Abstract
1. A study was made of functional and structural changes during degeneration of end-plates in the rat diaphragm after phrenic nerve section at two levels.
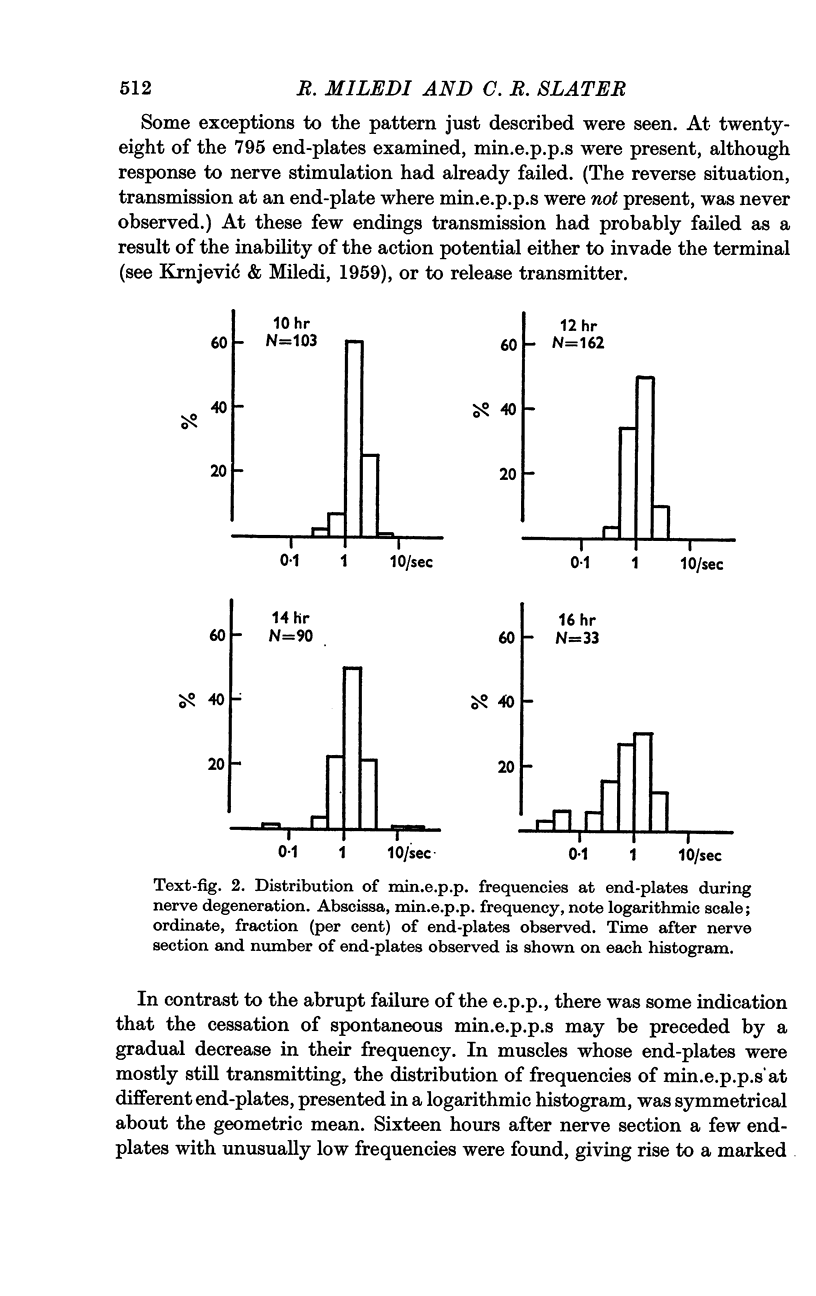
2. For 8-10 hr after cutting the nerve in the neck, all end-plates retain the ability to transmit impulses. During the following 8-10 hr, an increasing number of end-plates lose this ability so that after a total of about 20 hr, no end-plates can transmit.
