Abstract
The tra genes orf1 to orf11 of pIP501 were shown to be transcribed as a single operon of 11.3 kb in Enterococcus faecalis by reverse transcription-PCR. The transcriptional start site of the tra mRNA was mapped at 110 bp upstream from the predicted TTG start codon of the first gene of the operon, the traA relaxase. The TraA protein (660 amino acids) and a C-terminally truncated version of the TraA protein (293 amino acids) were purified as fusions with glutathione S-transferase. oriT cleavage activity of both TraA proteins was demonstrated in vitro on supercoiled plasmid pVA2241 DNA containing oriTpIP501. The activity of the DNA relaxase TraA is strictly dependent on the presence of Mg2+ or Mn2+ and is highest at temperatures of between 42 and 45°C.
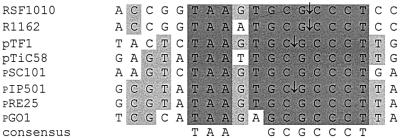
pIP501 is a 30.2-kb Inc18 broad-host-range conjugative plasmid that was originally isolated from Streptococcus agalactiae. It confers resistance to macrolide, lincosamide, and streptogramin B antibiotics and to chloramphenicol. In addition to self-transfer to a wide variety of gram-positive genera, pIP501 is able to mobilize non-self-transmissible plasmids such as pMV158, the prototype of family 2 of rolling-circle-replicating plasmids (6). Krah and Macrina (16) postulated that the determinants conferring pIP501-mediated conjugation are encoded by two separate regions, a so-called region A and another region, region B, which has not been further characterized. Wang and Macrina (25) reported the nucleotide sequence of the complete 8,121-bp region A. Analysis of this DNA sequence revealed six contiguous open reading frames (orf1 to orf6) and an oriT site with high sequence identity to oriT regions of plasmids from gram-negative bacteria (Fig. 1). The oriT of pIP501 belongs to the RSF1010 family (27). An alignment of the nick regions of the RSF1010 family (modified from reference 27), including two more plasmids of gram-positive origin, i.e., pGO1 from Staphylococcus aureus (5) and a new member, pRE25 from Enterococcus faecalis (GenBank accession no. X92945), is presented in Fig. 1. pRE25 is a 50.237-kb conjugative multiresistance plasmid whose putative tra region shows high overall identity with the tra region of pIP501 (B. Kurenbach and E. Grohmann, unpublished data).
FIG. 1.
Alignment of oriT nick regions, modified from reference 27. Nucleotides conserved in the nick regions of at least seven of eight plasmids of the RSF1010 oriT family are shown with dark grey shading. Nucleotides that are conserved in at least five of the eight plasmids are marked with light grey shading. A consensus sequence is also shown. The cleavage sites determined experimentally are indicated by arrows. GenBank/EMBL accession numbers are as follows: RSF1010, M28829; R1162, M13380; pTF1, X52699; pTiC58, M95646; pSC101, X01654; pIP501, L39769; pRE25, X92945; and pGO1, U50629.
The precise nick site within oriTpIP501 was mapped by primer extension analysis on relaxed (open circular) pVA1857 DNA containing the entire tra region (26). To only one of the genes encoded by the pIP501 tra region A was a putative function ascribed. Wang and Macrina (26) demonstrated nicking of the oriTpIP501 site with plasmid pVA1860 carrying only oriTpIP501 and the traA gene, which gave rise to amounts of open circular plasmid DNA similar to those with pVA1857 containing the entire tra region.
The six proteins encoded by tra region A of pIP501 show high overall similarity (between 24 and 36% identity over the whole amino acid sequence) with the gene products of the tra regions of the staphylococcal plasmids pGO1 (5) and pSK41 (3) and of the lactococcal conjugative plasmid pMRC01 (7). The TraA protein encoded by pIP501 belongs to the family of IncQ-type relaxases, which includes the relaxases of the gram-positive plasmids pGO1, pSK41, and pMRC01 as well as those of plasmids RSF1010, pSC101, and pTF1 of gram-negative origin (27). The prototype of this relaxase family is the MobA protein encoded by the mobilizable plasmid RSF1010. MobA is a multifunctional protein consisting of an N-terminal relaxase domain and a C-terminal DNA primase domain. Both domains are involved in vegetative plasmid replication (21) as well as in transfer replication (13). The RSF1010 relaxase family shows the conserved active-site tyrosine (Tyr-26 for TraA of pIP501) that is located at the very N terminus of the respective proteins, similar to the case for the other relaxase types. The tyrosine in the catalytic center of MobA has been identified by sequencing a proteolytic peptide that remained covalently attached to a single-stranded DNA substrate (22). IncQ-type relaxases also contain motif III, which is present not only in all conjugative DNA relaxases but also in several other rolling-circle initiator proteins. Motif III was first identified in the N-terminal moiety of IncP-type relaxases (2). It is specified by two histidines (His-134 and -136 in TraA) separated by a hydrophobic residue. A model for the possible role of these essential histidine residues was presented (14). Those authors proposed that the histidine residues are involved in coordination of the Mg2+ ions that are required for oriT cleavage activity.
