Abstract
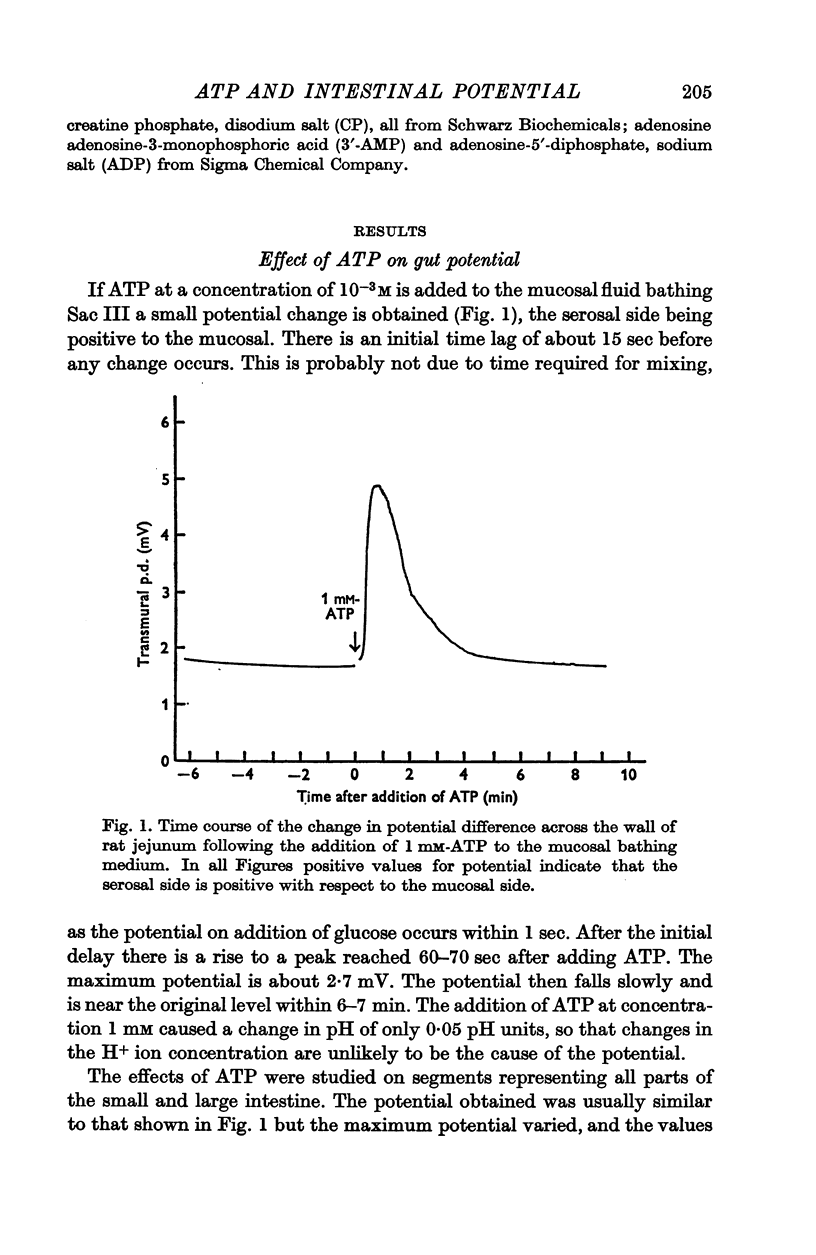
1. ATP either in the mucosal or serosal fluid caused a transient increase in the potential difference and short-circuit current across the wall of rat jejunum in vitro, the serosal solution becoming more positive.
