Abstract
1. Voltage clamp experiments on sartorius muscle fibres at 3° C showed that the potassium current is divisible into three components, namely:
(a) Current in the delayed rectifier channel, which reached a maximum in about 0·1 sec at -30 mV, and declined with a time constant of about 4 msec when the fibre was repolarized to -100 mV; this component had an approximately linear instantaneous current—voltage relation and an equilibrium potential E1 at 10-15 mV positive to the resting potential.
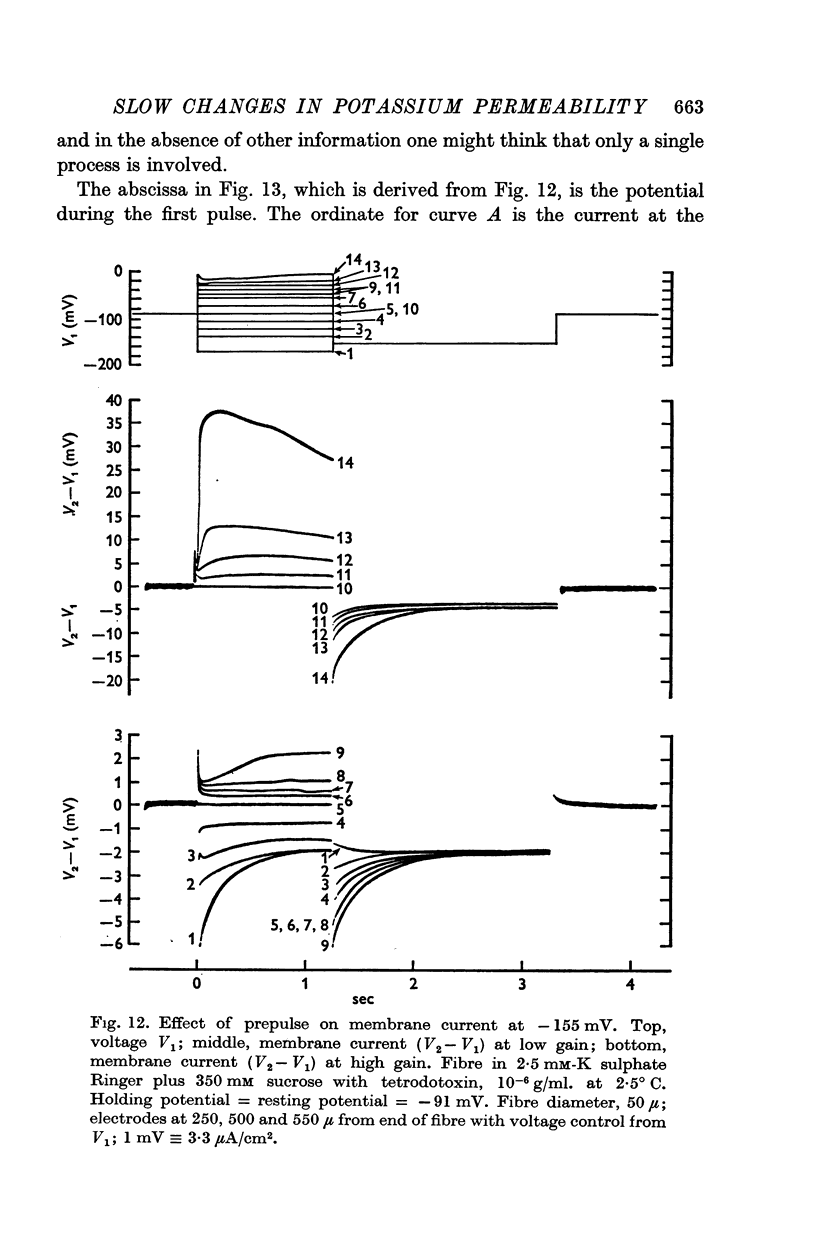
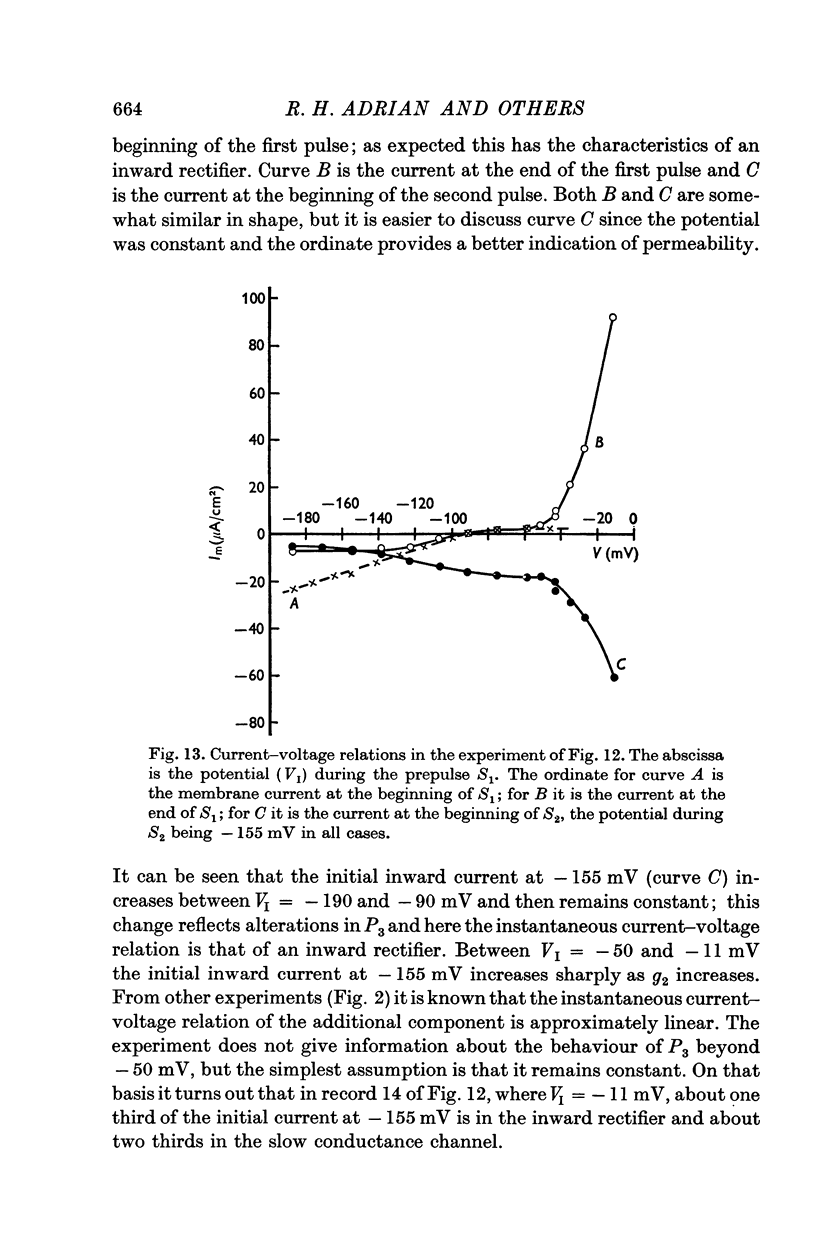
(b) A slow component which reached a maximum in about 3 sec at -30 mV, and declined with a time constant of about 0·5 sec when the fibre was repolarized to -100 mV; this component had an approximately linear instantaneous current—voltage relation and a mean equilibrium potential E2 at -83 mV in fibres where E1 averaged -75 mV.