In this study we show cotranscription of the pIP501 tra genes orf1 to orf11. The transcriptional start site of the tra mRNA was mapped. The catalytic activity of the purified TraA protein was demonstrated by in vitro cleavage of supercoiled pIP501 oriT DNA.
The transfer genes orf1 to orf11 are transcribed as a single operon.
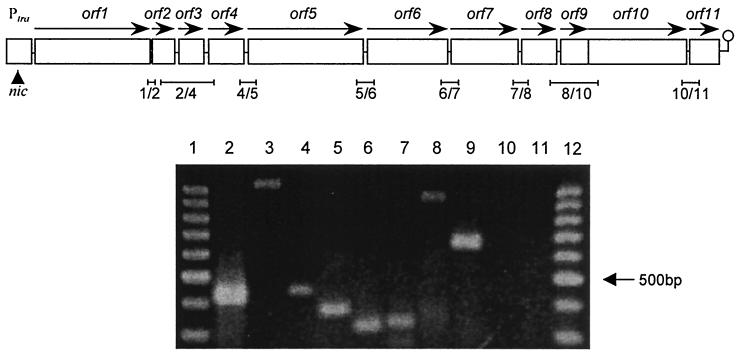
In order to test the putative cotranscription of the tra genes, we first performed reverse transcription-PCR (RT-PCR) assays with primer pairs designed to amplify two or three successive open reading frames within region A. To further characterize the pIP501 tra region, we sequenced the entire region B, which proved to be virtually identical to the corresponding part of the putative pRE25 tra region. The nucleotide sequence of region B will be submitted to GenBank, and a detailed analysis of the encoded gene products will be presented elsewhere (Kurenbach and Grohmann, unpublished data). Further RT-PCR assays with primer pairs selected to amplify two or three successive open reading frames within region B were performed in order to determine the end of the tra operon. The sequences of the primers used for RT-PCR will be made available upon request. Total RNA of E. faecalis JH2-2 (15) harboring pIP501 was isolated by three freeze-thaw cycles. The RNA was extracted twice with hot acid-phenol (70°C) and once with a mixture of hot acid-phenol and chloroform (1:1), precipitated with ethanol at −70°C, and dissolved in aqua bidest diethyl pyrocarbonate. Prior to use in RT-PCR, RNA was treated with DNase I (Promega). A 0.5-μg quantity of RNA was used in each reaction. RT-PCR was performed with the Access RT-PCR kit (Promega). Fifty picomoles of each primer was used in the RT-PCR assays. RNA samples were denatured for 2 min at 70°C prior to addition of polymerases. Control reactions were performed with RNA from the plasmid-free isogenic strain E. faecalis JH2-2 (Fig. 2, lane 10), without template RNA (data not shown), and with RNA template but with the RT step omitted (Fig. 2, lane 11). RT-PCR-amplified products, with the expected sizes, across the boundaries of two (orf1 and -2, orf4 and -5, orf5 and -6, orf6 and -7, orf7 and -8, and orf10 and -11) or three (orf2 to -4 and orf8 to -10) adjacent genes are shown in Fig. 2. By inspection of the sequence downstream from the translational termination codon of orf11 with the GCG TERMINATOR search program MFold (Wisconsin package version 10.2; Genetics Computer Group, Madison, Wis.), we found a potential rho-independent transcription terminator (28) with a free energy of −6 kcal mol−1 approximately 200 bp downstream the orf11 stop codon (not shown). A putative transcription terminator was also found in the intergenic region between orf11 (traK) and orf12 (traL) in the transfer region of pSK41 (9).
FIG. 2.
