Abstract
1. 42K has been used to study the ouabain-sensitive component of potassium efflux from intact human red cells.
2. Ouabain-sensitive efflux of potassium was observed only in media containing either sodium ions in moderate or high concentration or potassium ions.
3. The effects of sodium and potassium in the medium were not additive. Potassium ions always increased the ouabain-sensitive potassium efflux, but in media containing 4·2 mM-K an increase in sodium concentration from 4·5 to 131 mM had little effect.
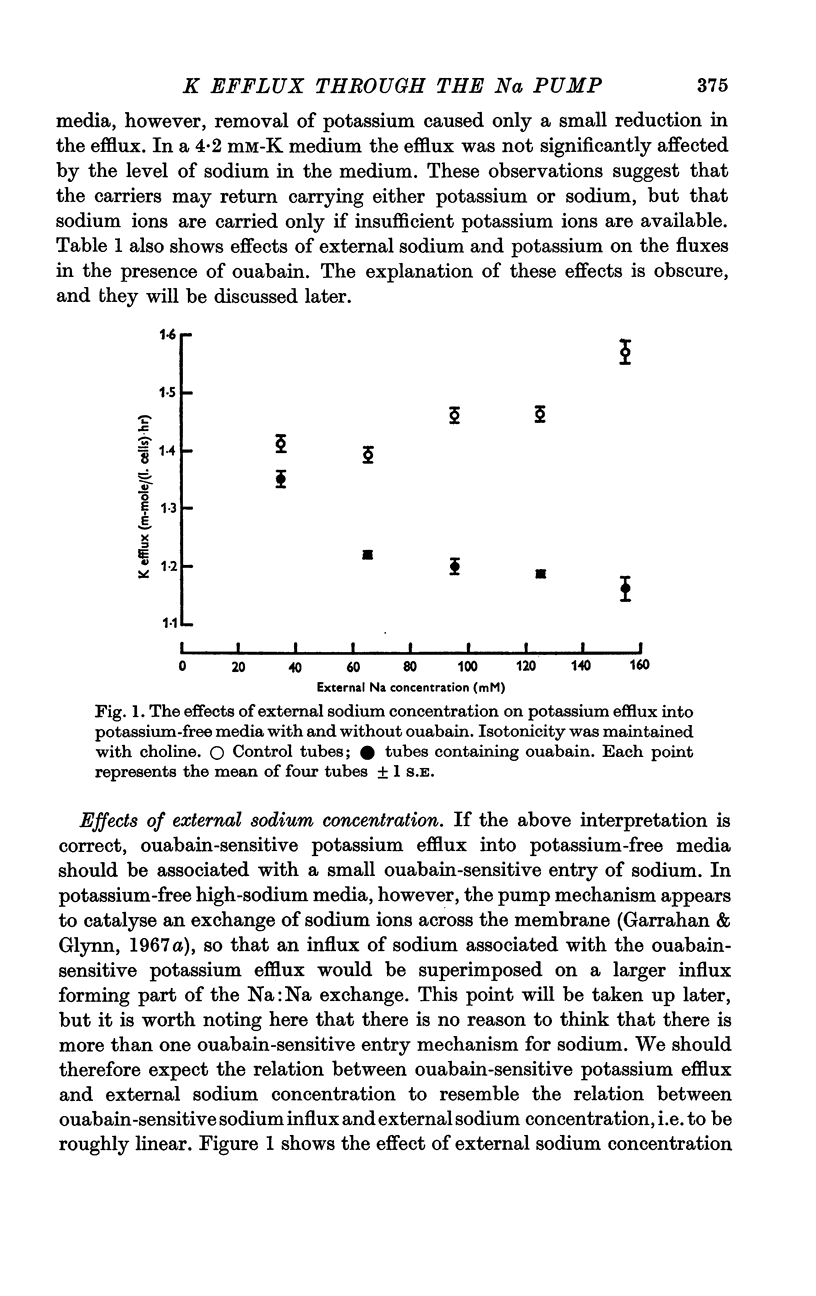
4. In potassium-free media, ouabain-sensitive potassium efflux increased roughly linearly as the external sodium concentration was increased from 35 to 155 mM.
5. The sensitivity of potassium efflux to external potassium depended on the concentration of sodium in the medium. In a 5 mM-Na (choline) medium, ouabain-sensitive potassium efflux was half-maximal at about 0·27 mM-K. In a 150 mM-Na medium the effect of potassium was half-maximal at about 1 mM-K. The relation between external potassium concentration and ouabain-sensitive sodium efflux was similarly influenced by the concentration of sodium ions in the medium.
6. Inosine greatly reduced the ouabain-sensitive efflux of potassium into both 5 mM-K and potassium-free media. It probably acted by reducing the intracellular phosphate concentration to a low level.
7. Ouabain-sensitive potassium efflux was not affected by the concentration of inorganic phosphate outside the cells and was not associated with a ouabain-sensitive efflux of phosphate of comparable magnitude. A small associated efflux could not be excluded.
8. Simultaneous measurements of sodium efflux and of potassium efflux from identical batches of cells incubated in potassium-free media showed that inosine reduced ouabain-sensitive potassium efflux at the same time as it increased ouabain-sensitive sodium efflux.
