Abstract
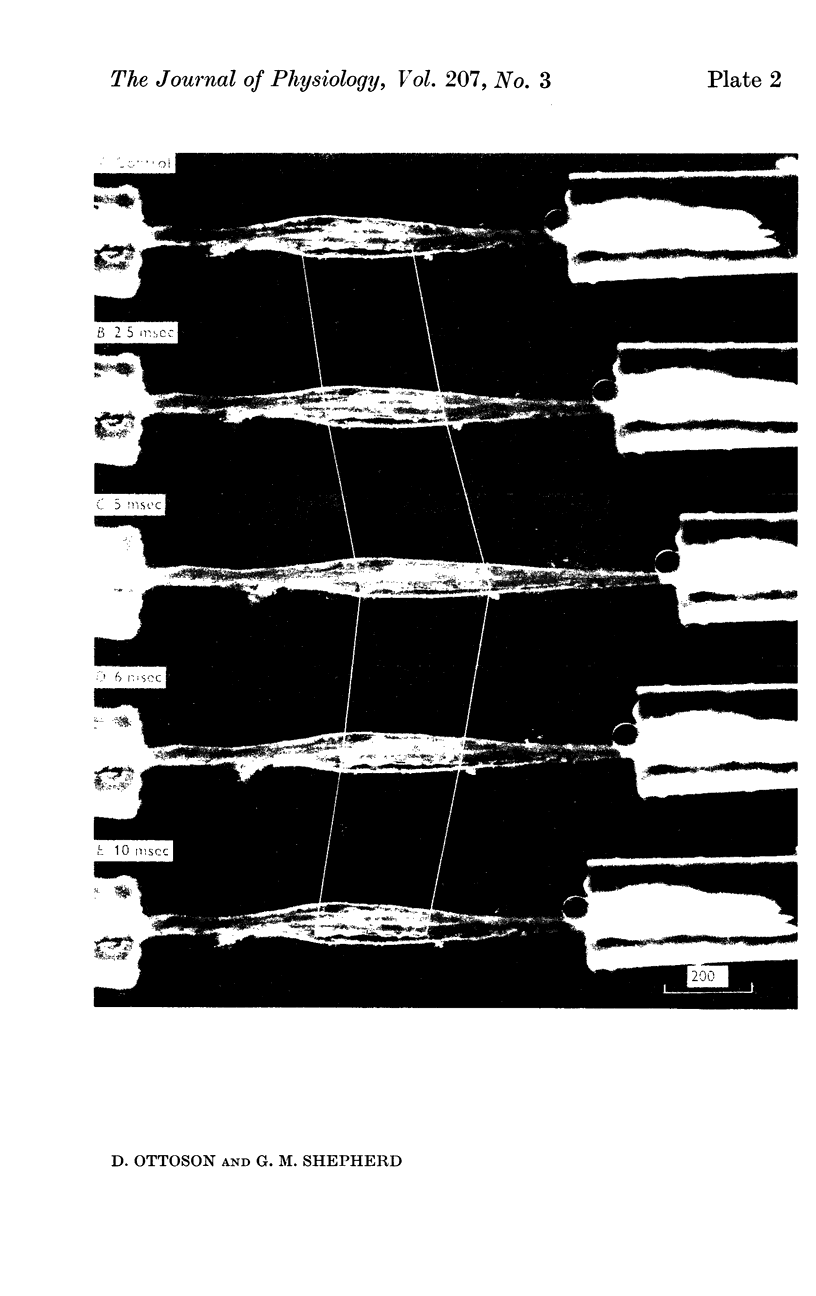
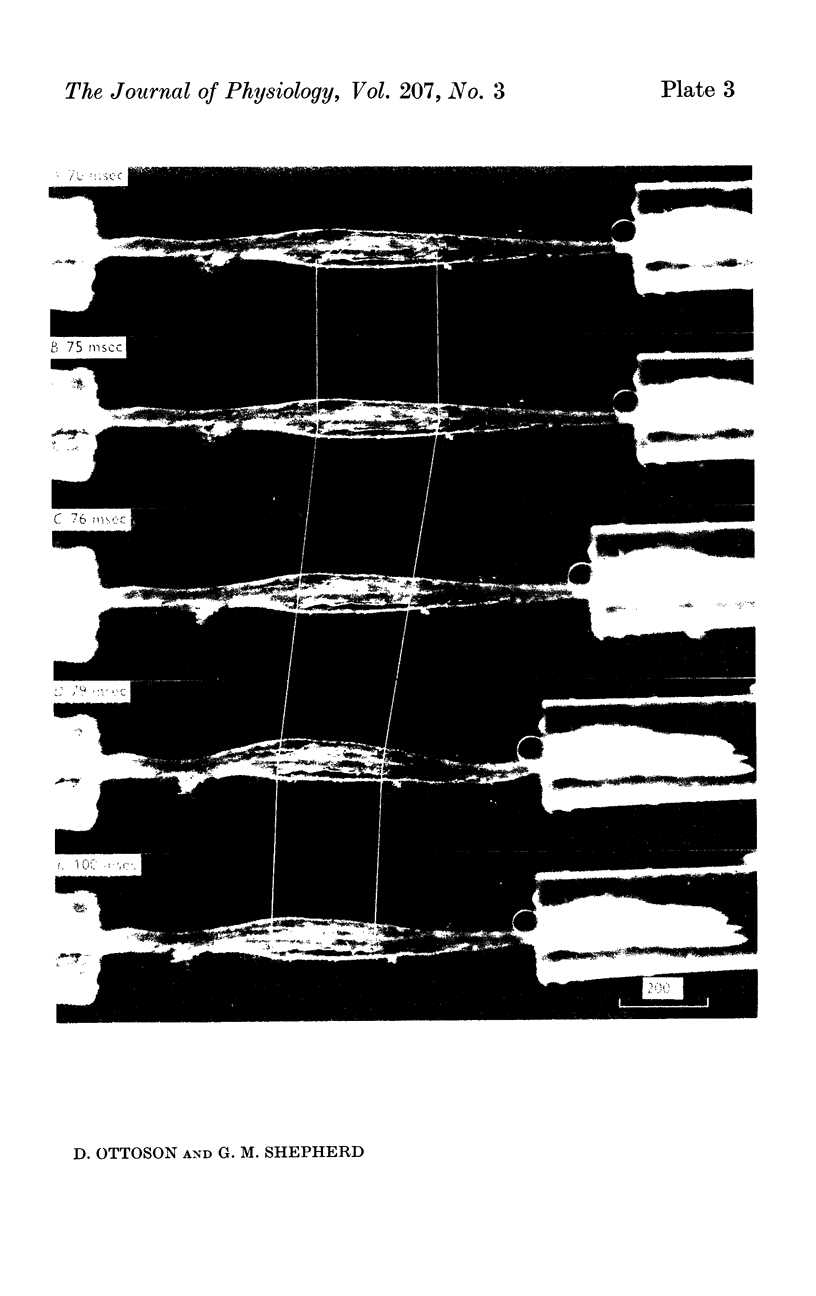
1. The length changes within the frog muscle spindle during stretch have been studied by stroboscopic photomicroscopy. Attention was focused on the length changes within the central reticular zone and these changes were related to the features of the receptor potential.