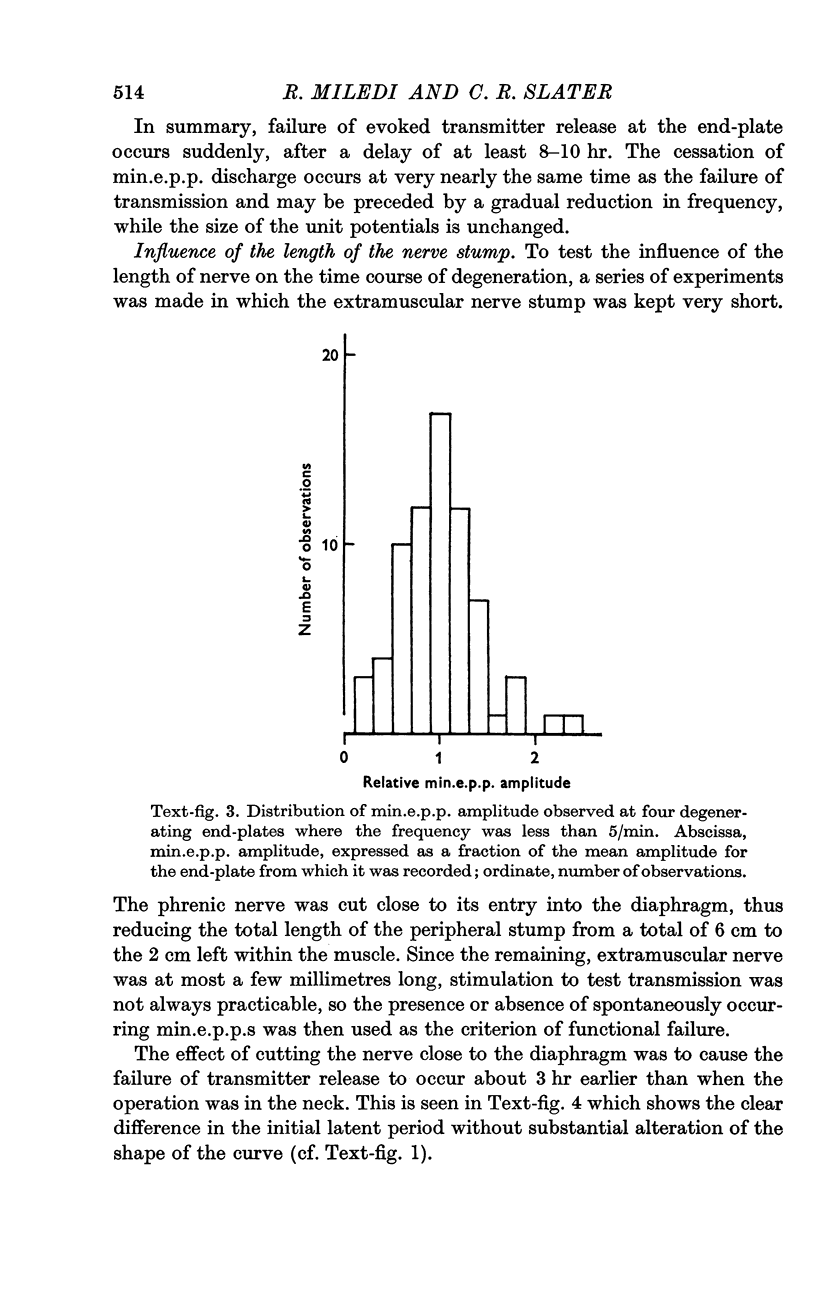
3. Transmission failure occurs abruptly at most end-plates. This failure is usually accompanied by cessation of spontaneous miniature end-plate potentials (min.e.p.p.s), though in a few cases min.e.p.p.s persist after junctional transmission has failed. Several degenerating junctions were observed where the frequency of min.e.p.p.s was very low, suggesting an intermediate stage in min.e.p.p. failure.
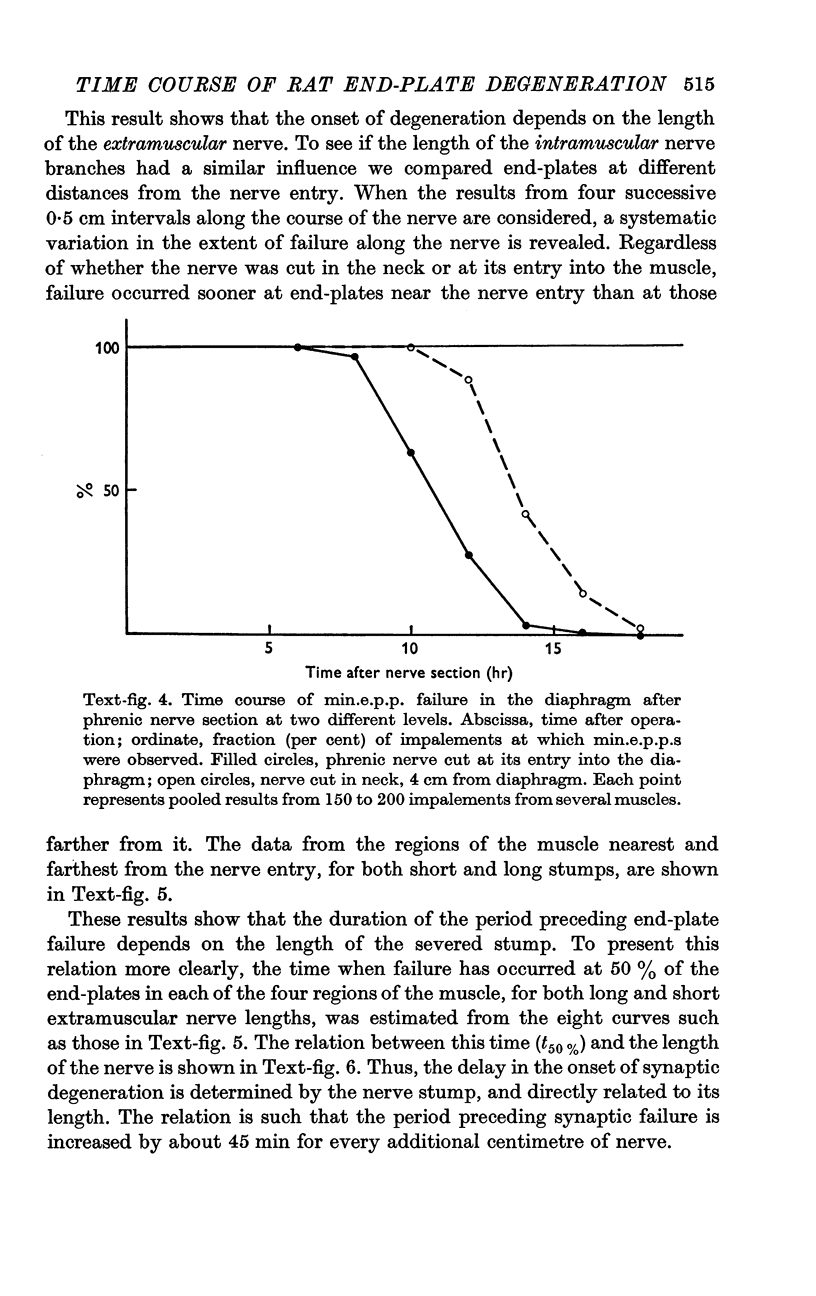
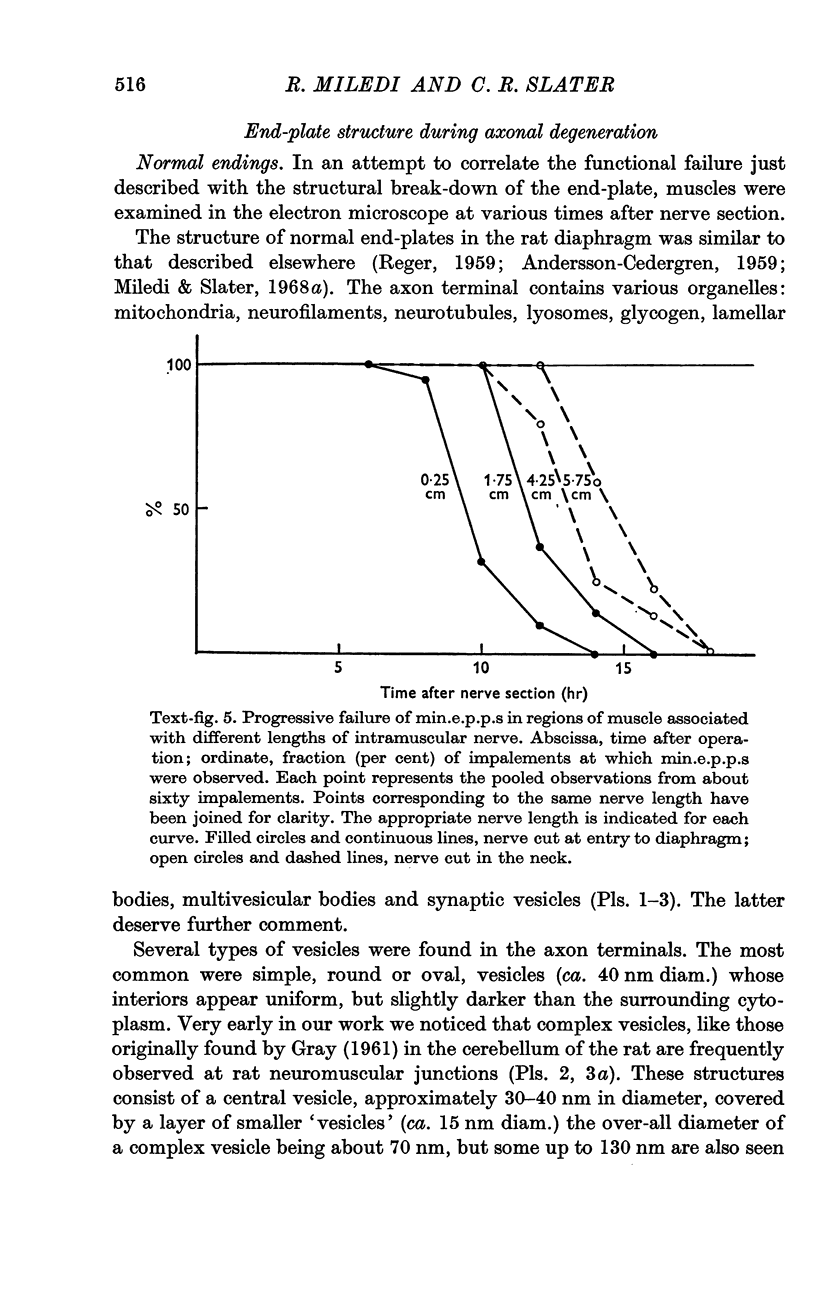
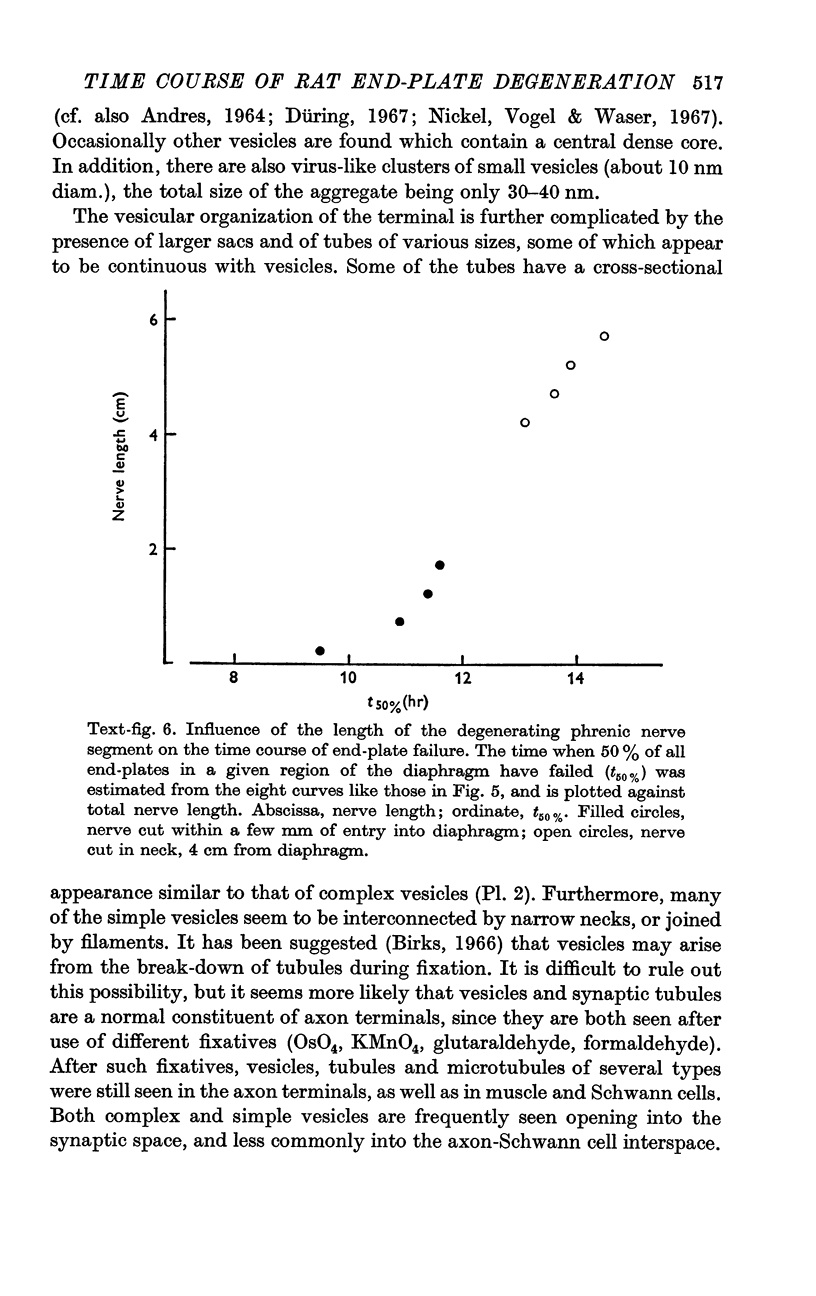
4. The time of junctional failure depends on the length of the degenerating nerve stump. For each additional centimetre of nerve, failure is delayed about 45 min.
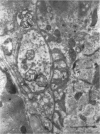
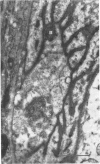
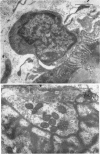
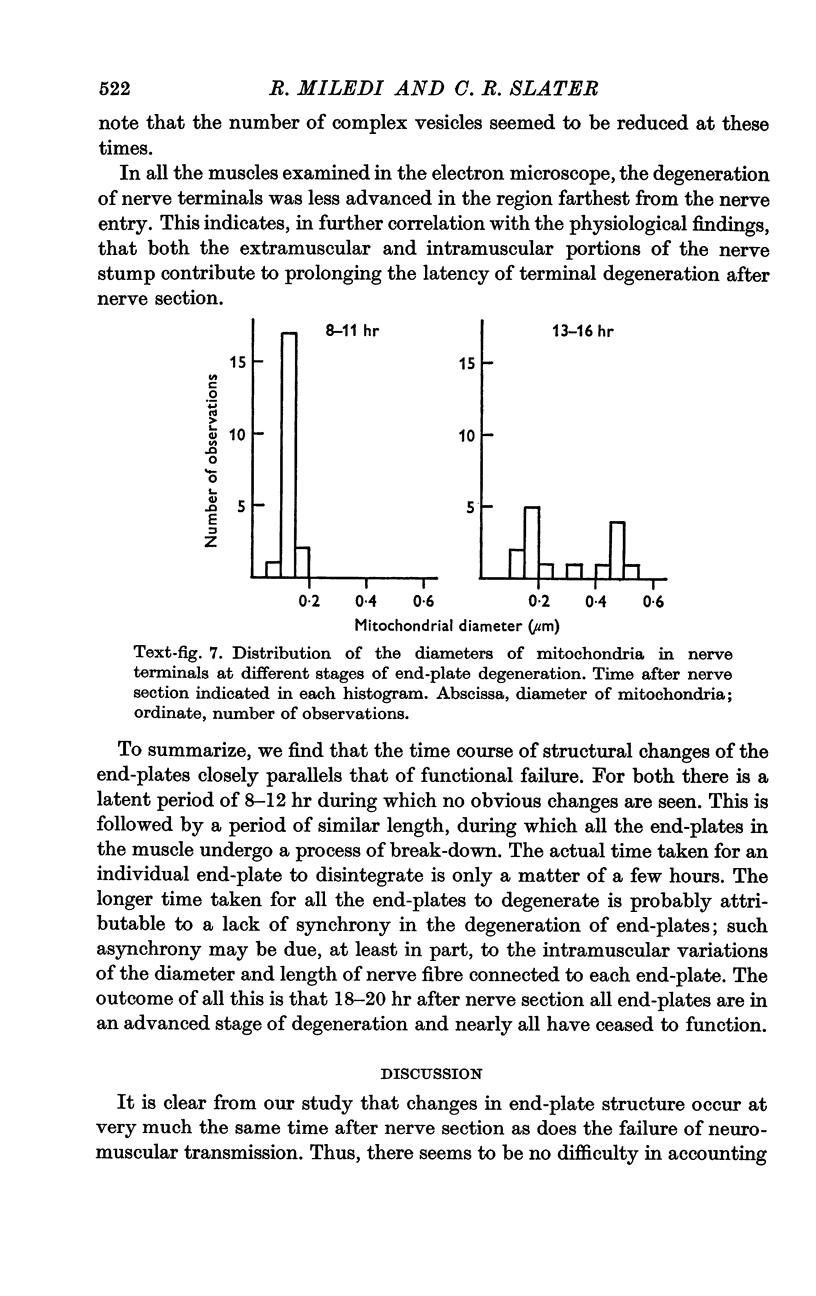
5. Changes in ultrastructure of nerve endings closely parallel those of function. For about 8-12 hr after cutting the nerve, nearly all end-plates appear normal. During the period when transmission is failing, some end-plates are clearly undergoing structural break-down. By the time functional failure is complete, all end-plates appear grossly abnormal.