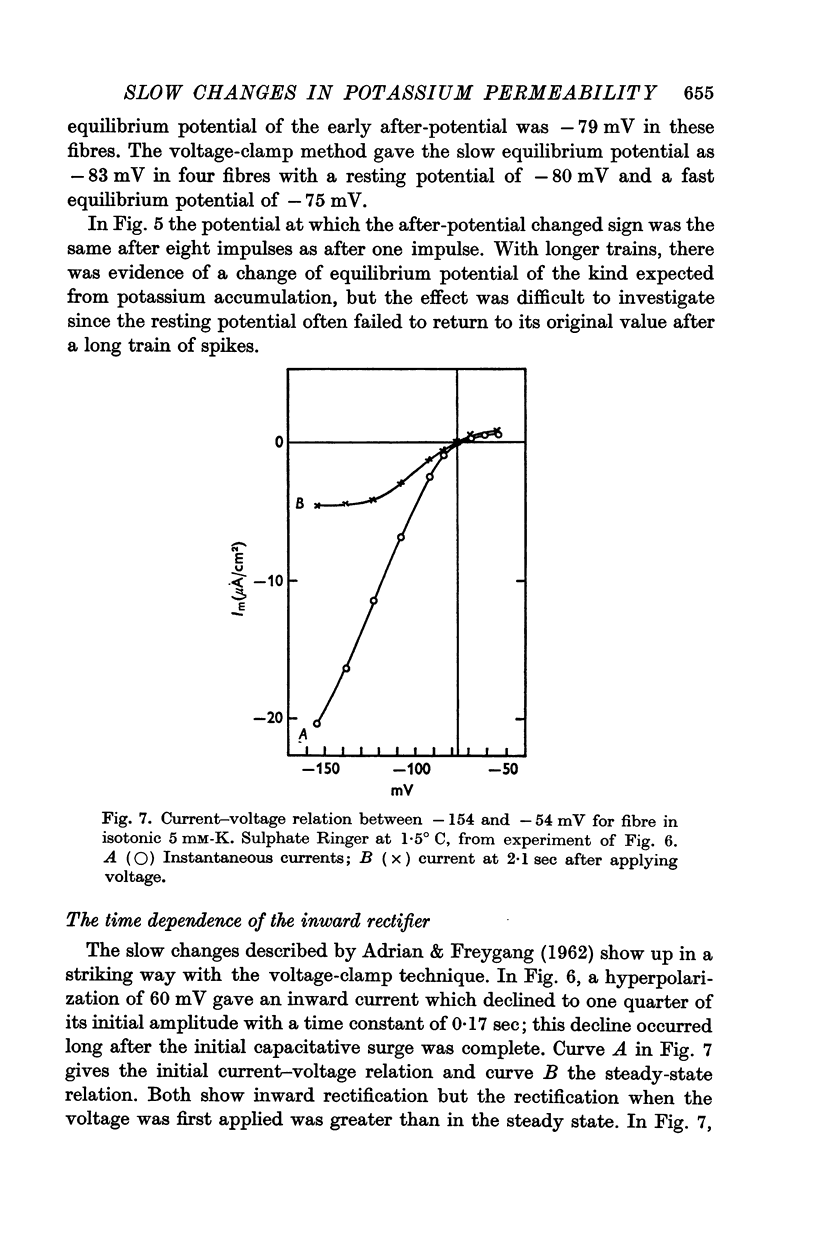
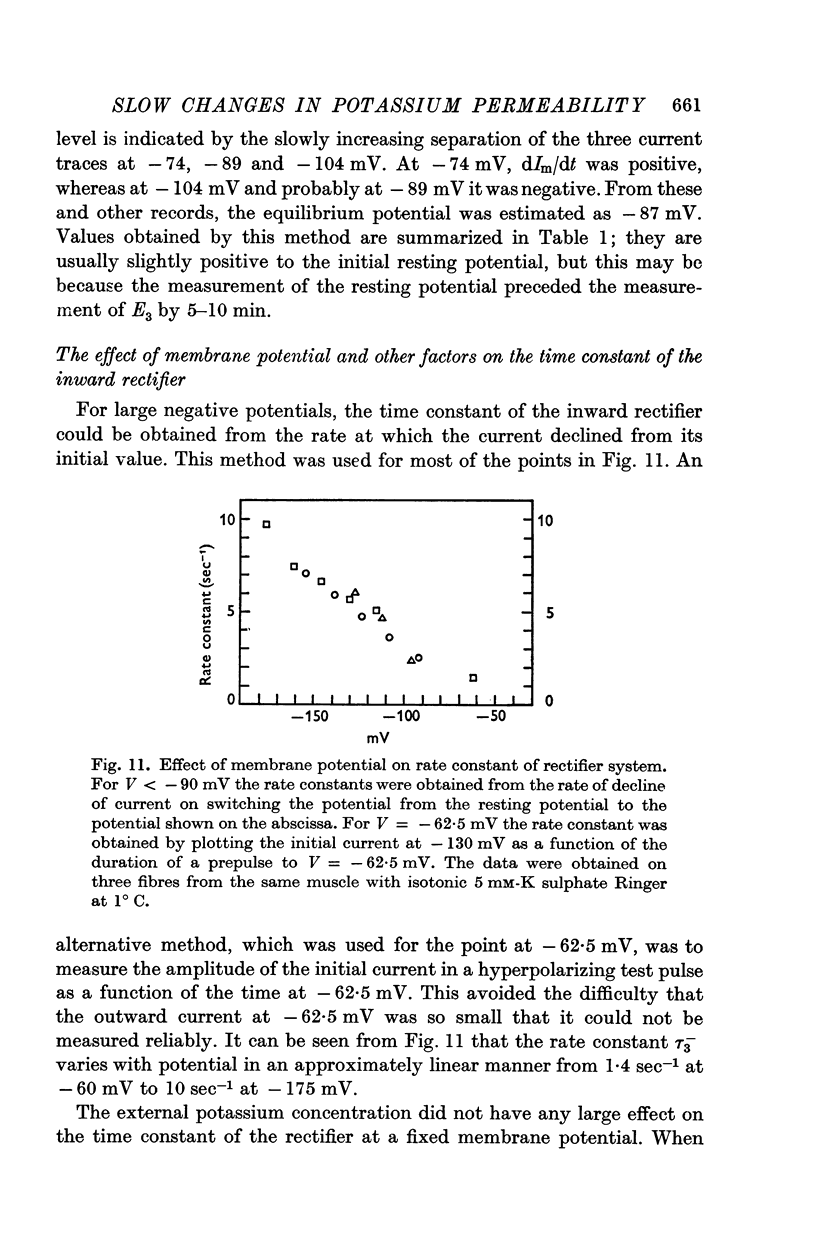
(c) Current in the inward rectifier channel which decreased with a time constant of about 0·25 sec when the fibre was hyperpolarized to -150 mV. This component had an equilibrium potential close to the resting potential and an instantaneous current—voltage relation which was that of an inward rectifier.
