Abstract
1. The innervation of carotid body Type I cells has been investigated in seventeen cats. At a sterile operation the glossopharyngeal and vagus nerve roots were cut intracranially on one side.
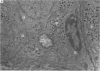
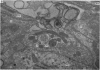
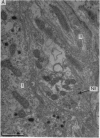
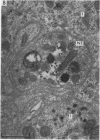
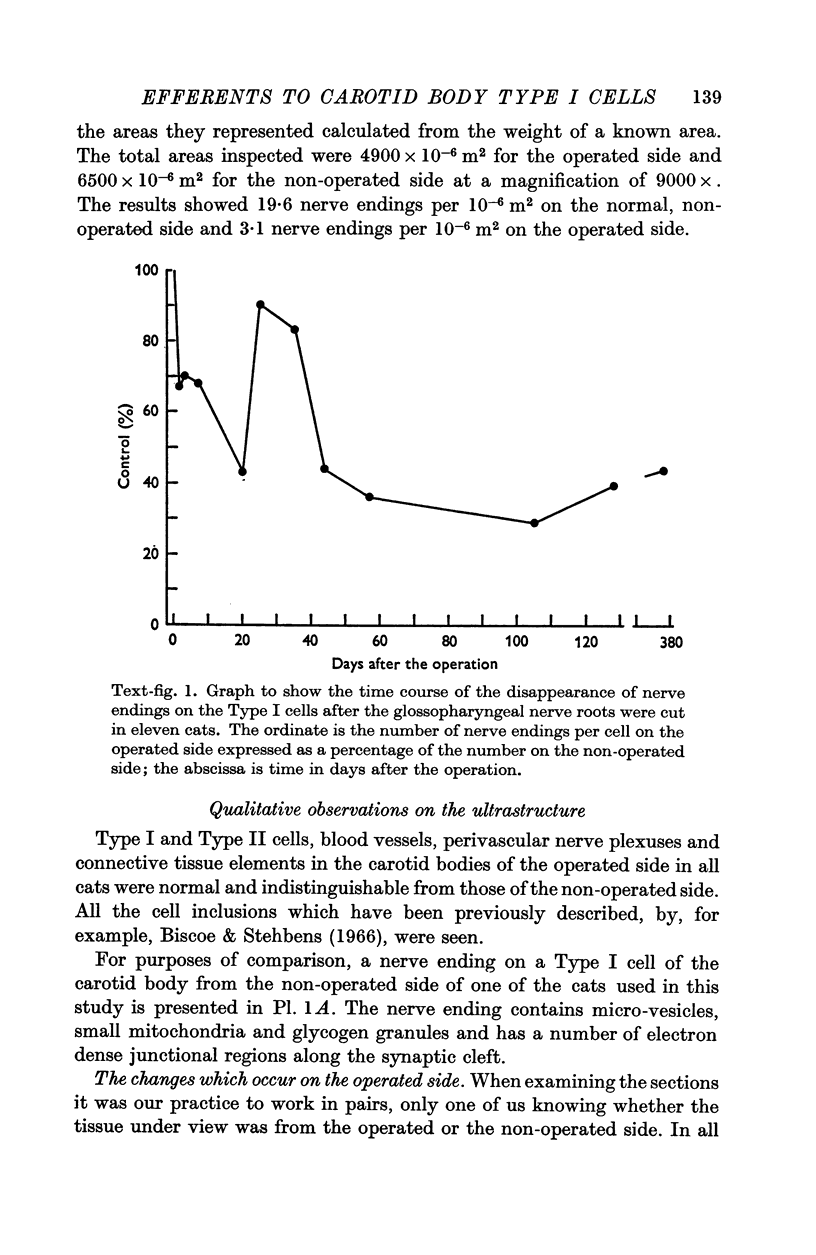