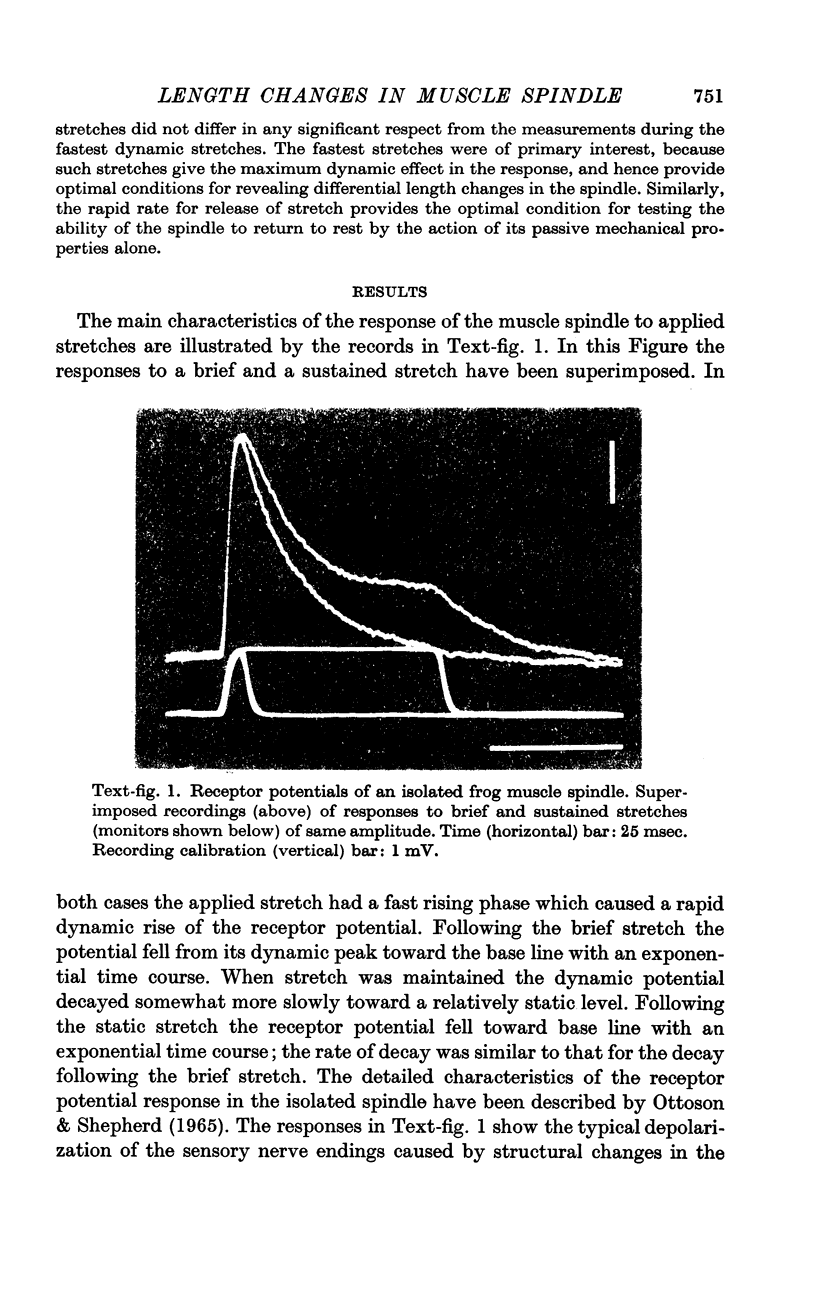
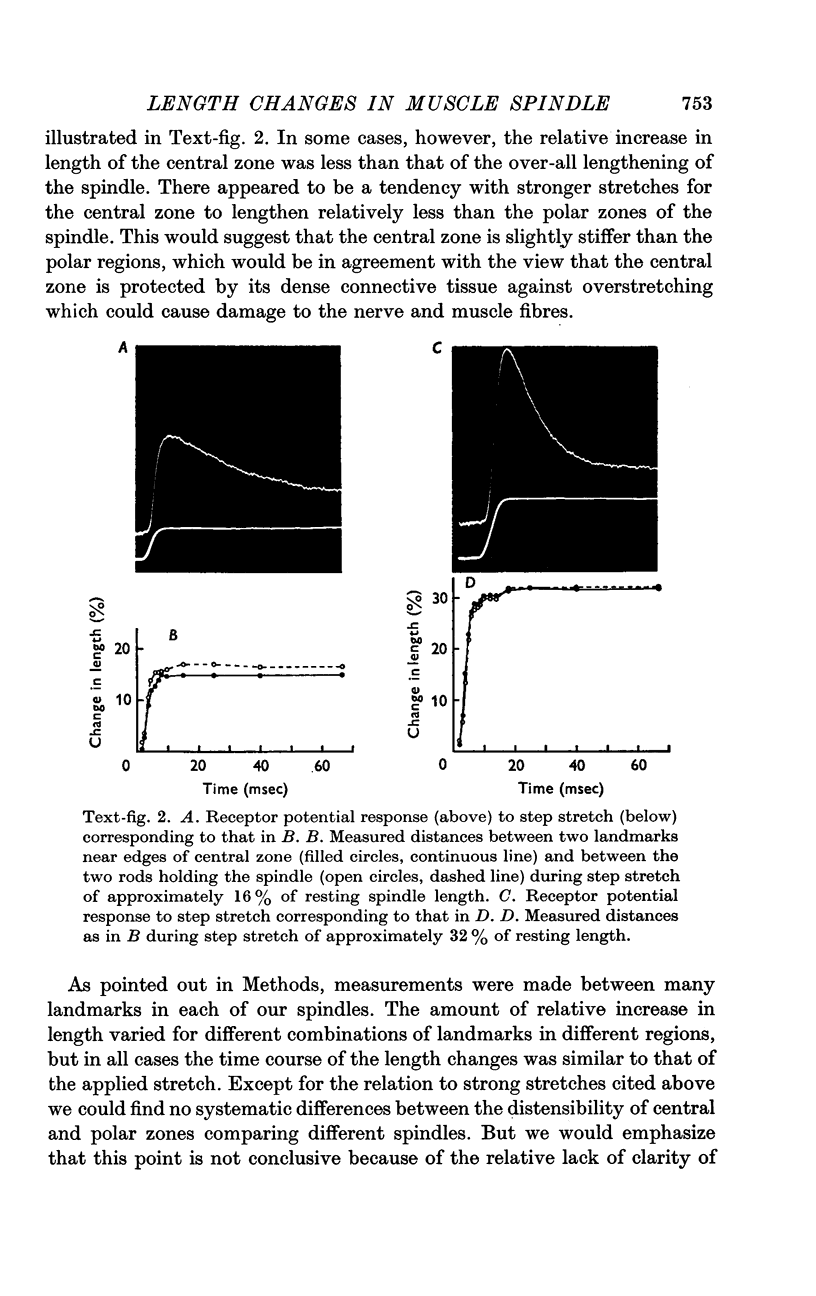
2. It was found that the length changes of the central reticular zone closely followed the applied stretch in time course and magnitude. The results suggest that the length changes of the polar zones are generally similar to those in the central zone.