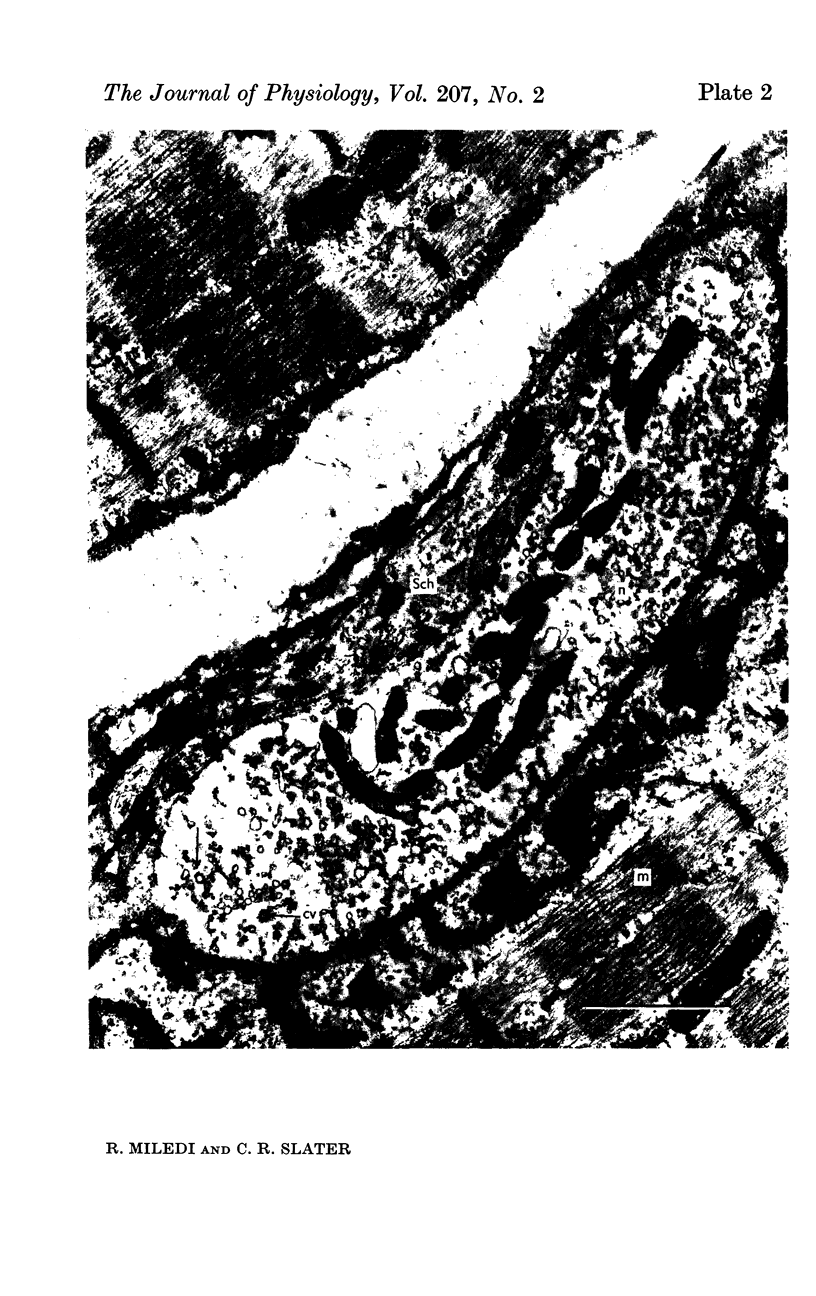
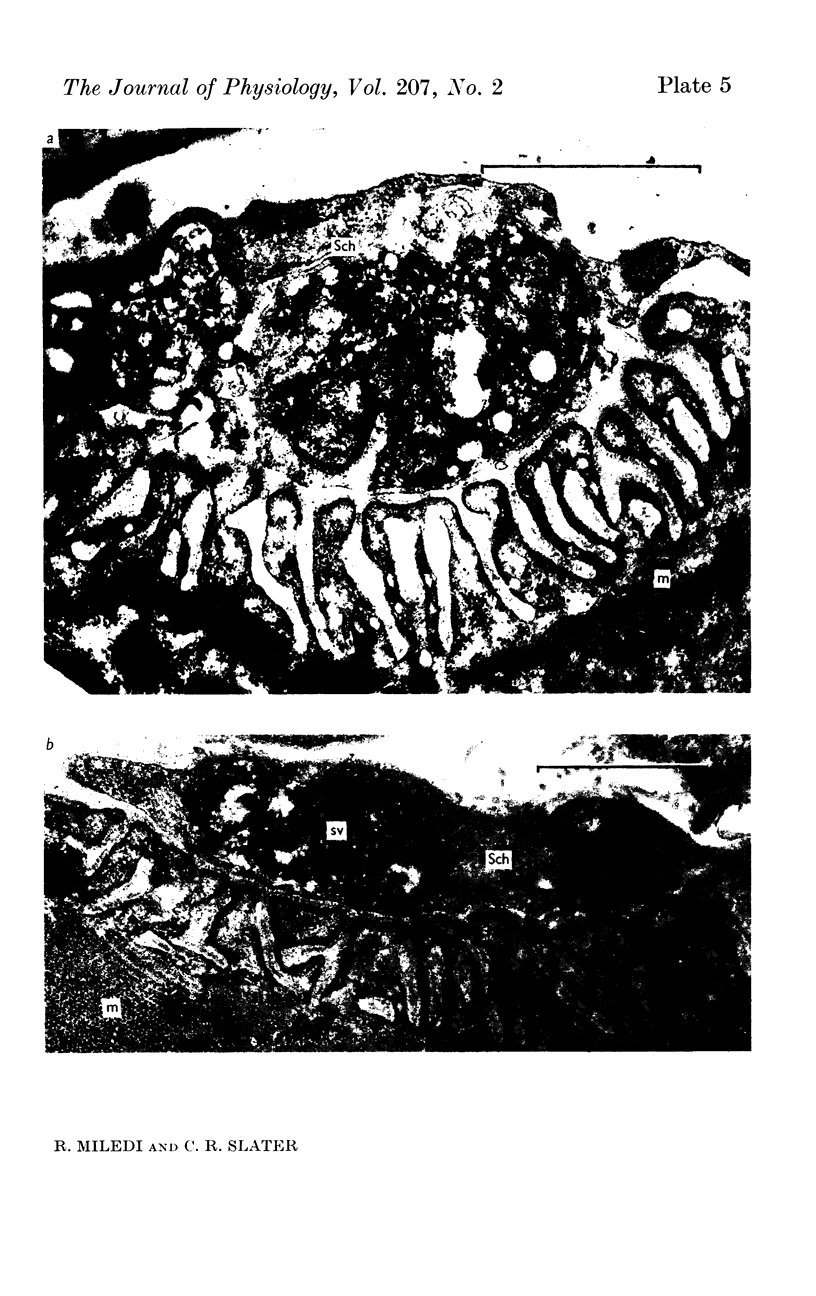
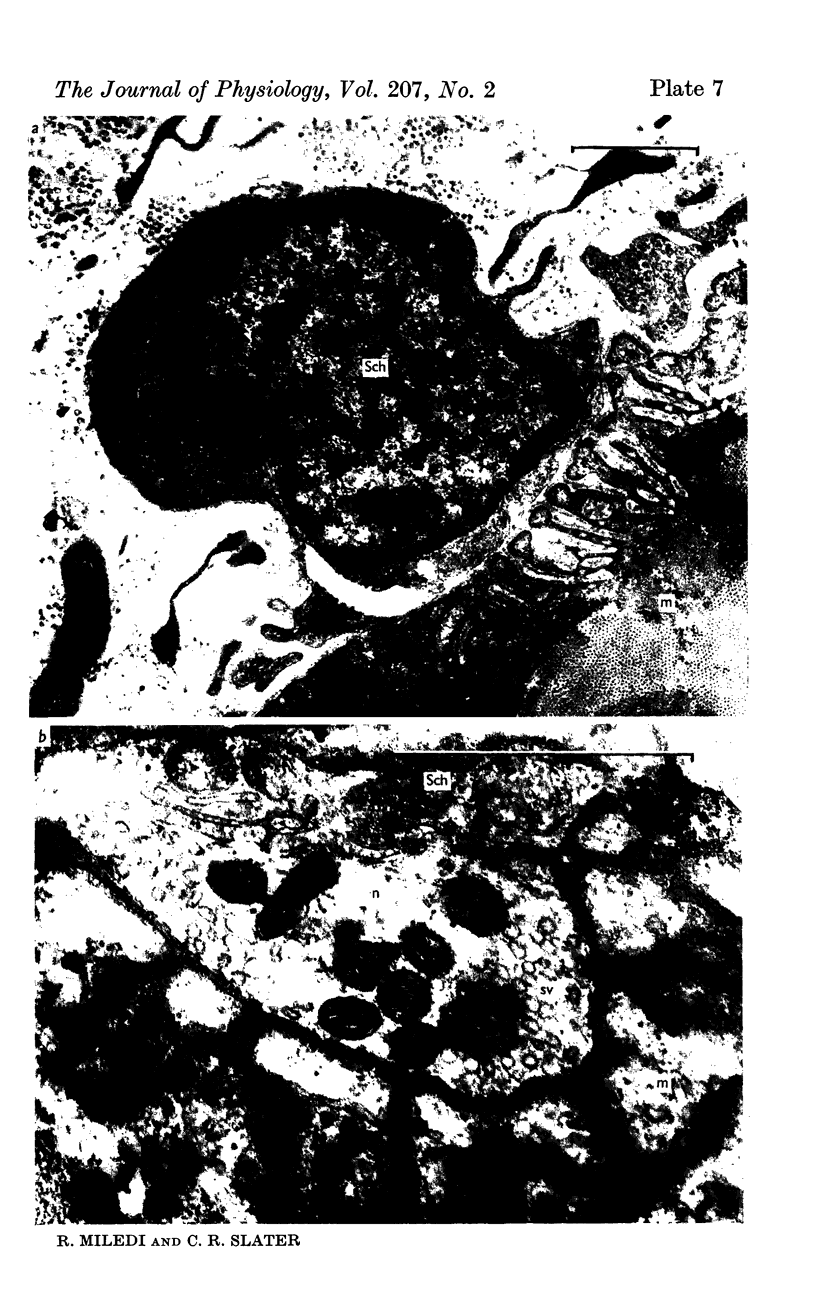
6. During degeneration, the contents of the axoplasm undergo disruption and the nerve terminal breaks up into small fragments. In contrast, the Schwann cell appears to become very active and its processes extend into the synaptic cleft to surround fragments of the nerve terminal. Ultimately, the Schwann cell completely replaces the axon at the end-plate.
7. Increasing the length of the peripheral nerve stump delays the onset of structural break-down. Disruption of end-plates near the site of nerve entry into the muscle occurs before those farther away.
8. It is suggested that end-plate degeneration is triggered by a signal which passes from the site of injury to the nerve terminal. The duration of the period after transection when end-plates appear to be normal would then reflect the time required for this signal to travel the length of the isolated nerve stump.
Full text
PDF


















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN R. D., COOLEDGE J. W., HALL P. J. Streaming in cytoplasm dissociated from the giant amoeba, Chaos chaos. Nature. 1960 Sep 10;187:896–899. doi: 10.1038/187896a0. [DOI] [PubMed] [Google Scholar]