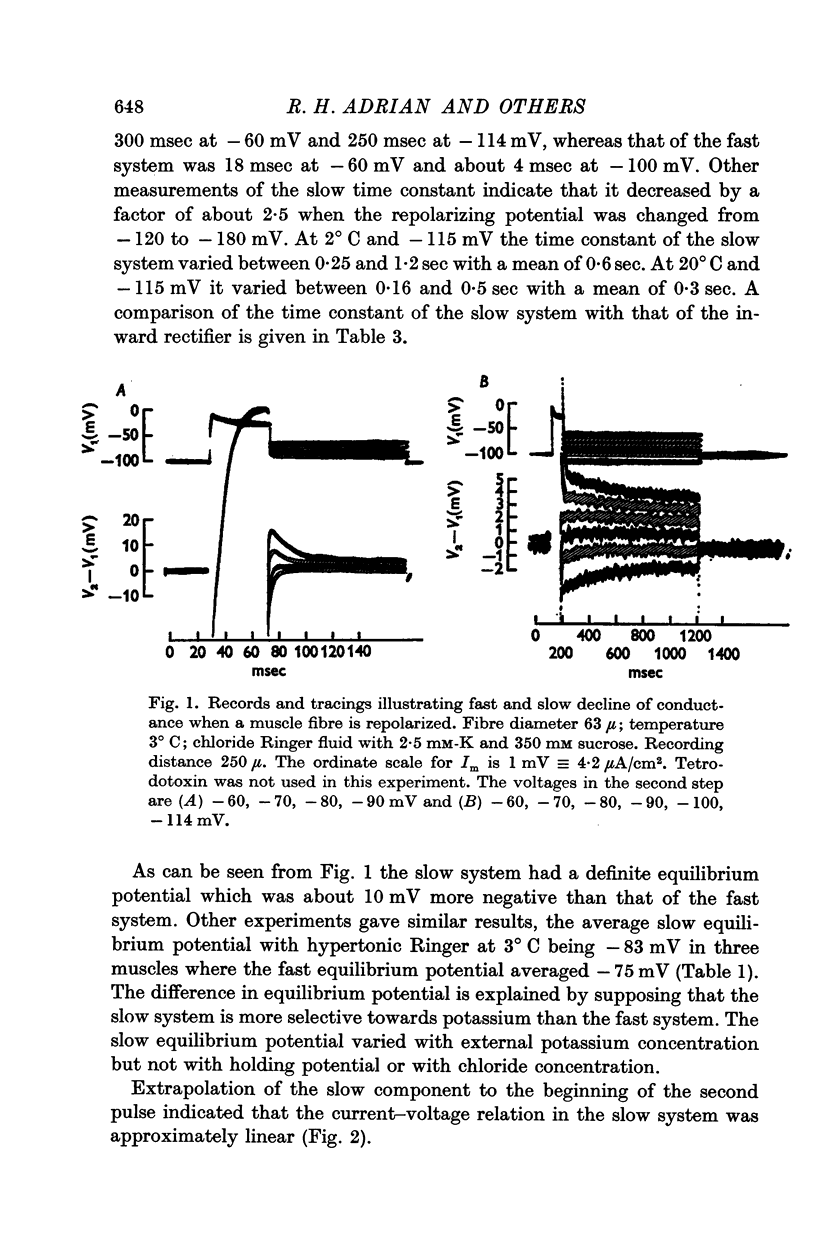
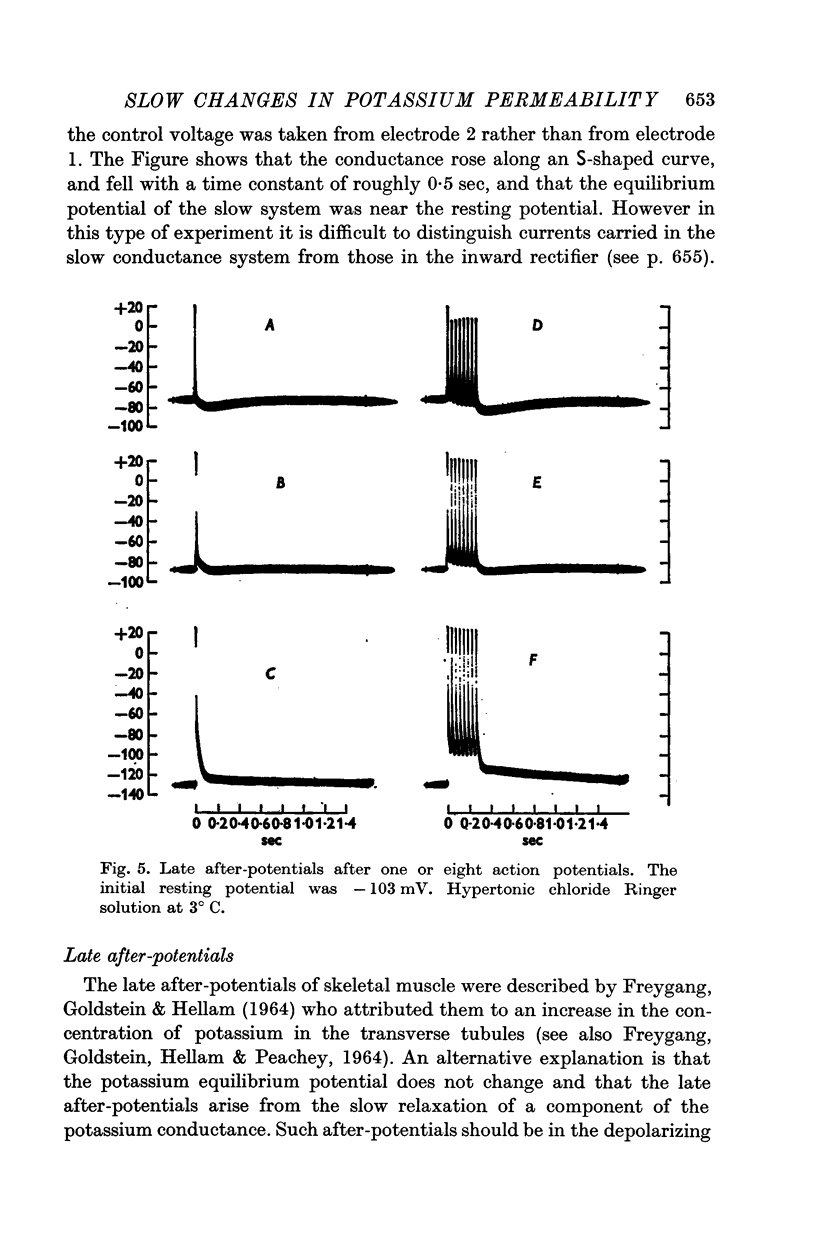
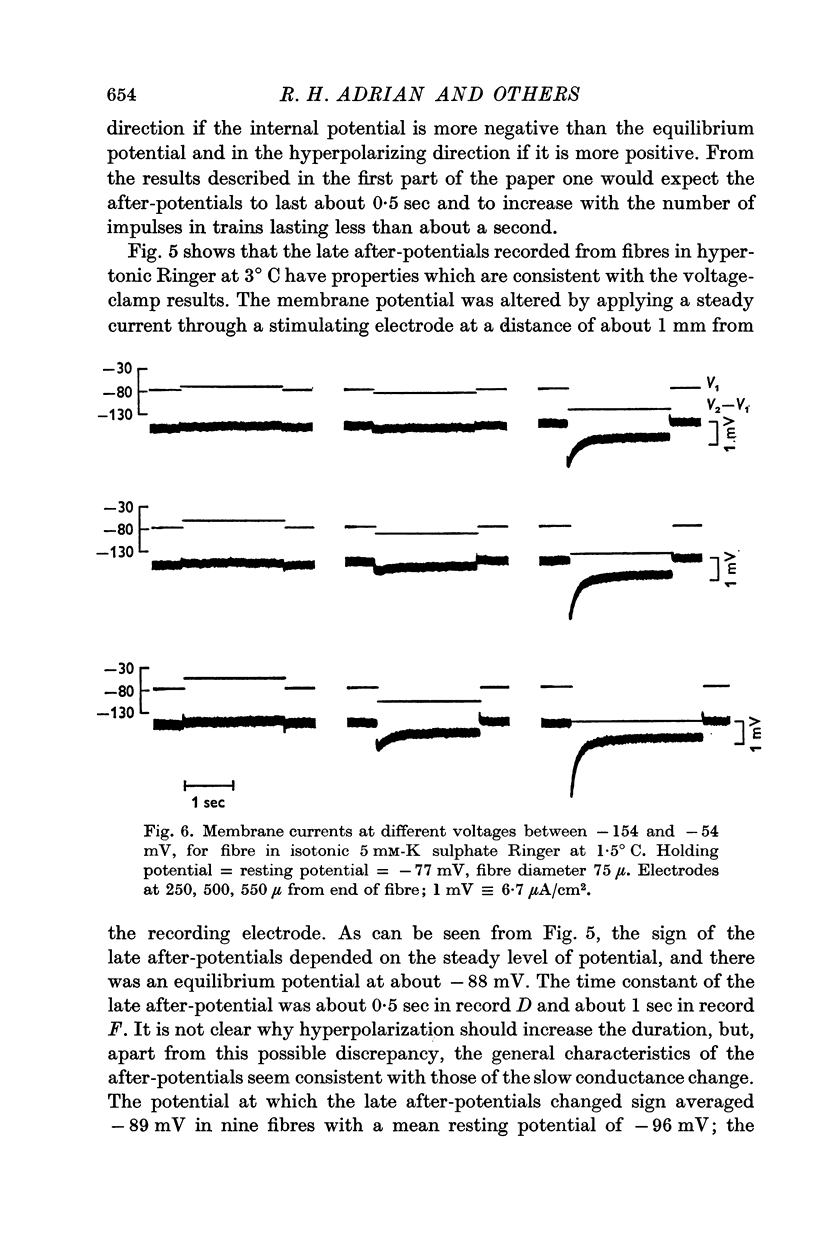
2. The general characteristics of the late after-potential in muscles in hypertonic solutions at 3° C are consistent with those of the slow conductance change. The sign of the late after-potentials was reversed by depolarizing below -80 mV.