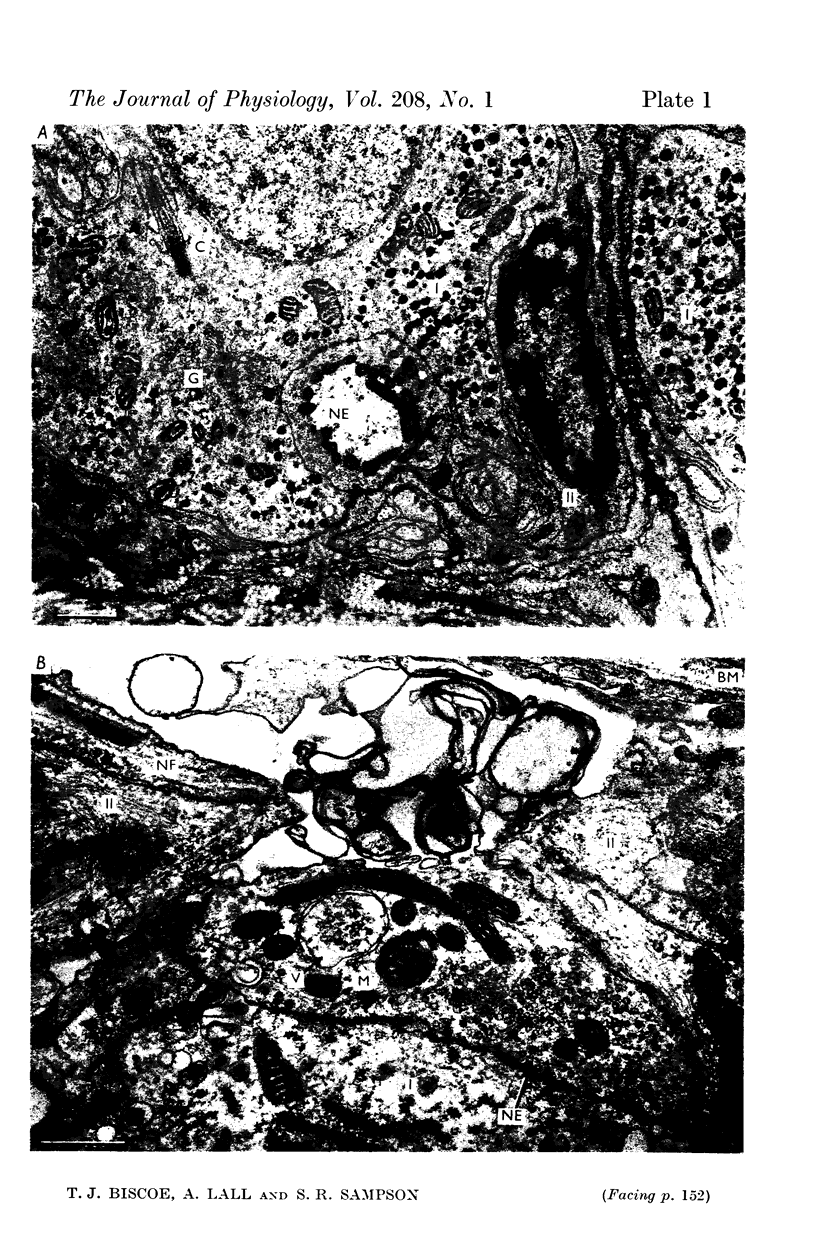
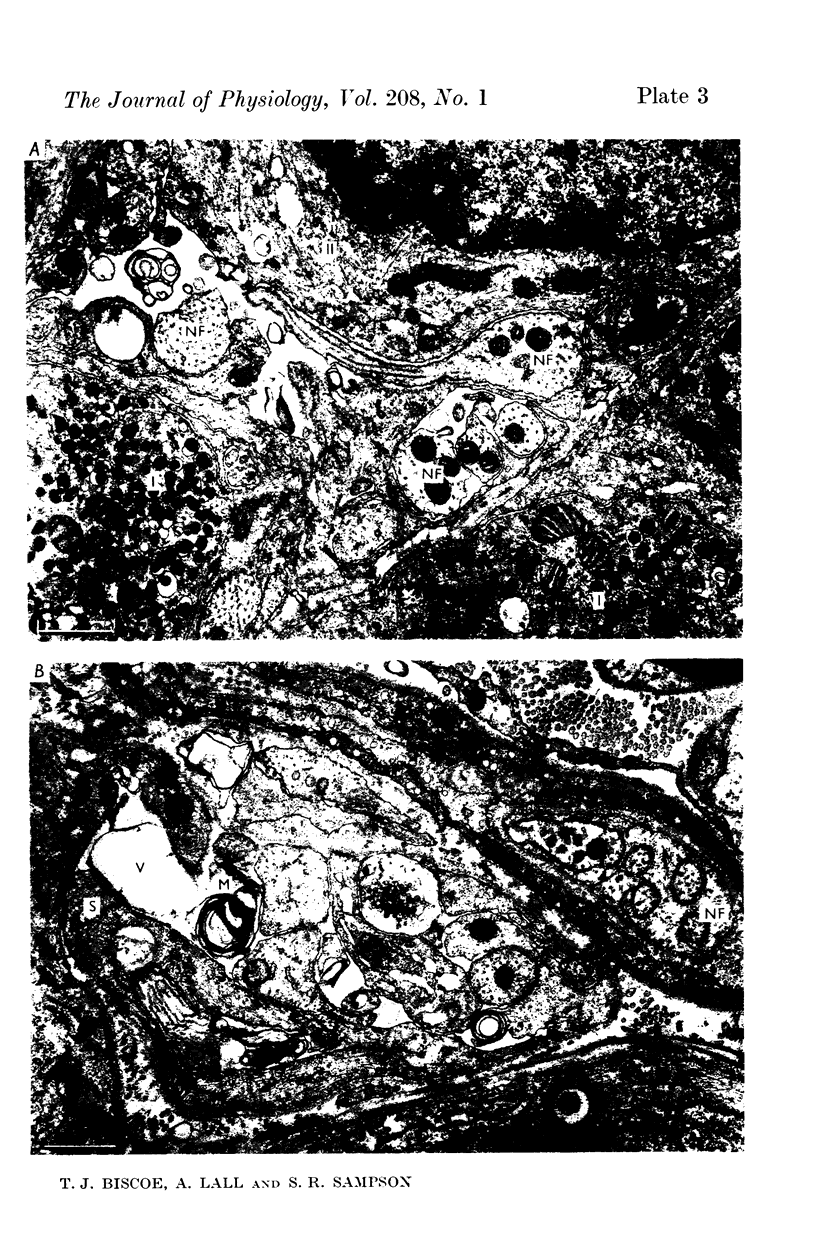
2. From 1½ to 378 days after the operation the carotid bodies were fixed in situ and prepared for electron microscopy. Nerve endings on Type I cells