Abstract
1. Repeated flashes of diffuse light were presented to the cat's eye over long periods of time (hours) while the mixed ganglion cell response was recorded from single axons in the optic tract. This was done for cats receiving gallamine triethiodide intravenously and for cats who did not receive the drug.
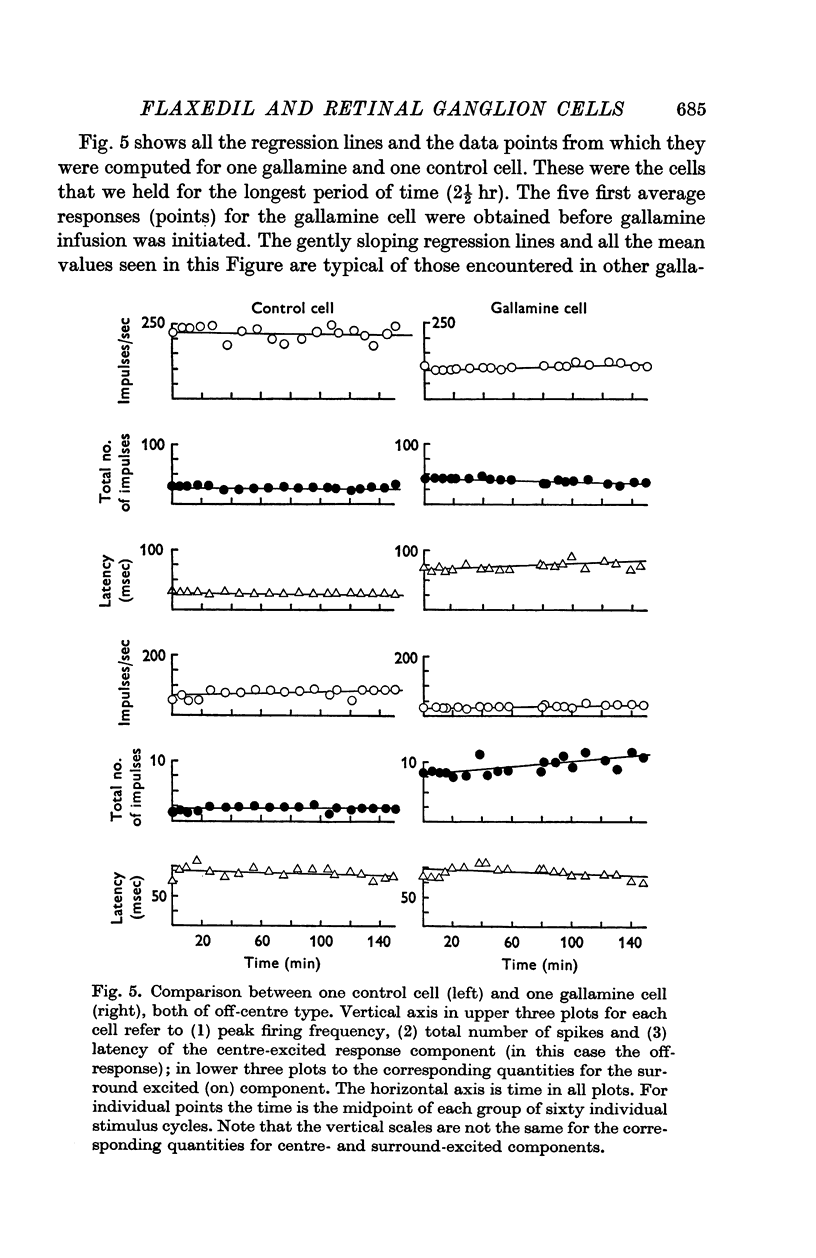
2. All responses were transformed into instantaneous pulse density tracings. Such tracings from control and gallamine cells were compared to observe possible effects of gallamine: (a) the shape (time course) did not change during gallamine administration; (b) maximum firing frequency, total number of spikes and latency was measured for both the on- and the off-component on the pulse density tracings and plotted versus time. Statistical methods failed to reveal any difference between the manner in which these response features of control cells and of gallamine cells varied with time.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow H. B., Levick W. R. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969 Jun;202(3):699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Cleland B. G., Cooper G. F., Enroth-Cugell C. The angular selectivity of visual cortical cells to moving gratings. J Physiol. 1968 Sep;198(1):237–250. doi: 10.1113/jphysiol.1968.sp008604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow K. L., Lindsley D. F. Influences of residual eye movements in single-unit studies of the visual system. Brain Res. 1968 May;8(2):385–388. doi: 10.1016/0006-8993(68)90060-7. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-Cugell C. Quantitative aspects of gain and latency in the cat retina. J Physiol. 1970 Jan;206(1):73–91. doi: 10.1113/jphysiol.1970.sp008998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-cugell C. Quantitative aspects of sensitivity and summation in the cat retina. J Physiol. 1968 Sep;198(1):17–38. doi: 10.1113/jphysiol.1968.sp008591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJong R. H., Robles R., Morikawa K. I. Actions of immobilizing drugs on synaptic transmission. Exp Neurol. 1968 Jun;21(2):213–218. doi: 10.1016/0014-4886(68)90139-8. [DOI] [PubMed] [Google Scholar]
- Galindo A., Krnjević K., Schwartz S. Patterns of firing in cuneate neurones and some effects of Flaxedil. Exp Brain Res. 1968;5(2):87–101. doi: 10.1007/BF00238699. [DOI] [PubMed] [Google Scholar]
- Halpern L. M., Black R. G. Flaxedil (gallamine triethiodide): evidence for a central action. Science. 1967 Mar 31;155(3770):1685–1687. doi: 10.1126/science.155.3770.1685. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Pettigrew J. D., Bishop P. O., Nikara T. Residual eye movements in receptive-field studies of paralyzed cats. Vision Res. 1967 Jan;7(1):107–110. doi: 10.1016/0042-6989(67)90031-4. [DOI] [PubMed] [Google Scholar]
- Stone J., Fabian M. Summing properties of the cat's retinal ganglion cell. Vision Res. 1968 Aug;8(8):1023–1040. doi: 10.1016/0042-6989(68)90075-8. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N. Receptive fields of ganglion cells in the cat's retina. J Physiol. 1960 Oct;153:583–594. doi: 10.1113/jphysiol.1960.sp006557. [DOI] [PMC free article] [PubMed] [Google Scholar]