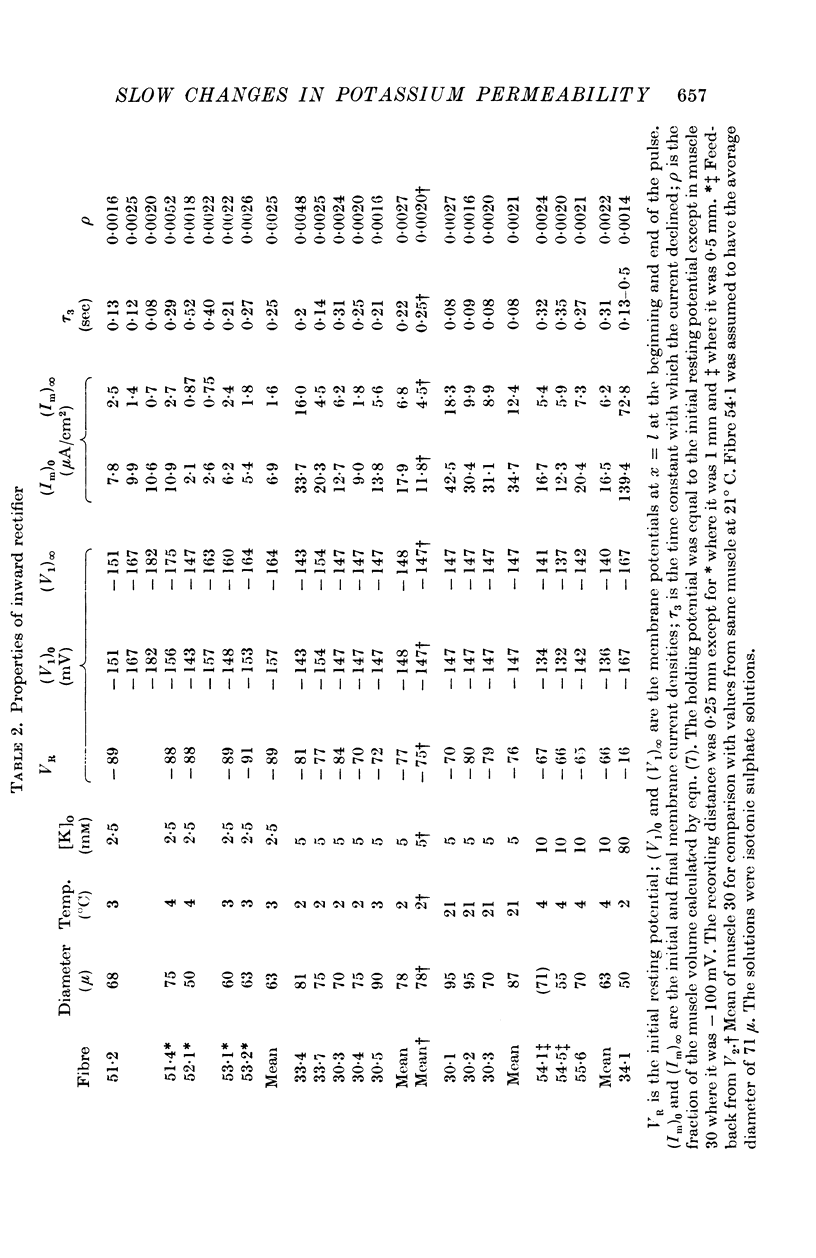
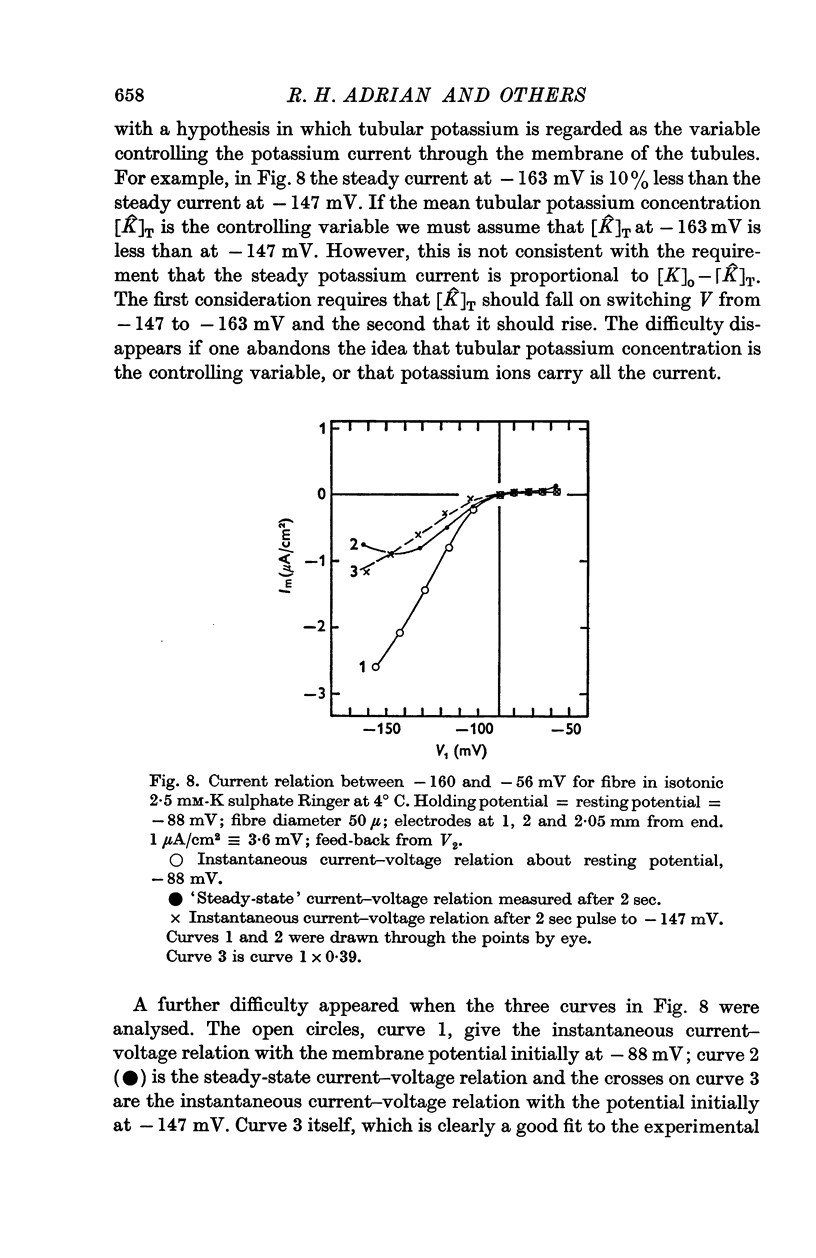
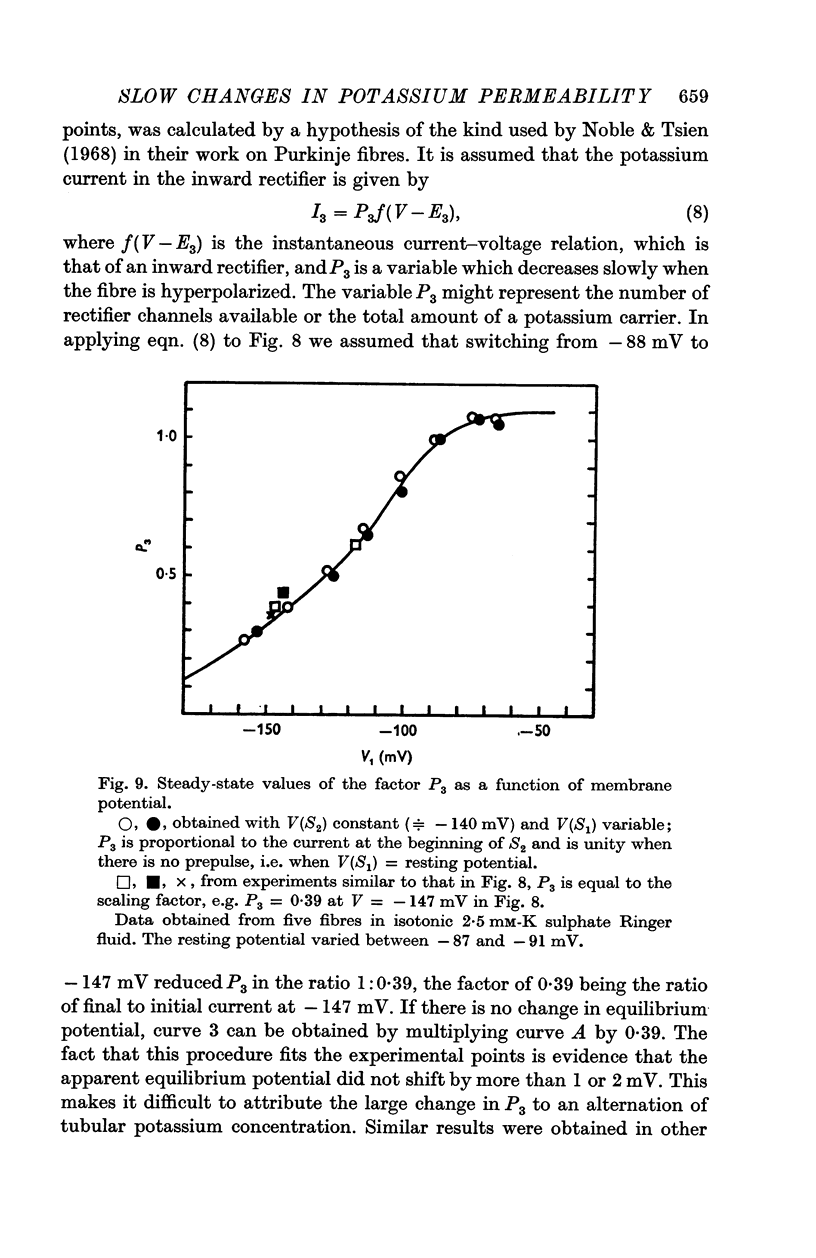
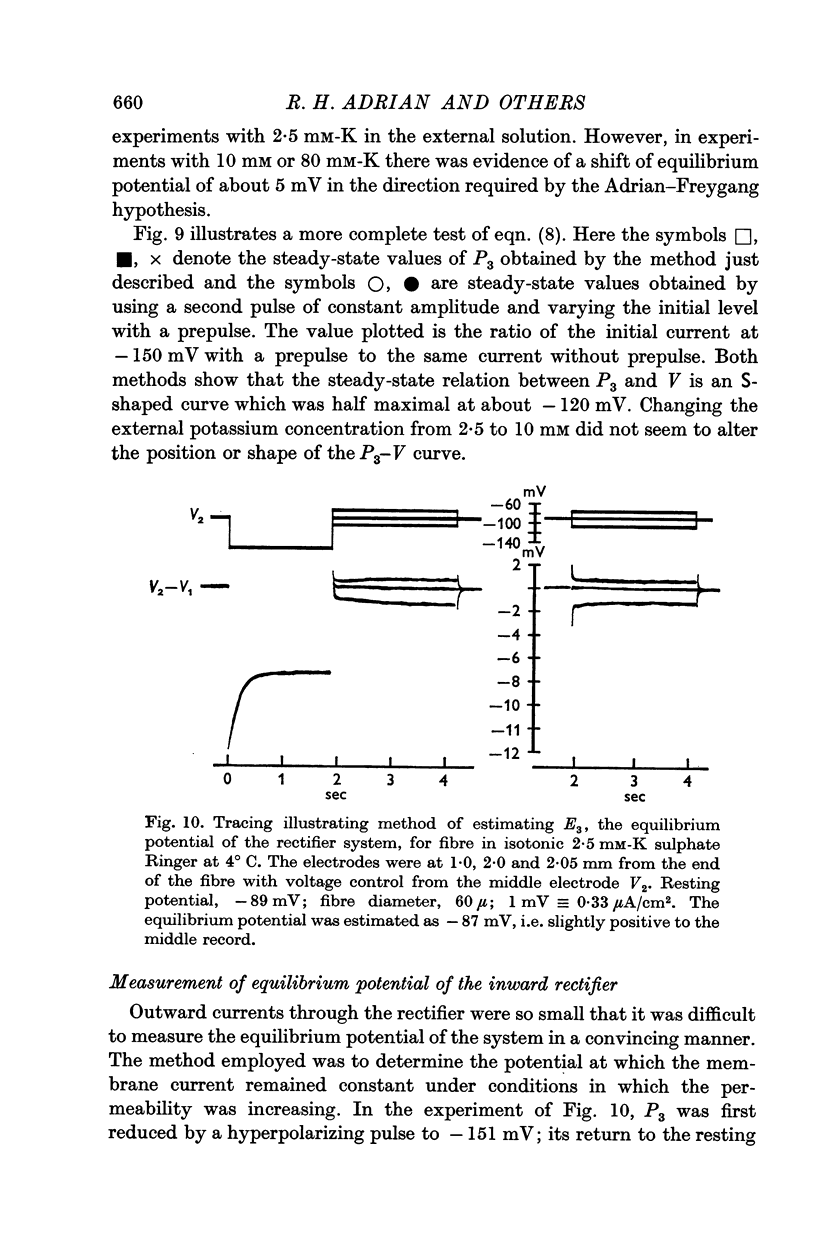
3. The decline of current during a maintained hyperpolarization cannot be attributed solely to a decrease in tubular potassium concentration, since there may be a large decrease in current without much alteration of equilibrium potential. The negative slope conductance often seen at -150 mV is also difficult to reconcile with the tubular depletion hypothesis.