2. Similar responses were observed in the ileum and colon, and in in vivo preparations of small intestine.
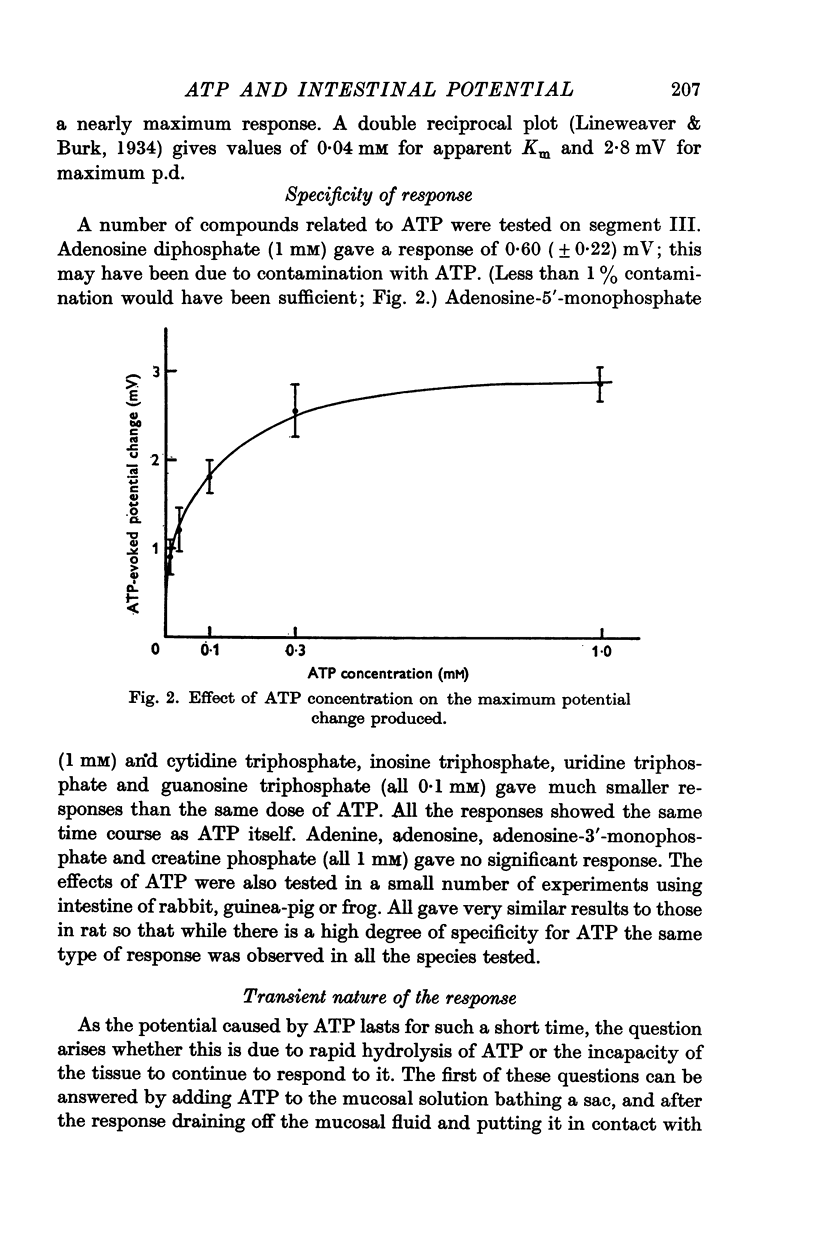
3. The response is relatively specific for ATP.
4. The transient nature of the response is not due to rapid hydrolysis of extracellular ATP.
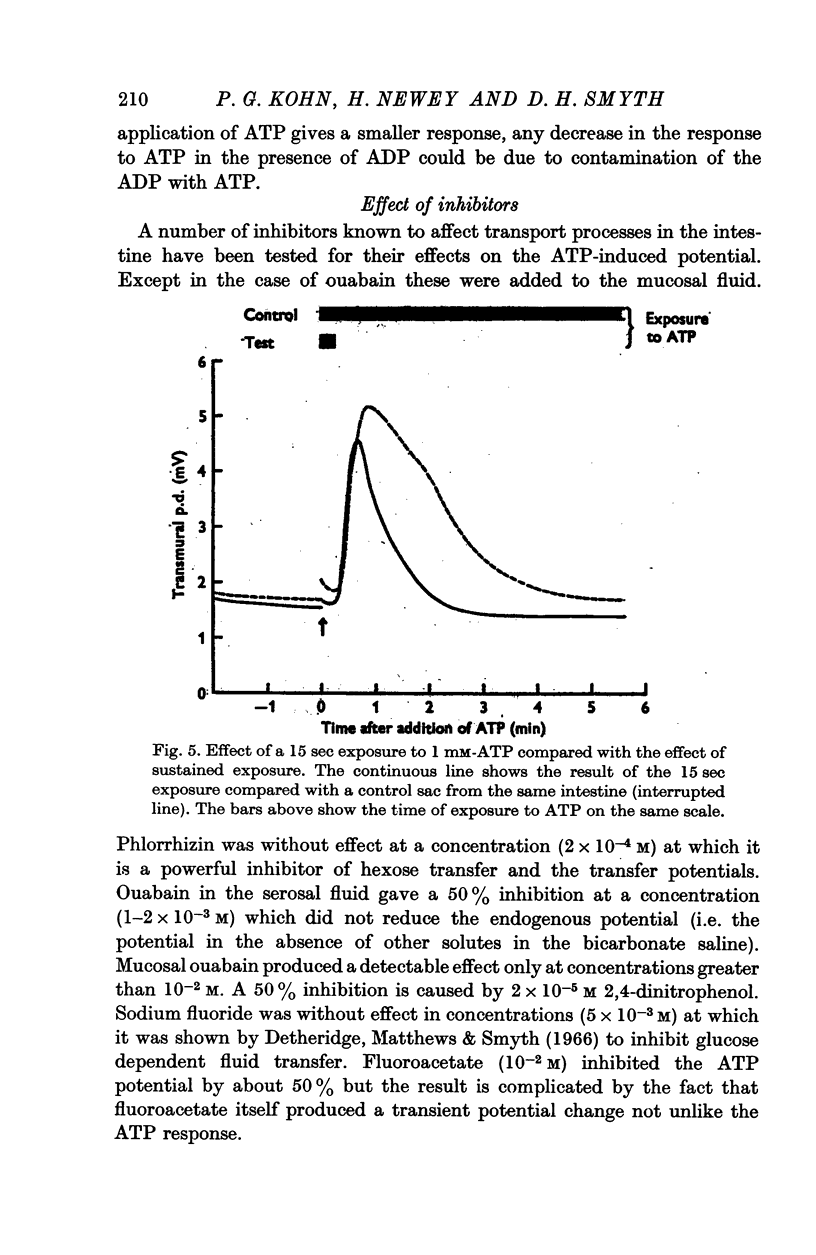
5. High concentrations of K+ in the mucosal medium, serosal ouabain or mucosal 2,4-dinitrophenol all inhibit the response without altering the time course.
6. Significant inhibition was not observed in the presence of ADP or in Mg2+-free salines.
7. The results are consistent with an intracellular action of ATP in the epithelium to stimulate net ion transport. The results do not demonstrate whether or not extracellular ATP can act as an energy donor for an electrogenic ion pump.
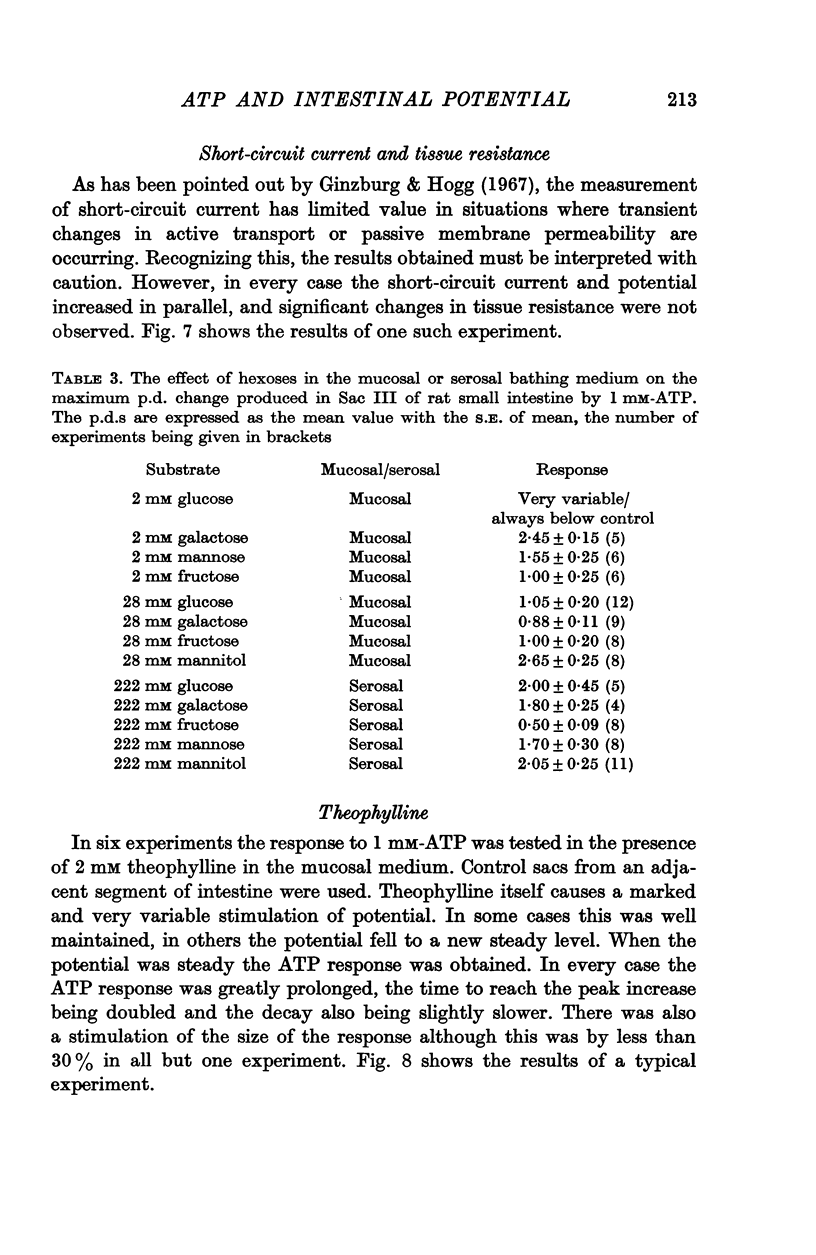
8. Theophylline prolongs the time course of the response, and the involvement of the adenyl cyclase system cannot be excluded as an explanation for the findings.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRY B. A., MATTHEWS J., SMYTH D. H. Transfer of glucose and fluid by different parts of the small intestine of the rat. J Physiol. 1961 Jul;157:279–288. doi: 10.1113/jphysiol.1961.sp006721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRY R. J., DIKSTEIN S., MATTHEWS J., SMYTH D. H., WRIGHT E. M. ELECTRICAL POTENTIALS ASSOCIATED WITH INTESTINAL SUGAR TRANSFER. J Physiol. 