- ANDRES K. H. MIKROPINOZYTOSE IM ZENTRALNERVENSYSTEM. Z Zellforsch Mikrosk Anat. 1964 Sep 17;64:63–73. [PubMed] [Google Scholar]
- BIRKS R., HUXLEY H. E., KATZ B. The fine structure of the neuromuscular junction of the frog. J Physiol. 1960 Jan;150:134–144. doi: 10.1113/jphysiol.1960.sp006378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRKS R., KATZ B., MILEDI R. Physiological and structural changes at the amphibian myoneural junction, in the course of nerve degeneration. J Physiol. 1960 Jan;150:145–168. doi: 10.1113/jphysiol.1960.sp006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I. The fine structure of motor nerve endings at frog myoneural junctions. Ann N Y Acad Sci. 1966 Jan 26;135(1):8–19. doi: 10.1111/j.1749-6632.1966.tb45458.x. [DOI] [PubMed] [Google Scholar]
- COERS C. Contribution à l'étude de la jonction neuro-musculaire. II. Topographie zonale de l'innervation motrice terminale dans les muscles striés. Arch Biol (Liege) 1953;64(4):495–508. [PubMed] [Google Scholar]
- COLONNIER M. EXPERIMENTAL DEGENERATION IN THE CEREBRAL CORTEX. J Anat. 1964 Jan;98:47–53. [PMC free article] [PubMed] [Google Scholar]