(Top) Organization of the tra region encompassing orf1 to orf11. Open reading frames are shown as boxes with horizontal arrows indicating the direction of transcription. The oriT nick site is indicated by an arrowhead. RT-PCR products are shown below the diagram. They are named according to the genes amplified, counting from orf1 to orf11. (Bottom) Cotranscription of the tra genes. Except for the amplification of the products from orf2 to orf4 and orf8 to orf10, the conditions were as follows. Incubation at 48°C for 40 min for RT was followed by inactivation of avian myeloblastosis virus reverse transcriptase and denaturation of the template at 94°C for 90 s. A cycle program consisting of 94°C for 30 s, 60°C for 90 s, and 68°C for 2 min was then applied for 30 cycles and terminated by a final elongation step of 7 min. For the amplification of products from orf2 to orf4 and orf8 to orf10, the RT step lasted 60 min and a cycle program consisting of 94°C for 30 s, 55°C for 90 s (51°C for orf8 to orf10), and 68°C for 4 min was applied for 35 cycles and terminated by a final elongation step of 8 min. The RT-PCR products were loaded onto 2.2% Tris-acetate-EDTA gels. Lanes 1 and 12, 100-bp DNA ladder (Promega); lane 2, product from orf1 and -2 (expected size, 406 bp); lane 3, product from orf2 to -4 (expected size, 1,035 bp); lane 4, product from orf4 and -5 (expected size, 433 bp); lane 5, product from orf5 and -6 (expected size, 359 bp); lane 6, product from orf6 and -7 (expected size, 322 bp); lane 7, product from orf7 and -8 (expected size, 326 bp); lane 8, product from orf8 to -10 (expected size, 932 bp); lane 9, product from orf10 and -11 (expected size, 690 bp). No detectable products were obtained in control reactions with each pair of primers with RNA from the plasmid-free isogenic strain E. faecalis JH2-2 (lane 10, with primers for orf1 and orf2) and with RNA from E. faecalis JH2-2(pIP501) with the RT step omitted (lane 11, with primers for orf1 and orf2).
Transcription initiation site of the tra operon.
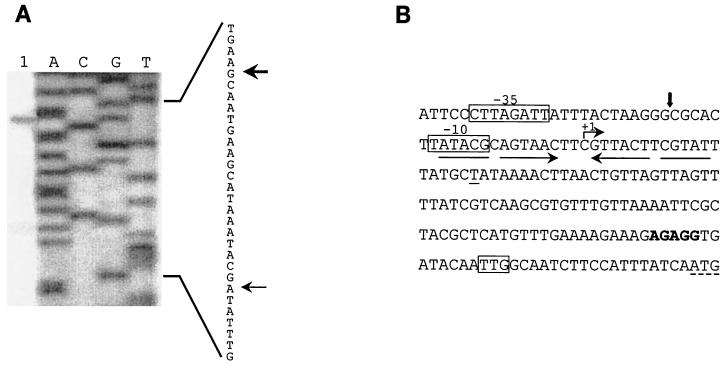
Primer extension reactions were carried out essentially as described previously (1) with minor modifications. One picomole of primer traP (5′-GGAAGATTGCCATGGGTATCACCTC-3′ [pIP501 positions 1382 to 1406]) was annealed at 65°C for 5 min to 15 μg of RNA purified from overnight cultures of E. faecalis JH2-2 harboring pIP501. E. faecalis JH2-2(pIP501) was grown in brain heart infusion medium (Difco) supplemented with 20 μg of chloramphenicol per ml. The primer extension reaction was started by the addition of 30 U of Moloney murine leukemia virus reverse transcriptase (Promega), and the reaction mixture was incubated for 1 h at 37°C. Reaction mixtures were phenol treated and ethanol precipitated, and the products were dissolved in 8 μl loading buffer (Amersham Pharmacia Biotech) and loaded onto 8% sequencing gels. Primer traP was also used for plasmid pIP501 DNA sequencing reactions with the Sequenase version 2.0 DNA sequencing kit (U.S. Biochemicals). Primer extension reactions (Fig. 3A, lane 1) resulted in one major product, showing the transcriptional start site at a C residue 110 bases upstream of the traA TTG start codon. A second, much weaker extension product started 91 bases upstream of the traA translation initiation codon, located just between two regions with potential to form a hairpin-like structure. Inspection of the DNA sequence around the transcription initiation point showed a promoter (trap) with weak −35 and −10 regions located nine nucleotides from the initiation site of tra mRNA. The transcriptional start site (+1) is situated between the two halves of an inverted repeat structure in oriT with potential to generate a secondary structure (Fig. 3B). The −10 box of trap coincides with the first half of the inverted repeat structure. The nucleotide sequence (coding strand) of the region encompassing the pIP501 oriT, including trap, the start codon, and the putative ribosome binding site of traA, is shown in Fig. 3B.