1964 Jun;171:316–338. doi: 10.1113/jphysiol.1964.sp007379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry R. J., Smyth D. H., Wright E. M. Short-circuit current and solute transfer by rat jejunum. J Physiol. 1965 Nov;181(2):410–431. doi: 10.1113/jphysiol.1965.sp007770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg G. G., Szekerczes J. The sodium and potassium activated ATPase. II. Comparative study of intestinal epithelium and red cells. J Cell Physiol. 1966 Jun;67(3):487–500. doi: 10.1002/jcp.1040670313. [DOI] [PubMed] [Google Scholar]
- DUNHAM E. T., GLYNN I. M. Adenosinetriphosphatase activity and the active movements of alkali metal ions. J Physiol. 1961 Apr;156:274–293. doi: 10.1113/jphysiol.1961.sp006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detheridge J. F., Matthews J., Smyth D. H. The effect of inhibitors on intestinal transfer of glucose and fluid. J Physiol. 1966 Mar;183(2):369–377. doi: 10.1113/jphysiol.1966.sp007871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Plotkin G. R., Silen W. Effects of vasopressin, theophylline and cyclic adenosine monophosphate on short-circuit current across isolated rabbit ileal mucosa. Nature. 1968 Feb 3;217(5127):469–471. doi: 10.1038/217469a0. [DOI] [PubMed] [Google Scholar]
- Kohn P. G., Newey H., Smyth D. H. Electrical potential across the rat small intestine stimulated by adenosine triphosphate. Nature. 1967 Sep 23;215(5108):1395–1395. doi: 10.1038/2151395a0. [DOI] [PubMed] [Google Scholar]
- Leaf A. Transepithelial transport and its hormonal control in toad bladder. Ergeb Physiol. 1965;56:216–263. [PubMed] [Google Scholar]