- EYZAGUIRRE C., ESPILDORA J., LUCO J. V. Alterations of neuromuscular synapsis during Wallerian degeneration. Acta Physiol Lat Am. 1952 Dec;2(4):213–227. [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- GRAY E. G. Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. J Anat. 1959 Oct;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- GRAY E. G. The granule cells, mossy synapses and Purkinje spine synapses of the cerebellum: light and electron microscope observations. J Anat. 1961 Jul;95:345–356. [PMC free article] [PubMed] [Google Scholar]
- Gray E. G., Guillery R. W. Synaptic morphology in the normal and degenerating nervous system. Int Rev Cytol. 1966;19:111–182. doi: 10.1016/s0074-7696(08)60566-5. [DOI] [PubMed] [Google Scholar]
- HUGHES A. The growth of embryonic neurites; a study of cultures of chick neural tissues. J Anat. 1953 Apr;87(2):150–162. [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., NELSON P. G. STRUCTURAL AND FUNCTIONAL CHANGES IN THE FROG SYMPATHETIC GANGLION FOLLOWING CUTTING OF THE PRESYNAPTIC NERVE FIBRES. J Physiol. 1965 Mar;177:1–20. doi: 10.1113/jphysiol.1965.sp007571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy R. R., Bittner G. D., Kennedy D. Regeneration in crustacean motoneurons: evidence for axonal fusion. Science. 