3. There was no evidence of a relative shortening of the central zone in the early phase of maintained stretch, corresponding to the decline of the receptor potential from its dynamic peak to the static level.
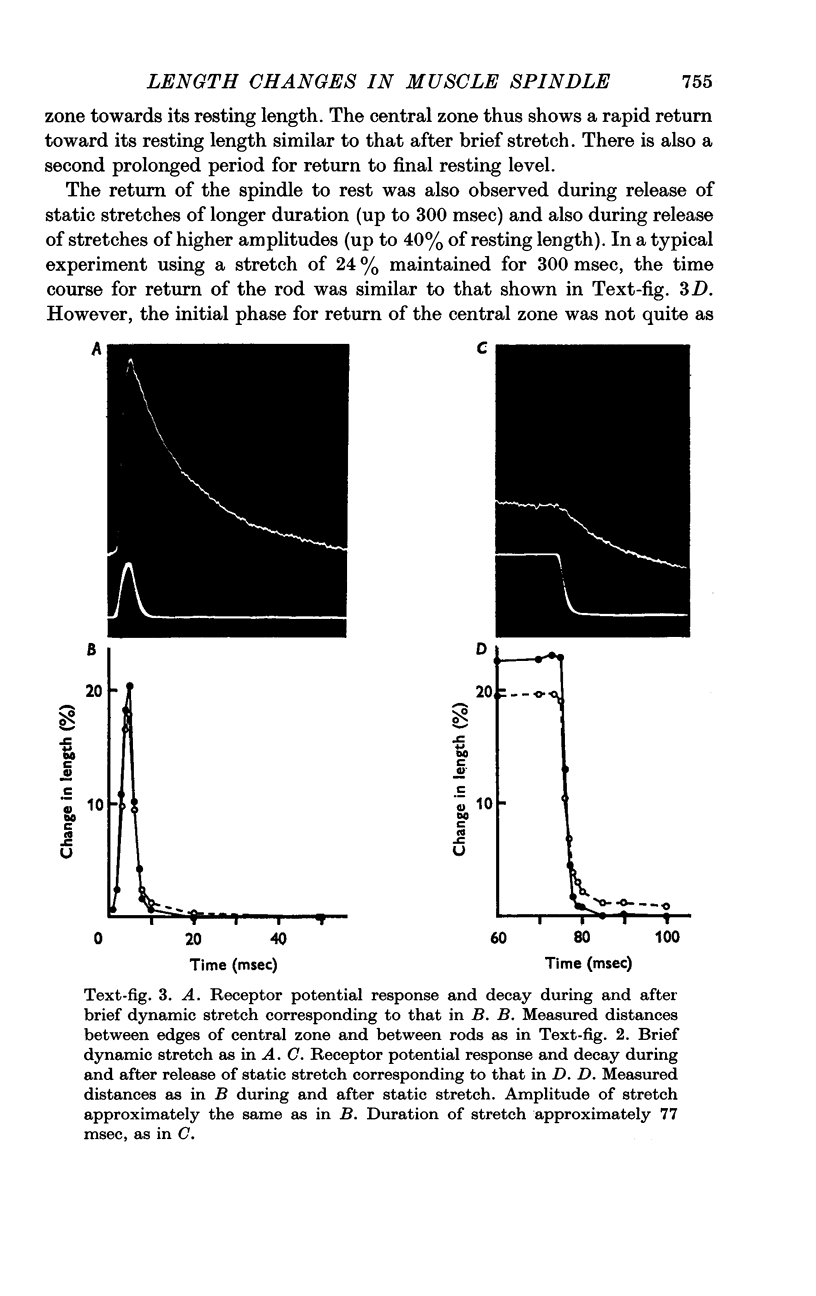
4. Following release of stretch the central zone returned to its original resting length within a few msec. The rapid return of the spindle was in sharp contrast to the relatively slow exponential decay of the receptor potential. With strong or prolonged stretches the return became slower and resting length was not completely restored until 100-150 msec after release of stretch. No corresponding change in the decay of the receptor potential was seen.