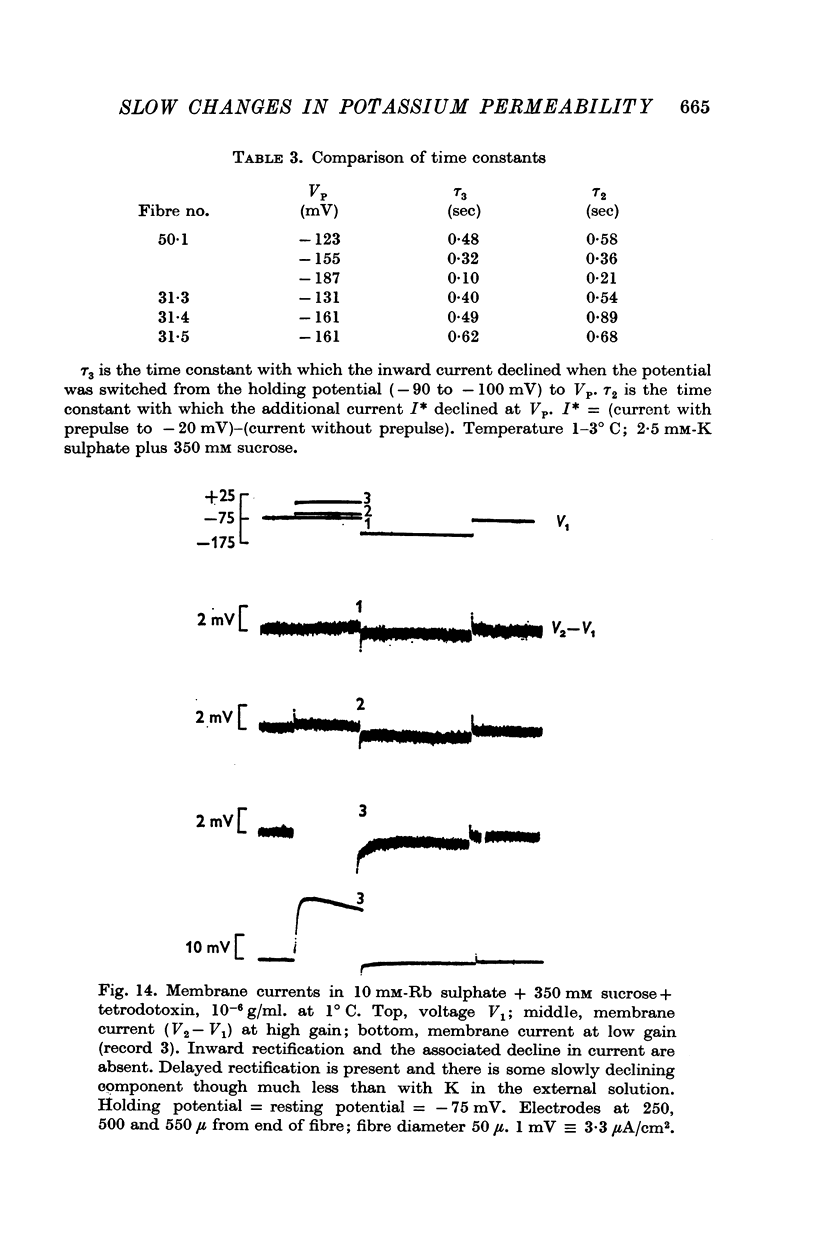
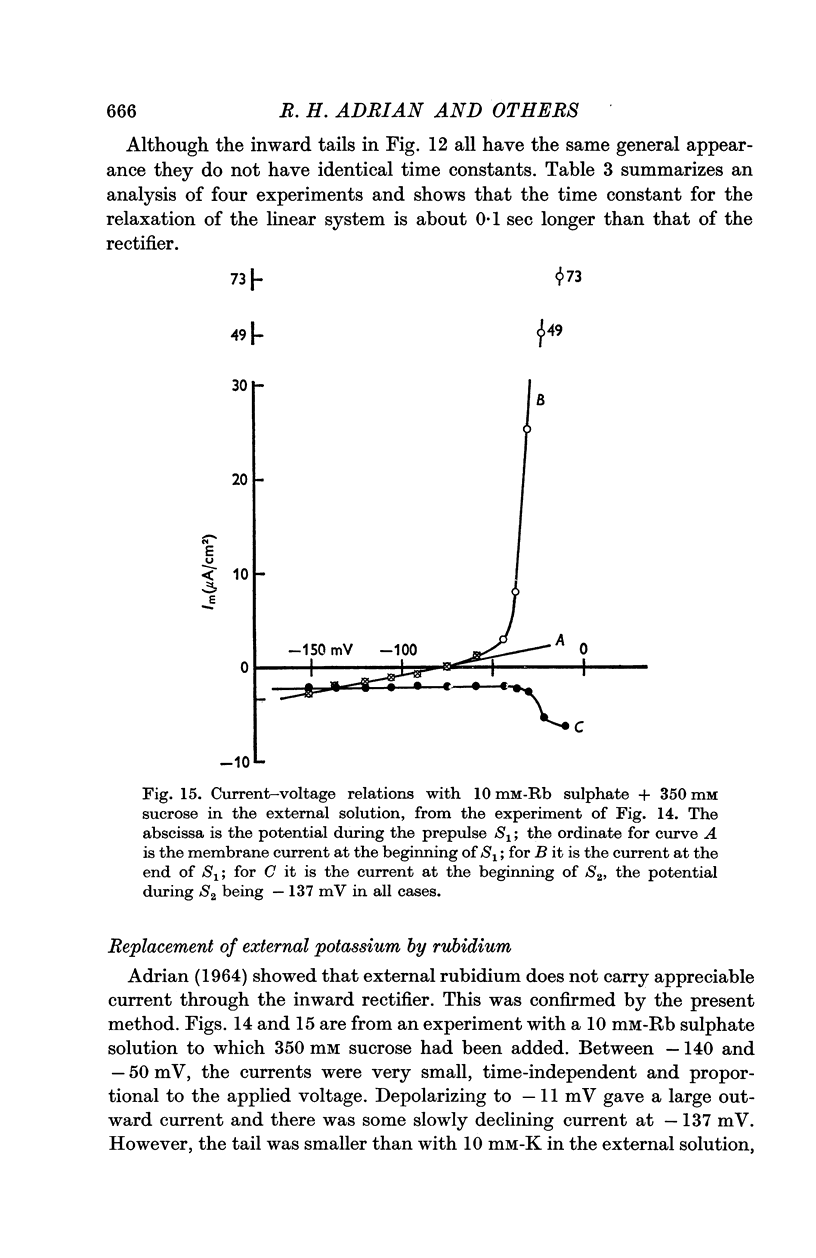
4. Replacement of 10 mM-K by 10 mM-Rb abolished inward rectification but had less effect on the fast and slow components of the potassium conductance.
