L., KEYNES R. D., SHAW T. I. Partial inhibition of the active transport of cations in the giant axons of Loligo. J Physiol. 1960 Jul;152:591–600. doi: 10.1113/jphysiol.1960.sp006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C., HODGKIN A. L., KEYNES R. D., SHAW T. I. THE RATE OF FORMATION AND TURNOVER OF PHOSPHORUS COMPOUNDS IN SQUID GIANT AXONS. J Physiol. 1964 May;171:119–131. doi: 10.1113/jphysiol.1964.sp007366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C., HODGKIN A. L., KEYNES R. D., SHAW T. L. The effects of injecting 'energy-rich' phosphate compounds on the active transport of ions in the giant axons of Loligo. J Physiol. 1960 Jul;152:561–590. doi: 10.1113/jphysiol.1960.sp006509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C. The phosphorus metabolism of squid axons and its relationship to the active transport of sodium. J Physiol. 1960 Jul;152:545–560. doi: 10.1113/jphysiol.1960.sp006508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C. The separation of the phosphate esters of muscle by paper chromatography. Biochem J. 1953 Oct;55(3):458–467. doi: 10.1042/bj0550458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAUSEY G., HARRIS E. J. The uptake and loss of phosphate by frog muscle. Biochem J. 1951 Jul;49(2):176–182. doi: 10.1042/bj0490176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLECKENSTEIN A., JANKE J., DAVIES R. E. Der Austausch von radioaktivem Phosphat mit dem alpha-, beta- und gamma-Phosphor von ATP und mit Kreatinphosphat bei der Kontraktur des Froschrectus durch Acetylcholin, Nicotin und Succinylbischolin. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1956;228(6):596–614. [PubMed] [Google Scholar]
- GERLACH E., DEUTICKE B., DUHM J. PHOSPHAT-PERMEABILITAET UND PHOSPHAT-STOFFWECHSEL MENSCHLICHER ERYTHROCYTEN UND MOEGLICHKEITEN IHRER EXPERIMENTELLEN BEEINFLUSSUNG. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Jul 30;280:243–274. [PubMed] [Google Scholar]
- GLYNN I. M. THE ACTION OF CARDIAC GLYCOSIDES ON ION MOVEMENTS. Pharmacol Rev. 1964 Dec;16:381–407. [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Active transport of cations in giant axons from Sepia and Loligo. J Physiol. 1955 Apr 28;128(1):28–60. doi: 10.1113/jphysiol.1955.sp005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. The potassium permeability of a giant nerve fibre. J Physiol. 1955 Apr 28;128(1):61–88. doi: 10.1113/jphysiol.1955.sp005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke J., Fleckenstein A., Marmier P., Koenig L. Steigerung der absoluten ATP-Umsetzungsrate in der isolierten Skeletmuskulatur unter dem einfluss von 2,4-Dinitrophenol. Turnover-Studien mit P32-markiertem Orthophosphat und H2O18. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;287(1):9–28. [PubMed] [Google Scholar]