FIG. 3.
(A) Lane 1, primer extension of mRNA from E. faecalis JH2-2 harboring plasmid pIP501. The experiment was performed with total RNA and primer traP, which overlaps the putative start codon of traA. Lanes A, C, G, and T, corresponding DNA sequence reactions to size the extension products which are marked by arrows. The corresponding pIP501 sequence (complement of the sequence depicted in panel B) is shown on the right. (B) oriT region of pIP501 (nucleotides 1238 to 1417). The transcriptional start site of tra mRNA is marked with a bent arrow and numbered +1. The start of the very weak second extension product (T at position 1304) is underlined. The oriT nick site is indicated by a vertical arrow. The putative −10 and −35 promoter elements are boxed. Arrows below the sequence mark an imperfect inverted repeat with potential to generate a secondary structure. The two possible start codons of traA are indicated, and the putative ribosome binding site is shown in boldface.
The TraA protein specifically cleaves supercoiled pVA2241 DNA containing oriTpIP501.
Plasmid pVA2241 (containing a 309-bp fragment of pVA1702 encompassing the oriTpIP501 [26]) was purified with the Plasmid Maxi Kit (Qiagen) and used as a substrate in an in vitro cleavage assay with purified TraA protein. The traA gene was amplified as a 2,003-bp fragment by PCR. The traA coding region was cloned into the unique BamHI and EcoRI sites of plasmid pGEX-2T (Amersham Pharmacia Biotech). Similarly, the region of the traA gene corresponding to the N terminus (coding for the first 293 amino acids of a total of 660 amino acids and designated traA*) was amplified as a 893-bp fragment by PCR, and the traA* coding region was also inserted into the BamHI and EcoRI sites of plasmid pGEX-2T. The sequences of the primers used for traA and traA* amplification and cloning will be made available upon request. The sequences of the insertions in the recombinant plasmids pGEX-2T-traA and pGEX-2T-traA* were controlled by dideoxy chain termination sequencing in an automated sequencer (ABI Prism 310; Perkin-Elmer).
Both proteins were overexpressed in Escherichia coli BL21 and purified by affinity chromatography. E. coli BL21 cultures harboring pGEX-2T-traA and pGEX-2T-traA* were grown in Luria-Bertani medium, induced with 0.1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), and lysed by the addition of 1.5% Sarkosyl followed by 1 min of sonication on ice (Branson Sonifier 250; power level 7, 35% duty cycle) as described previously (10). The lysates were clarified by centrifugation at 10,000 × g (5 min, 4°C), and the supernatants were adjusted to 2% Triton X-100 and applied to glutathione-Sepharose 4B columns. The purification procedure was according to the instructions of the supplier (Amersham Pharmacia Biotech) with minor modifications: all purification steps were performed at 4°C, and incubation of the protein-loaded column with elution buffer was extended to 30 min. The purification procedure was followed by electrophoresis on sodium dodecyl sulfate-10% polyacrylamide gels (not shown). Protein concentrations were determined by the Bradford dye-binding procedure (Bio-Rad).
To assess the cleavage activity of TraA and to establish the optimal reaction conditions, the protein was incubated with purified pVA2241 supercoiled DNA (500 ng; form FI) under various conditions. Control reactions were performed with the oriT− plasmid pDL277 (16, 17). The relaxed products (form FII) of the TraA-mediated activity were visualized by stopping the reaction with 10 mM EDTA and then incubating with sodium dodecyl sulfate and proteinase K. The optimal temperature range was 42 to 45°C, with the reactions being less efficient at temperatures of below 37°C. The TraA-mediated cleavage of supercoiled DNA was strictly dependent on the presence of Mg2+ or Mn2+. The Mg2+ optimum was 5 mM, and the optimal Mn2+ concentration was 10 mM. The requirement for a divalent cation was not fulfilled by Ca2+ or Zn2+. As was the case with MobM of pMV158 (12), the TraI-TraJ-oriT complexes described for plasmid RP4 (20), the TrwC protein from R388 (18), and TraI of F (19), the maximum amount of form FII generated by TraA was about 55%. Total DNA relaxation was not achieved.