were found to degenerate with a prolonged time course. In each cat there was a decrease in the number of nerve endings on the operated side as compared with the non-operated side.
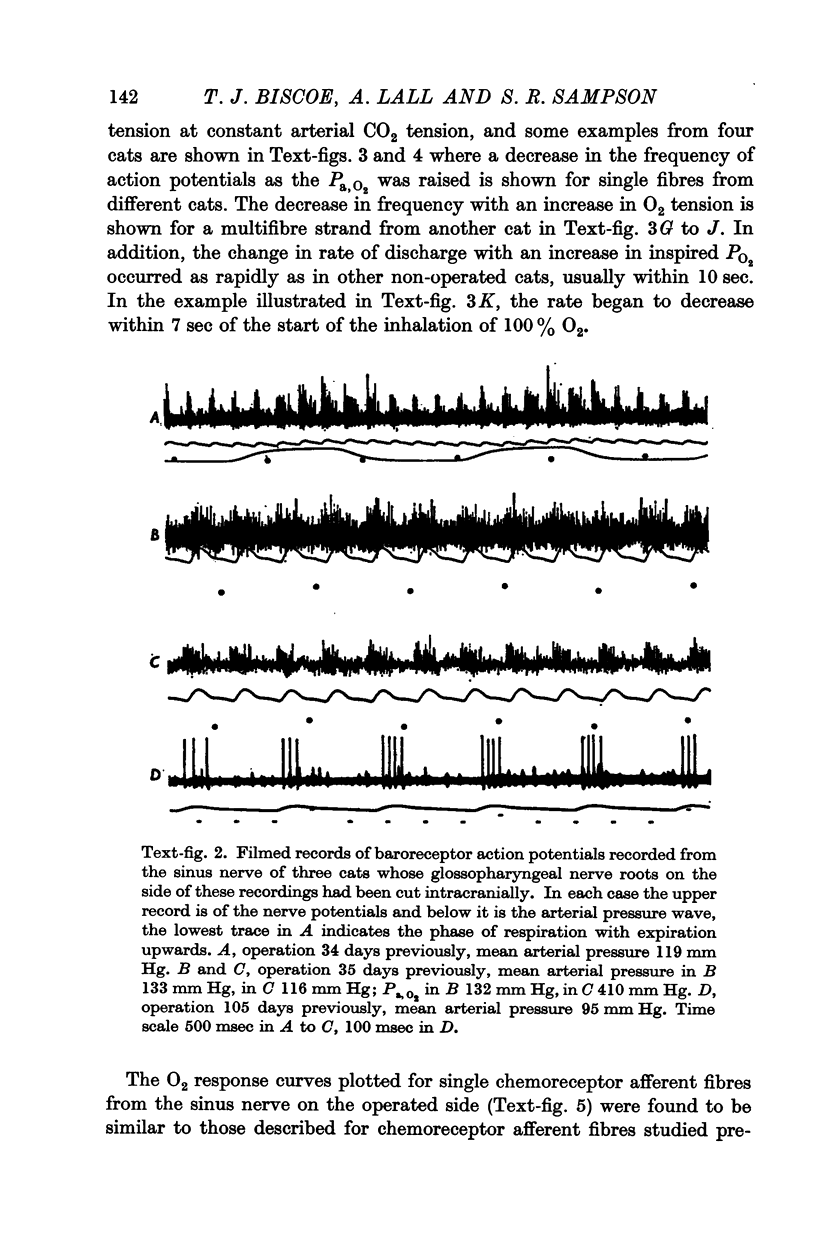