Full text
PDF


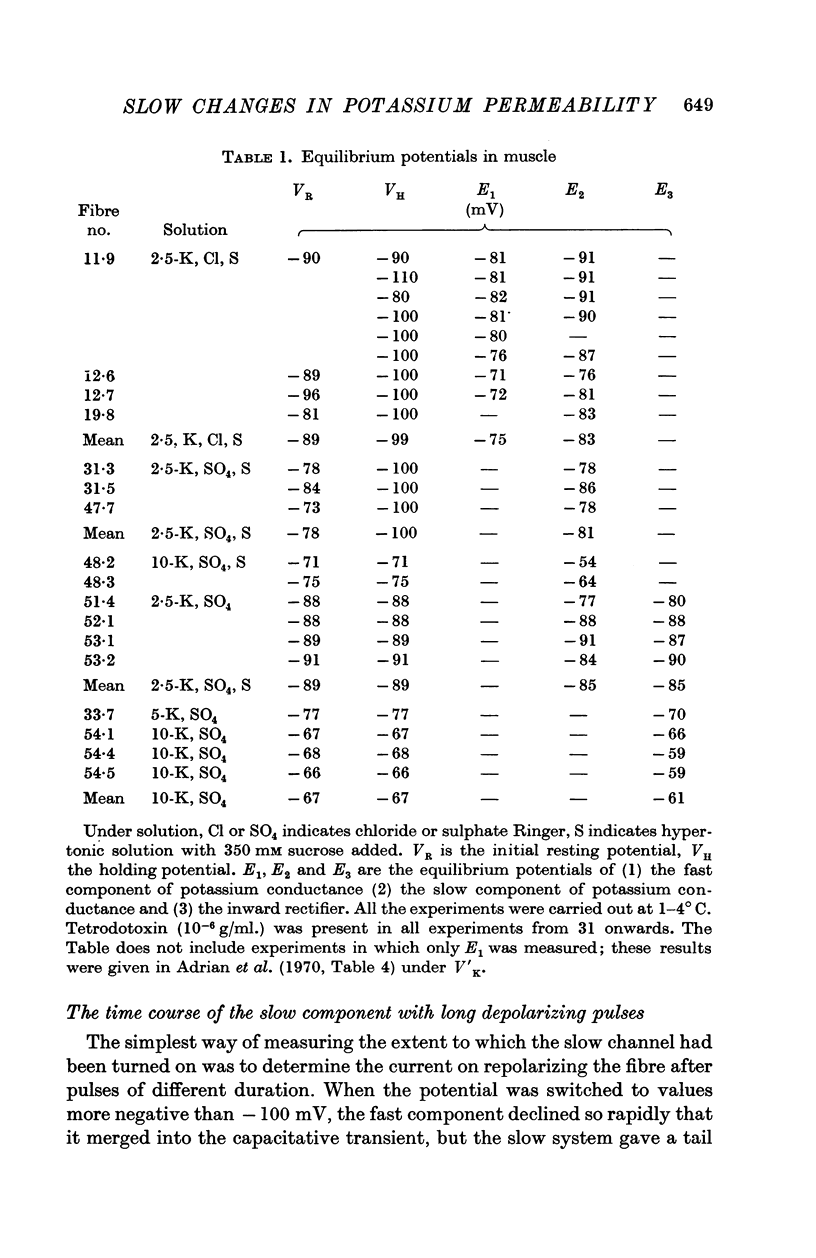
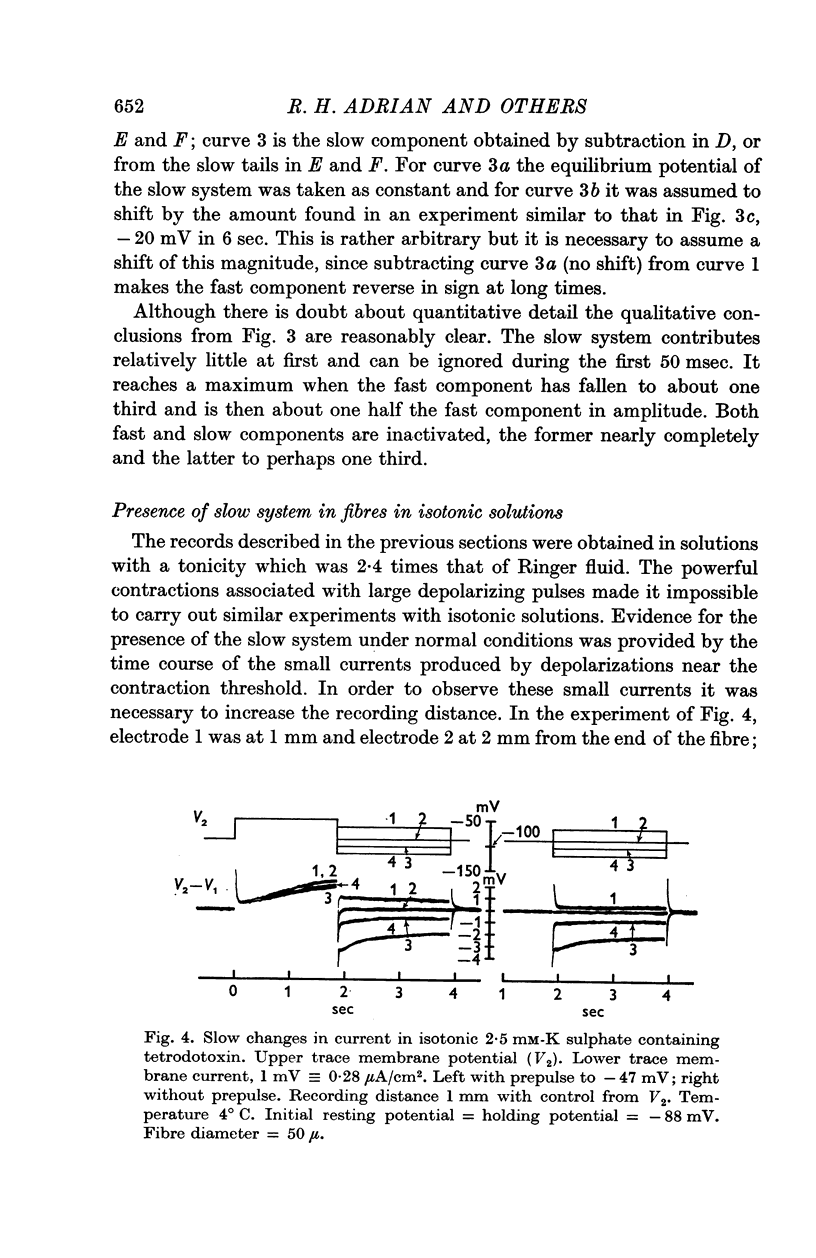




Selected References
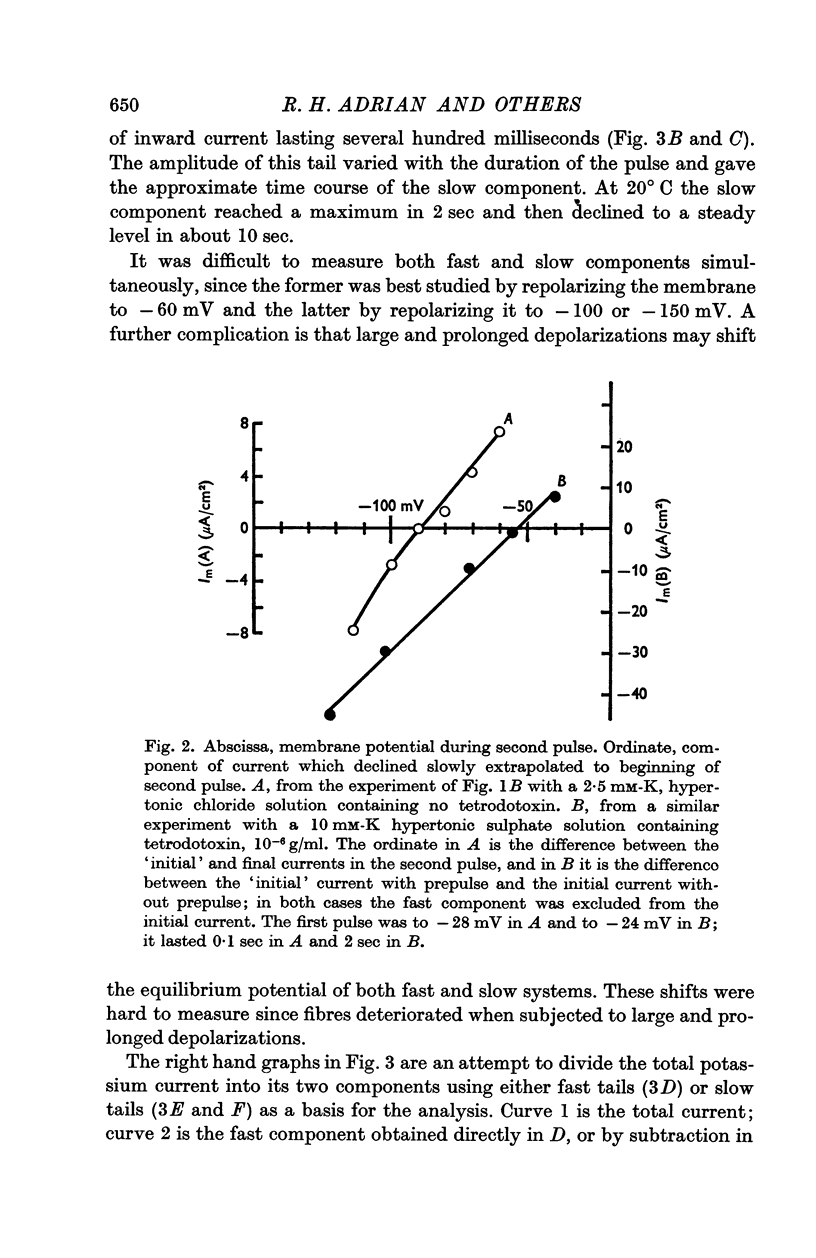
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. THE RUBIDIUM AND POTASSIUM PERMEABILITY OF FROG MUSCLE MEMBRANE. J Physiol. 1964 Dec;175:134–159. doi: 10.1113/jphysiol.1964.sp007508. [DOI] [PMC free article] [PubMed] [Google Scholar]
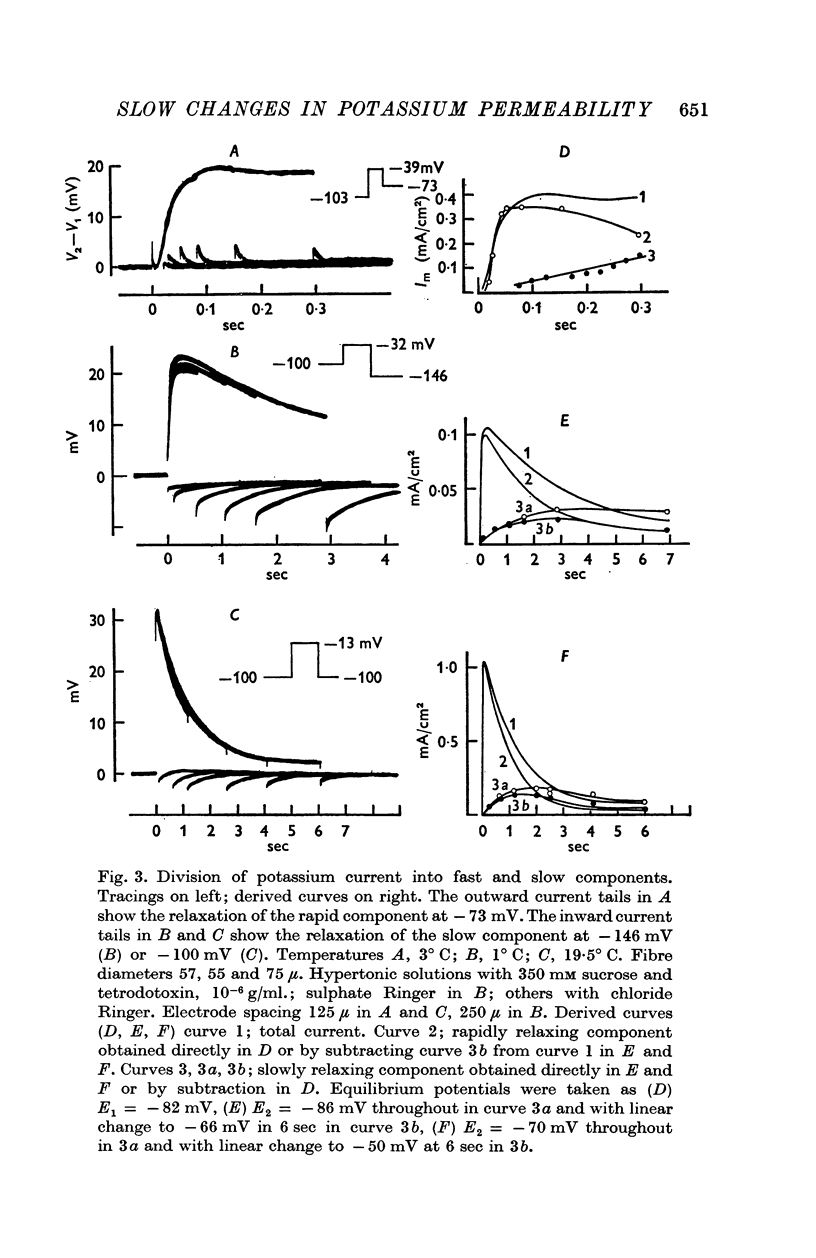
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian R. H., Freygang W. H. The potassium and chloride conductance of frog muscle membrane. J Physiol. 1962 Aug;163(1):61–103. doi: 10.1113/jphysiol.1962.sp006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREYGANG W. H., Jr, GOLDSTEIN D. A., HELLAM D. C., PEACHEY L. D. THE RELATION BETWEEN THE LATE AFTER-POTENTIAL AND THE SIZE OF THE TRANSVERSE TUBULAR SYSTEM OF FROG MUSCLE. J Gen Physiol. 1964 Nov;48:235–263. doi: 10.1085/jgp.48.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREYGANG W. H., Jr, GOLDSTEIN D. A., HELLAM D. C. THE AFTER-POTENTIAL THAT FOLLOWS TRAINS OF IMPULSES IN FROG MUSCLE FIBERS. J Gen Physiol. 1964 May;47:929–952. doi: 10.1085/jgp.47.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Eisenberg R. S. Action potentials, afterpotentials, and excitation-contraction coupling in frog sartorius fibers without transverse tubules. J Gen Physiol. 1969 Mar;53(3):298–310. doi: 10.1085/jgp.53.3.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY H. E. EVIDENCE FOR CONTINUITY BETWEEN THE CENTRAL ELEMENTS OF THE TRIADS AND EXTRACELLULAR SPACE IN FROG SARTORIUS MUSCLE. Nature. 1964 Jun 13;202:1067–1071. doi: 10.1038/2021067b0. [DOI] [PubMed] [Google Scholar]
- Noble D., Tsien R. W. The kinetics and rectifier properties of the slow potassium current in cardiac Purkinje fibres. J Physiol. 1968 Mar;195(1):185–214. doi: 10.1113/jphysiol.1968.sp008454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]