1967 Apr 14;156(3772):251–252. doi: 10.1126/science.156.3772.251. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Presynaptic failure of neuromuscular propagation in rats. J Physiol. 1959 Dec;149:1–22. doi: 10.1113/jphysiol.1959.sp006321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCO J. V., EYZAGUIRRE C. Fibrillation and hypersensitivity to ACh in denervated muscle: effect of length of degenerating nerve fibers. J Neurophysiol. 1955 Jan;18(1):65–73. doi: 10.1152/jn.1955.18.1.65. [DOI] [PubMed] [Google Scholar]
- Lasek R. Axoplasmic transport in cat dorsal root ganglion cells: as studied with [3-H]-L-leucine. Brain Res. 1968 Mar;7(3):360–377. doi: 10.1016/0006-8993(68)90003-6. [DOI] [PubMed] [Google Scholar]
- MIANI N. ANALYSIS OF THE SOMATO-AXONAL MOVEMENT OF PHOSPHOLIPIDS IN THE VAGUS AND HYPOGLOSSAL NERVES. J Neurochem. 1963 Dec;10:859–874. doi: 10.1111/j.1471-4159.1963.tb11913.x. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Grafstein B. Fast and slow components in axonal transport of protein. J Cell Biol. 1968 Sep;38(3):494–508. doi: 10.1083/jcb.38.