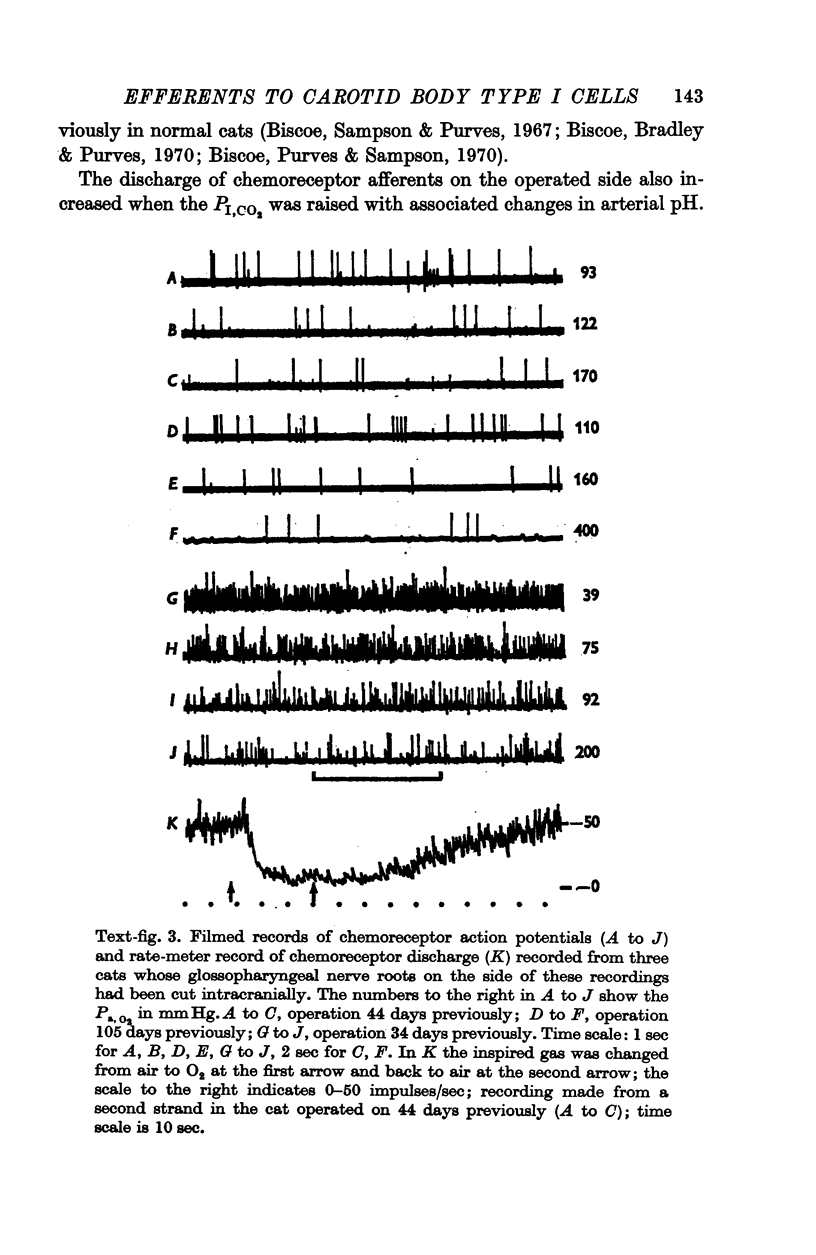
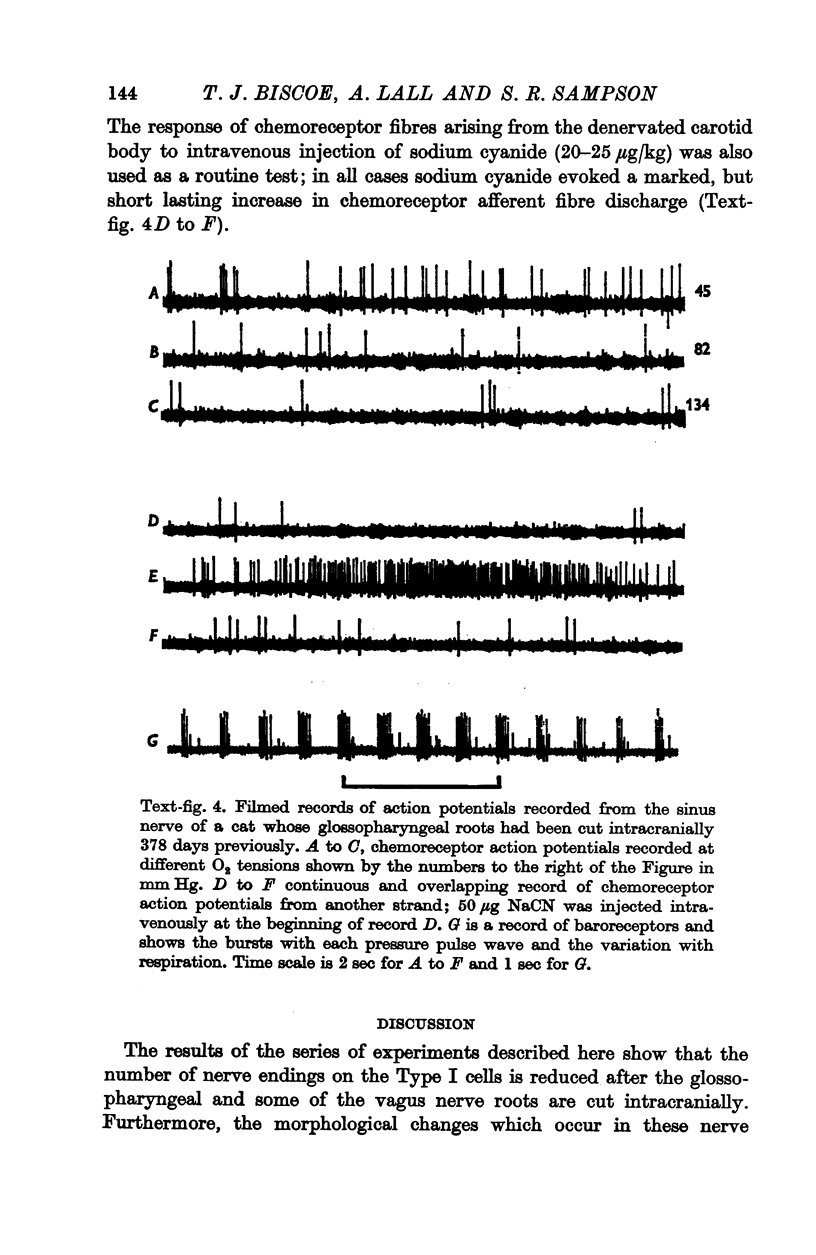
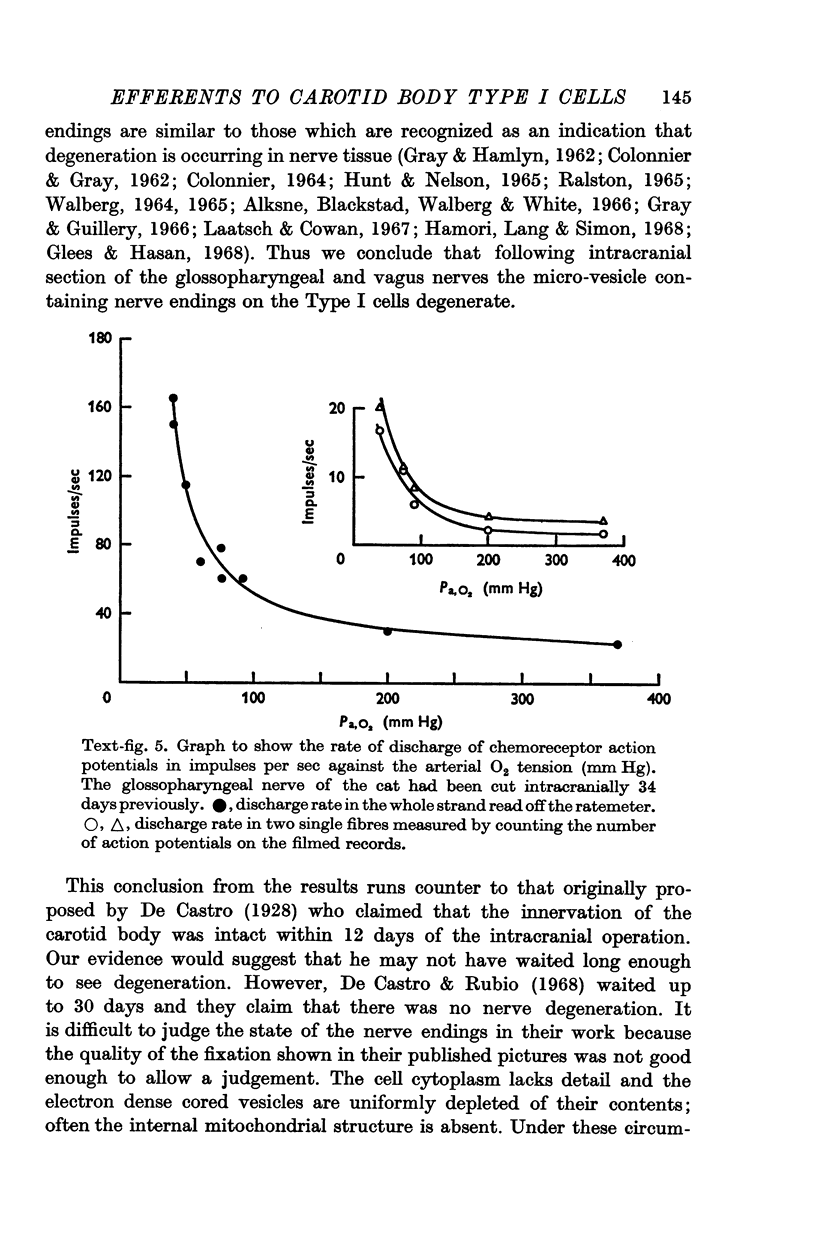
3. Before the carotid bodies were fixed, recordings were made from chemoreceptor, and baroreceptor, afferent fibres in the sinus nerve on the operated side. The chemoreceptors responded in the usual way to changes in arterial O2, CO2 and pH; the injection of cyanide evoked a brisk response.