Interestingly, the amino-terminal portion of TraA comprising the first 293 amino acids also cleaves supercoiled pVA2241 DNA, although less efficiently (about 25% conversion) than the full-length protein. These data are in good agreement with the results obtained for the MobA protein of plasmid RSF1010. Observations with a C-terminally truncated MobA protein indicated that the MobA-dependent oriT nicking activity resides within the first 34% of the 78-kDa MobA polypeptide (243 amino acids) (23).
The highest homology of the newly identified putative pIP501 transfer genes orf7 to orf11 to known bacterial transfer proteins on the amino acid level was found for orf10. It was shown to belong to the TraG-TraD family of bacterial transfer proteins, with highest similarity to the Agrobacterium tumefaciens conjugal transfer protein TraG and the Agrobacterium T-DNA transfer system protein VirD4. VirD4 and TraG are members of the so-called coupling protein family, which appear to be necessary for presentation of the DNA substrate to the mating channel. Recently there has been accumulating evidence that these coupling proteins contribute not only to DNA transport but also to type IV-mediated protein export (reference 4 and references therein).
The compact organization of the pIP501 oriT region resembles that of the rolling-circle-replicating plasmid pMV158, which can be efficiently mobilized by pIP501 (reference 24 and our unpublished data). These are similar, in the sense that the nick region, where the DNA relaxase MobM binds to its cognate DNA (11), lies within the promoter structure (trap [Fig. 3B] and mobp [8]). This configuration makes autoregulation of the tra operon by the DNA relaxase TraA likely. For both DNA relaxases, MobM and TraA, this assumption is currently under investigation.
Acknowledgments
We thank F. Macrina (Virginia Commonwealth University, Richmond) for his kind gift of plasmids pVA2241 and pDL277 and M. Espinosa and E. L. Zechner for critical reading of the manuscript. We are very grateful to M. Meixner (DLMBC) for sequencing the pIP501 region B and to K. F. Genser for the transcriptional terminator search.
This research was funded by a grant from the Deutsche Forschungsgemeinschaft (Gr1792/1-1).
REFERENCES
- 1.Acebo, P., A. M. Hernández-Arriaga, M. G. Kramer, M. Espinosa, and G. del Solar. 1998. Identification of a new gene in the streptococcal plasmid pLS1. Plasmid 40:214-224. [DOI] [PubMed] [Google Scholar]
- 2.Balzer, D., W. Pansegrau, and E. Lanka. 1994. Essential motifs of relaxase (TraI) and TraG proteins involved in conjugative transfer of plasmid RP4. J. Bacteriol. 176:4285-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg, T., N. Firth, S. Apisiridej, A. Hettiaratchi, A. Leelaporn, and R. A. Skurray. 1998. Complete nucleotide sequence of pSK41: evolution of staphylococcal conjugative multiresistance plasmids. J. Bacteriol. 180:4350-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christie, P. J. 2001. Type IV secretion: intercellular transfer of macromolecules by systems ancestrally related to conjugation machines. Mol. Microbiol. 40:294-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Climo, M. W., V. K. Sharma, and G. L. Archer. 1996. Identification and characterization of the origin of conjugative transfer (oriT) and a gene (nes) encoding a single-stranded endonuclease of the staphylococcal plasmid pGO1. J. Bacteriol. 178:4975-4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.del Solar, G., M. Moscoso, and M. Espinosa. 1993. Rolling circle-replicating plasmids from gram-positive and gram-negative bacteria: a wall falls. Mol. Microbiol. 8:789-796. [DOI] [PubMed] [Google Scholar]
- 7.Dougherty, B. A., C. Hill, J. F. Weidman, D. R. Richardson, J. C. Venter, and R. P. Ross. 1998. Sequence and analysis of the 60 kb conjugative, bacteriocin-producing plasmid pMRC01 from Lactococcus lactis DPC3147. Mol. Microbiol. 29:1029-1038. [DOI] [PubMed] [Google Scholar]
- 8.Farías, M. E., E. Grohmann, and M. Espinosa. 1999. Expression of the mobM gene of the streptococcal plasmid pMV158 in Lactococcus lactis subsp. lactis. FEMS Microbiol. Lett. 176:403-410. [DOI] [PubMed] [Google Scholar]