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan U. J. Fine structure of synapses in the dorsal nucleus of the lateral geniculate body of normal and blinded rats. Z Zellforsch Mikrosk Anat. 1967;76(1):116–146. doi: 10.1007/BF00337036. [DOI] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. Electrophysiology and electron-microscopy of rat neuromuscular junctions after nerve degeneration. Proc R Soc Lond B Biol Sci. 1968 Feb 27;169(1016):289–306. doi: 10.1098/rspb.1968.0012. [DOI] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. Some mitochondrial changes in denervated muscle. J Cell Sci. 1968 Mar;3(1):49–54. doi: 10.1242/jcs.3.1.49. [DOI] [PubMed] [Google Scholar]
- Nickel E., Vogel A., Waser P. G. Coated Vesicles in der Umgebung der neuro-muskulären Synapsen. Z Zellforsch Mikrosk Anat. 1967;78(2):261–266. [PubMed] [Google Scholar]
- Nickel E., Waser P. G. Elektronenmikroskopische Untersuchungen am Diaphragma der Maus nach einseitiger Phrenikotomie. I. Die degenerierende motorische Endplatte. Z Zellforsch Mikrosk Anat. 1968;88(2):278–296. [PubMed] [Google Scholar]
- Ochs S., Sabri M. I., Johnson J. Fast transport system of materials in mammalian nerve fibers. Science. 1969 Feb 14;163(3868):686–687. doi: 10.1126/science.163.3868.686. [DOI] [PubMed] [Google Scholar]
- Potter L. T., Glover V. A., Saelens J. K. Choline acetyltransferase from rat brain. J Biol Chem. 1968 Jul 25;243(14):3864–3870. [PubMed] [Google Scholar]