4. It is concluded that the nerve endings on Type I cells are efferent rather than afferent and the cell bodies of their axons are probably in the brain stem.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alksne J. F., Blackstad T. W., Walberg F., White L. E., Jr Electron microscopy of axon degeneration: a valuable tool in experimental neuroanatomy. Ergeb Anat Entwicklungsgesch. 1966;39(1):3–32. doi: 10.1007/978-3-662-30450-1. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Bradley G. W., Purves M. J. The relation between carotid body chemoreceptor discharge, carotid sinus pressure and carotid body venous flow. J Physiol. 1970 May;208(1):99–120. doi: 10.1113/jphysiol.1970.sp009108. [DOI] [PMC free article] [PubMed] [Google Scholar]
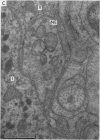
- Biscoe T. J., Lall A., Sampson S. R. On the nerve endings associated with the carotid body glomus cells of the cat. J Physiol. 1969 Feb;200(2):131P–132P. [PubMed] [Google Scholar]
- Biscoe T. J., Purves M. J. Observations on the rhythmic variation in the cat carotid body chemoreceptor activity which has the same period as respiration. J Physiol. 1967 Jun;190(3):389–412. doi: 10.1113/jphysiol.1967.sp008217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Purves M. J., Sampson S. R. The frequency of nerve impulses in single carotid body chemoreceptor afferent fibres recorded in vivo with intact circulation. J Physiol. 1970 May;208(1):121–131. doi: 10.1113/jphysiol.1970.sp009109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Sampson S. R. Rhythmical and non-rhythmical spontaneous activity recorded from the central cut end of the sinus nerve. J Physiol. 1968 May;196(2):327–338. doi: 10.1113/jphysiol.1968.sp008510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe T. J., Sampson S. R. Spontaneous activity recorded from the central cut end of the carotid sinus nerve of the cat. Nature. 1967 Oct 21;216(5112):294–295. doi: 10.1038/216294a0. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Sampson S. R. Stimulus response curves of single carotid body chemoreceptor afferent fibres. Nature. 1967 Aug 5;215(5101):654–655. doi: 10.1038/215654a0. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J., Stehbens W. E. Ultrastructure of the carotid body. J Cell Biol. 1966 Sep;30(3):563–578. doi: 10.1083/jcb.30.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstad T. W. Mapping of experimental axon degeneration by electron microscopy of Golgi preparations. Z Zellforsch Mikrosk Anat. 1965 Sep 17;67(6):819–834. doi: 10.1007/BF00339303. [DOI] [PubMed] [Google Scholar]
- COLONNIER M. EXPERIMENTAL DEGENERATION IN THE CEREBRAL CORTEX. J Anat. 1964 Jan;98:47–53. [PMC free article] [PubMed] [Google Scholar]
- COLONNIER M., GUILLERY R. W. SYNAPTIC ORGANIZATION IN THE LATERAL GENICULATE NUCLEUS OF THE MONKEY. Z Zellforsch Mikrosk Anat. 1964 Apr 9;62:333–355. doi: 10.1007/BF00339284. [DOI] [PubMed] [Google Scholar]
- COTTLE M. K. DEGENERATION STUDIES OF PRIMARY AFFERENTS OF IXTH AND XTH CRANIAL NERVES IN THE CAT. J Comp Neurol. 1964 Jun;122:329–345. doi: 10.1002/cne.901220304. [DOI] [PubMed] [Google Scholar]