9. When cells that had been largely depleted of energy stores by pre-incubation with 2-deoxyglucose were incubated in high-sodium media, with and without potassium, it was observed that potassium in the medium increased the ouabain-sensitive potassium efflux but reduced the ouabain-sensitive efflux of sodium.
10. Simultaneous measurements of the ouabain-sensitive efflux of potassium and influx and efflux of sodium across the membranes of starved cells incubated in potassium-free media, with and without inosine, suggested that ouabain-sensitive potassium efflux was associated with a net ouabain-sensitive entry of sodium.
11. The results are best explained by supposing that the ouabain-sensitive efflux of potassium does not reflect lack of discrimination by the mechanism responsible for sodium expulsion, but is brought about by the reversal of steps in the pump cycle normally responsible for potassium entry.
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers R. W., Koval G. J., Siegel Studies on the interaction of ouabain and other cardio-active steroids with sodium-potassium-activated adenosine triphosphatase. Mol Pharmacol. 1968 Jul;4(4):324–336. [PubMed] [Google Scholar]
- GLYNN I. M. Sodium and potassium movements in human red cells. J Physiol. 1956 Nov 28;134(2):278–310. doi: 10.1113/jphysiol.1956.sp005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLYNN I. M. The action of cardiac glycosides on sodium and potassium movements in human red cells. J Physiol. 1957 Apr 3;136(1):148–173. doi: 10.1113/jphysiol.1957.sp005749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. Facftors affecting the relative magnitudes of the sodium:potassium and sodium:sodium exchanges catalysed by the sodium pump. J Physiol. 1967 Sep;192(1):189–216. doi: 10.1113/jphysiol.1967.sp008296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The behaviour of the sodium pump in red cells in the absence of external potassium. J Physiol. 1967 Sep;192(1):159–174. doi: 10.1113/jphysiol.1967.sp008294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The sensitivity of the sodium pump to external sodium. J Physiol. 1967 Sep;192(1):175–188. doi: 10.1113/jphysiol.1967.sp008295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Lew V. L. Affinities or apparent affinities of the transport adenosine triphosphatase system. J Gen Physiol. 1969 Jul 1;54(1):289–305. doi: 10.1085/jgp.54.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Lew V. L. Potassium efflux associated with partial or complete reversal of the sodium pump in intact human red cells. J Physiol. 1969 Jun;202(2):89P–90P. [PubMed] [Google Scholar]
- Glynn I. M., Lew V. L. Synthesis of adenosine triphosphate at the expense of downhill cation movements in intact human red cells. J Physiol. 1970 Apr;207(2):393–402. doi: 10.1113/jphysiol.1970.sp009068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Lüthi U. The Relation between Ouabain-Sensitive Potassium Efflux and the Hypothetical Dephosphorylation Step in the "Transport ATPase" System. J Gen Physiol. 1968 May 1;51(5):385–391. [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Lüthli U. Can the later stages of the 'transport ATPase' system be reversed independently of the earlier stages? J Physiol. 1967 Jul;191(2):104P–105P. [PubMed] [Google Scholar]
- Glynn I. M. Membrane adenosine triphosphatase and cation transport. Br Med Bull. 1968 May;24(2):165–169. doi: 10.1093/oxfordjournals.bmb.a070620. [DOI] [PubMed] [Google Scholar]
- HOFFMAN J. F. The active transport of sodium by ghosts of human red blood cells. J Gen Physiol. 1962 May;45:837–859. doi: 10.1085/jgp.45.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J. F., Kregenow F. M. The characterization of new energy dependent cation transport processes in red blood cells. Ann N Y Acad Sci. 1966 Jul 14;137(2):566–576. doi: 10.1111/j.1749-6632.1966.tb50182.x. [DOI] [PubMed] [Google Scholar]
- Lindenmayer G. E., Laughter A. H., Schwartz A. Incorporation of inorganic phosphate-32 into a Na+, K+-ATPase preparation: stimulation by ouabain. Arch Biochem Biophys. 1968 Sep 20;127(1):187–192. doi: 10.1016/0003-9861(68)90215-4. [DOI] [PubMed] [Google Scholar]
- POST R. L., MERRITT C. R., KINSOLVING C. R., ALBRIGHT C. D. Membrane adenosine triphosphatase as a participant in the active transport of sodium and potassium in the human erythrocyte. J Biol Chem. 1960 Jun;235:1796–1802. [PubMed] [Google Scholar]
- POST R. L., SEN A. K., ROSENTHAL A. S. A PHOSPHORYLATED INTERMEDIATE IN ADENOSINE TRIPHOSPHATE-DEPENDENT SODIUM AND POTASSIUM TRANSPORT ACROSS KIDNEY MEMBRANES. J Biol Chem. 1965 Mar;240:1437–1445. [PubMed] [Google Scholar]
- Priestland R. N., Whittam R. Sodium movements in human red cells in the absence of external potassium. J Physiol. 1969 Sep;204(1):49P–50P. [PubMed] [Google Scholar]
- Priestland R. N., Whittam R. The influence of external sodium ions on the sodium pump in erythrocytes. Biochem J. 1968 Sep;109(3):369–374. doi: 10.1042/bj1090369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIL-MALHERBE H., GREEN R. H. The catalytic effect of molybdate on the hydrolysis of organic phosphate bonds. Biochem J. 1951 Aug;49(3):286–292. [PMC free article] [PubMed] [Google Scholar]