- REGER J. F. Studies on the fine structure of normal and denervated neuromuscular junctions from mouse gastrocnemius. J Ultrastruct Res. 1959 Mar;2(3):269–282. doi: 10.1016/s0022-5320(59)80001-0. [DOI] [PubMed] [Google Scholar]
- ROTH T. F., PORTER K. R. YOLK PROTEIN UPTAKE IN THE OOCYTE OF THE MOSQUITO AEDES AEGYPTI. L. J Cell Biol. 1964 Feb;20:313–332. doi: 10.1083/jcb.20.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater C. R. Time course of failure of neuromuscular transmission after motor nerve section. Nature. 1966 Jan 15;209(5020):305–306. doi: 10.1038/209305b0. [DOI] [PubMed] [Google Scholar]
- TERRY R. D., HARKIN J. C. Wallerian degeneration and regeneration of peripheral nerves. Prog Neurobiol. 1959;4:303–320. [PubMed] [Google Scholar]
- THOMPSON C. M., WOLPERT L. THE ISOLATION OF MOTILE CYTOPLASM FROM AMOEBA PROTEUS. Exp Cell Res. 1963 Oct;32:156–160. doi: 10.1016/0014-4827(63)90078-8. [DOI] [PubMed] [Google Scholar]
- Usherwood P. N., Cochran D. G., Rees D. Changes in structural, physiological and pharmacological properties of insect excitatory nerve-muscle synapses after nerve section. Nature. 1968 May 11;218(5141):589–591. doi: 10.1038/218589a0. [DOI] [PubMed] [Google Scholar]