- Chiocchio S. R., Biscardi A. M., Tramezzani J. H. 5-hydroxytryptamine in the carotid body of the cat. Science. 1967 Nov 10;158(3802):790–791. doi: 10.1126/science.158.3802.790. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Cowan W. M. An electron microscope study of normal and degenerating centrifugal fiber terminals in the pigeon retina. Z Zellforsch Mikrosk Anat. 1966;71(1):14–28. doi: 10.1007/BF00339827. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. Cell junctions in amphibian skin. J Cell Biol. 1965 Jul;26(1):263–291. doi: 10.1083/jcb.26.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUILLERY R. W. SOME ELECTRON MICROSCOPICAL OBSERVATIONS OF DEGENERATIVE CHANGES IN CENTRAL NERVOUS SYNAPSES. Prog Brain Res. 1965;14:57–76. doi: 10.1016/s0079-6123(08)63739-5. [DOI] [PubMed] [Google Scholar]
- Glees P., Hasan M. The signs of synaptic degeneration--a critical appraisal. Acta Anat (Basel) 1968;69(2):153–167. doi: 10.1159/000143069. [DOI] [PubMed] [Google Scholar]
- Gray E. G., Guillery R. W. Synaptic morphology in the normal and degenerating nervous system. Int Rev Cytol. 1966;19:111–182. doi: 10.1016/s0074-7696(08)60566-5. [DOI] [PubMed] [Google Scholar]
- Gray E. G., Hamlyn L. H. Electron microscopy of experimental degeneration in the avian optic tectum. J Anat. 1962 Jul;96(Pt 3):309–316.5. [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., NELSON P. G. STRUCTURAL AND FUNCTIONAL CHANGES IN THE FROG SYMPATHETIC GANGLION FOLLOWING CUTTING OF THE PRESYNAPTIC NERVE FIBRES. J Physiol. 1965 Mar;177:1–20. doi: 10.1113/jphysiol.1965.sp007571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hámori J., Láng E., Simon L. Experimental degeneration of the preganglionic fibers in the superior cervical ganglion of the cat. An electron microscope study. Z Zellforsch Mikrosk Anat. 1968;90(1):37–52. doi: 10.1007/BF00496701. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil E., O'Regan R. G. Effects of sinus and aortic nerve efferents on arterial chemoreceptor function. J Physiol. 1969 Jan;200(1):69P–71P. [PubMed] [Google Scholar]
- PALADE G. E. A study of fixation for electron microscopy. J Exp Med. 1952 Mar;95(3):285–298. doi: 10.1084/jem.95.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston H. J., 3rd The organization of the substantia gelatinosa rolandi in the cat lumbosacral spinal cord. Z Zellforsch Mikrosk Anat. 1965 Jul 5;67(1):1–23. doi: 10.1007/BF00339273. [DOI] [PubMed] [Google Scholar]
- SMITH J. M., O'LEARY J. L., HARRIS A. B., GAY A. J. ULTRASTRUCTURAL FEATURES OF THE LATERAL GENICULATE NUCLEUS OF THE CAT. J Comp Neurol. 1964 Dec;123:357–377. doi: 10.1002/cne.901230305. [DOI] [PubMed] [Google Scholar]
- SPOENDLIN H. H., GACEK R. R. ELECTRON MICROSCOPIC STUDY OF THE EFFERENT AND AFFERENT INNERVATION OF THE ORGAN OF CORTI IN THE CAT. Ann Otol Rhinol Laryngol. 1963 Sep;72:660–686. doi: 10.1177/000348946307200307. [DOI] [PubMed] [Google Scholar]
- SZENTAGOTHAI J. THE USE OF DEGENERATION METHODS IN THE INVESTIGATION OF SHORT NEURONAL CONNEXIONS. Prog Brain Res. 1965;14:1–32. [PubMed] [Google Scholar]
- Smith C. A., Rasmussen G. L. Degeneration in the efferent nerve endings in the cochlea after axonal section. J Cell Biol. 1965 Jul;26(1):63–77. doi: 10.1083/jcb.26.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALBERG F. An electron microscopic study of the inferior olive of the cat. J Comp Neurol. 1963 Feb;120:1–17. doi: 10.1002/cne.901200102. [DOI] [PubMed] [Google Scholar]
- Walberg F. An electron microscopic study of terminal degenration in the inferior olive of the cat. J Comp Neurol. 1965 Oct;125(2):205–222. doi: 10.1002/cne.901250205. [DOI] [PubMed] [Google Scholar]
- el-Lami F., Murray R. G. Fine structure of the carotid body of normal and anoxic cats. Anat Rec. 1968 Apr;160(4):697–718. doi: 10.1002/ar.1091600405. [DOI] [PubMed] [Google Scholar]