- Orloff J., Handler J. The role of adenosine 3',5'-phosphate in the action of antidiuretic hormone. Am J Med. 1967 May;42(5):757–768. doi: 10.1016/0002-9343(67)90093-9. [DOI] [PubMed] [Google Scholar]
- PARSONS D. S., PATERSON G. R. Movements of fluid and glucose in an everted sac preparation of rat colonic mucosa. Biochim Biophys Acta. 1960 Jun 17;41:173–175. doi: 10.1016/0006-3002(60)90393-0. [DOI] [PubMed] [Google Scholar]
- Quigley J. P., Gotterer G. S. Properties of a high specific activity, (Na+-K+)-stimulated ATPase from rat intestinal mucosa. Biochim Biophys Acta. 1969 Apr;173(3):469–476. doi: 10.1016/0005-2736(69)90011-x. [DOI] [PubMed] [Google Scholar]
- SCHATZMANN H. J. Herzglykoside als Hemmstoffe für den aktiven Kalium- und Natriumtransport durch die Erythrocytenmembran. Helv Physiol Pharmacol Acta. 1953;11(4):346–354. [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F., Wright E. M. Interpretation of hexose-dependent electrical potential differences in small intestine. Nature. 1967 Apr 29;214(5087):509–510. doi: 10.1038/214509a0. [DOI] [PubMed] [Google Scholar]
- Smyth D. H. Symposium on membrane transport. Electrical activity in relation to intestinal transport. Proc R Soc Med. 1967 Apr;60(4):321–324. doi: 10.1177/003591576706000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISEMAN G. Absorption of amino-acids using an in vitro technique. J Physiol. 1953 Apr 28;120(1-2):63–72. doi: 10.1113/jphysiol.1953.sp004873. [DOI] [PMC free article] [PubMed] [Google Scholar]