5. The results suggest that the early adaptive fall of the receptor potential is not related to differential length changes between the central zone and the polar zones. It seems more likely that the contribution of mechanical factors to the early adaptation of the frog spindle have to be sought at the ultrastructural level.
6. The finding that the length changes closely follow the applied stretch suggests that the stimulus in terms of lengthening is transmitted to the endings with little distortion.
7. The results suggest that the elastic elements play a dominant role for the transmission of the stimulus to the endings and for the return of the spindle to resting length after release of stretch.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FUORTES M. G., POGGIO G. F. Transient responses to sudden illumination in cells of the eye of Limulus. J Gen Physiol. 1963 Jan;46:435–452. doi: 10.1085/jgp.46.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY J. A. B., MATTHEWS P. B. C. A comparison of the adaptation of the Pacinian corpuscle with the accommodation of its own axon. J Physiol. 1951 Aug;114(4):454–464. doi: 10.1113/jphysiol.1951.sp004636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBBARD S. J. A study of rapid mechanical events in a mechanoreceptor. J Physiol. 1958 Apr 30;141(2):198–218. doi: 10.1113/jphysiol.1958.sp005968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk J. C., Cornew R. W., Stark L. A model of adaptation in amphibian spindle receptors. J Theor Biol. 1966 Nov;12(2):196–215. doi: 10.1016/0022-5193(66)90113-5. [DOI] [PubMed] [Google Scholar]
- LOEWENSTEIN W. R., MENDELSON M. COMPONENTS OF RECEPTOR ADAPTATION IN A PACINIAN CORPUSCLE. J Physiol. 1965 Apr;177:377–397. doi: 10.1113/jphysiol.1965.sp007598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS P. B. MUSCLE SPINDLES AND THEIR MOTOR CONTROL. Physiol Rev. 1964 Apr;44:219–288. doi: 10.1152/physrev.1964.44.2.219. [DOI] [PubMed] [Google Scholar]
- Matthews B. H. The response of a single end organ. J Physiol. 1931 Jan 21;71(1):64–110. doi: 10.1113/jphysiol.1931.sp002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottoson D., Shepherd G. M. Changes of length within the frog muscle spindle during stretch as shown by stroboscopic photomicroscopy. Nature. 1968 Nov 30;220(5170):912–914. doi: 10.1038/220912a0. [DOI] [PubMed] [Google Scholar]
- Ottoson D., Shepherd G. M. Receptor potentials and impulse generation in the isolated spindle during controlled extension. Cold Spring Harb Symp Quant Biol. 1965;30:105–114. doi: 10.1101/sqb.1965.030.01.014. [DOI] [PubMed] [Google Scholar]
- Ozeki M., Sato M. Changes in the membrane potential and the membrane conductance associated with a sustained compression of the non-myelinated nerve terminal in Pacinian corpuscles. J Physiol. 1965 Sep;180(1):186–208. [PMC free article] [PubMed] [Google Scholar]
- Shepherd G. M., Ottoson D. Response of the isolated muscle spindle to different rates of stretching. Cold Spring Harb Symp Quant Biol. 1965;30:95–103. doi: 10.1101/sqb.1965.030.01.013. [DOI] [PubMed] [Google Scholar]
- Toyama K. An analysis of impulse discharges from the spindle receptor. Jpn J Physiol. 1966 Apr 15;16(2):113–125. doi: 10.2170/jjphysiol.16.113. [DOI] [PubMed] [Google Scholar]