- 9.Firth, N., K. P. Ridgway, M. E. Byrne, P. D. Fink, L. Johnson, I. T. Paulsen, and R. A. Skurray. 1993. Analysis of a transfer region from the staphylococcal conjugative plasmid pSK41. Gene 136:13-25. [DOI] [PubMed] [Google Scholar]
- 10.Frangioni, J. V., and B. G. Neel. 1993. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Anal. Biochem. 210:179-187. [DOI] [PubMed] [Google Scholar]
- 11.Grohmann, E., L. M. Guzmán, and M. Espinosa. 1999. Mobilisation of the streptococcal plasmid pMV158: interactions of MobM protein with its cognate oriT DNA region. Mol. Gen. Genet. 261:707-715. [DOI] [PubMed] [Google Scholar]
- 12.Guzmán, L., and M. Espinosa. 1997. The mobilization protein, MobM, of the streptococcal plasmid pMV158 specifically cleaves supercoiled DNA at the plasmid oriT. J. Mol. Biol. 266:688-702. [DOI] [PubMed] [Google Scholar]
- 13.Henderson, D., and R. J. Meyer. 1996. The primase of broad-host-range plasmid R1162 is active in conjugal transfer. J. Bacteriol. 178:6888-6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ilyina, T. V., and E. V. Koonin. 1992. Conserved sequence motifs in the initiator proteins for rolling circle DNA replication encoded by replicons from eubacteria, eucaryotes and archaebacteria. Nucleic Acids Res. 20:3279-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacob, A. E., and S. J. Hobbs. 1974. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 117:360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krah, E. R., III, and F. L. Macrina. 1989. Genetic analysis of the conjugal transfer determinants encoded by the streptococcal broad-host-range plasmid pIP501. J. Bacteriol. 171:6005-6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Blanc, D. J., L. N. Lee, and A. Abu-Al-Jaibat. 1992. Molecular, genetic, and functional analysis of the basic replicon of pVA380-1, a plasmid of oral streptococcal origin. Plasmid 28:130-145. [DOI] [PubMed] [Google Scholar]
- 18.Llosa, M., G. Grandoso, and F. de la Cruz. 1995. Nicking activity of TrwC directed against the origin of transfer of the IncW plasmid R388. J. Mol. Biol. 246:54-62. [DOI] [PubMed] [Google Scholar]
- 19.Matson, S. W., and B. S. Morton. 1991. Escherichia coli DNA helicase I catalyzes a site- and strand-specific nicking reaction at the F plasmid oriT. J. Biol. Chem. 266:16232-16237. [PubMed] [Google Scholar]
- 20.Pansegrau, W., D. Balzer, V. Kruft, and E. Lanka. 1990. In vitro assembly of relaxosomes at the transfer origin of plasmid RP4. Proc. Natl. Acad. Sci. USA. 87:6555-6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scherzinger, E., V. Haring, R. Lurz, and S. Otto. 1991. Plasmid RSF1010 DNA replication in vitro promoted by purified RSF1010 RepA, RepB and RepC proteins. Nucleic Acids Res. 19:1203-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scherzinger, E., V. Kruft, and S. Otto. 1993. Purification of the large mobilization protein of plasmid RSF1010 and characterization of its site-specific DNA cleaving/DNA joining activity. Eur. J. Biochem. 217:929-938. [DOI] [PubMed] [Google Scholar]
- 23.Scherzinger, E., R. Lurz, S. Otto, and B. Dobrinski. 1992. In vitro cleavage of double- and single-stranded DNA by plasmid RSF1010-encoded mobilization proteins. Nucleic Acids Res. 20:41-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Lelie, D., H. A. Wosten, S. Bron, L. Oskam, and G. Venema. 1990. Conjugal mobilization of streptococcal plasmid pMV158 between strains of Lactococcus lactis subsp. lactis. J. Bacteriol. 172:47-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, A., and F. L. Macrina. 1995. Characterization of six linked open reading frames necessary for pIP501-mediated conjugation. Plasmid 34:206-210. [DOI] [PubMed] [Google Scholar]
- 26.Wang, A., and F. L. Macrina. 1995. Streptococcal plasmid pIP501 has a functional oriT site. J. Bacteriol. 177:4199-4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zechner, E. L., F. de la Cruz, R. Eisenbrandt, A. M. Grahn, G. Koraimann, E. Lanka, G. Muth, W. Pansegrau, C. M. Thomas, B. M. Wilkins, and M. Zatyka. 2000. Conjugative-DNA transfer processes, p. 87-174. In C. M. Thomas (ed.), The horizontal gene pool. Bacterial plasmids and gene spread. Harwood Academic Publishers, Amsterdam, The Netherlands.
- 28.Zuker, M. 1989. On finding all suboptimal foldings of an RNA molecule. Science 244:48-52. [DOI] [PubMed] [Google Scholar]