Abstract
1. Extracellular recordings were made from caudate nucleus neurones in decerebrate cats using five-barrel micropipette electrodes.
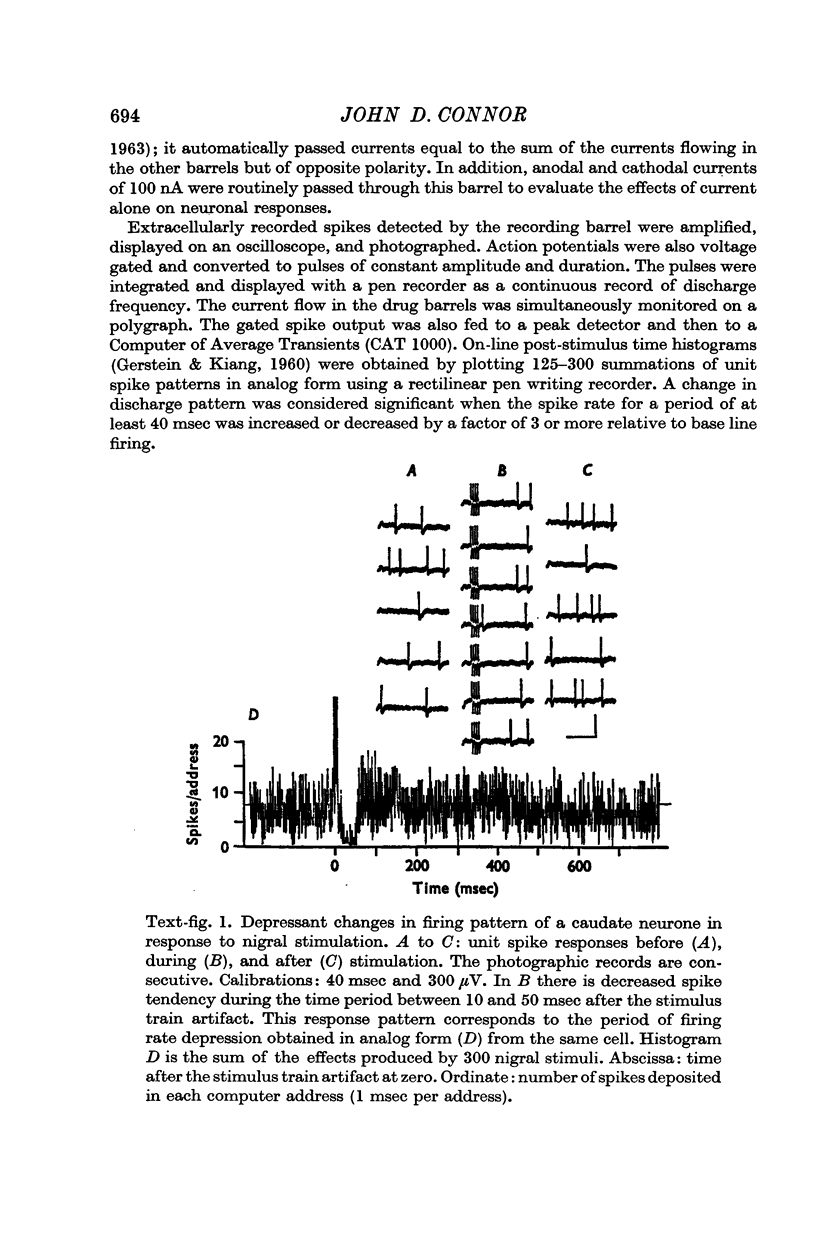
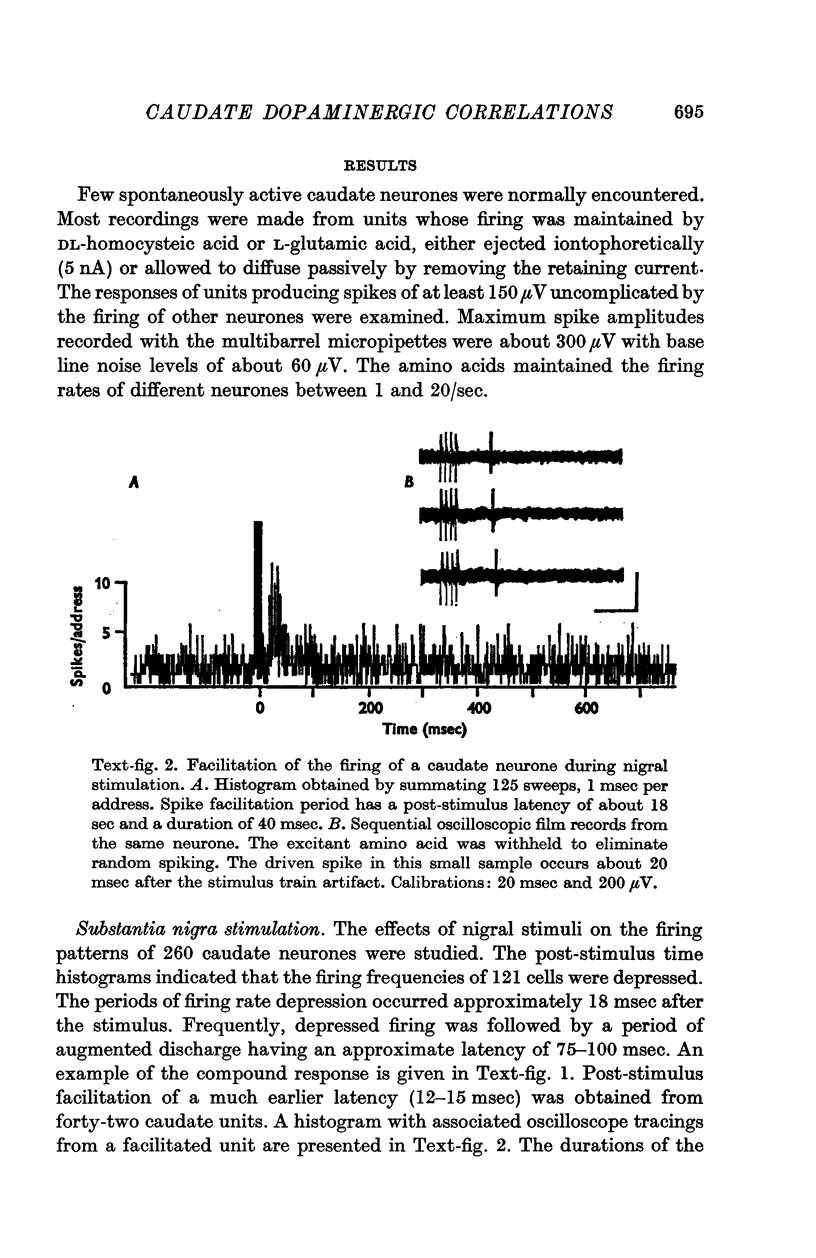
2. Electrical stimulation of the substantia nigra caused spike rate depressions in 121 of 260 caudate neurones as evidenced by changes in the post-stimulus histograms of their firing patterns. Spike rate facilitation occurred with forty-two caudate units.
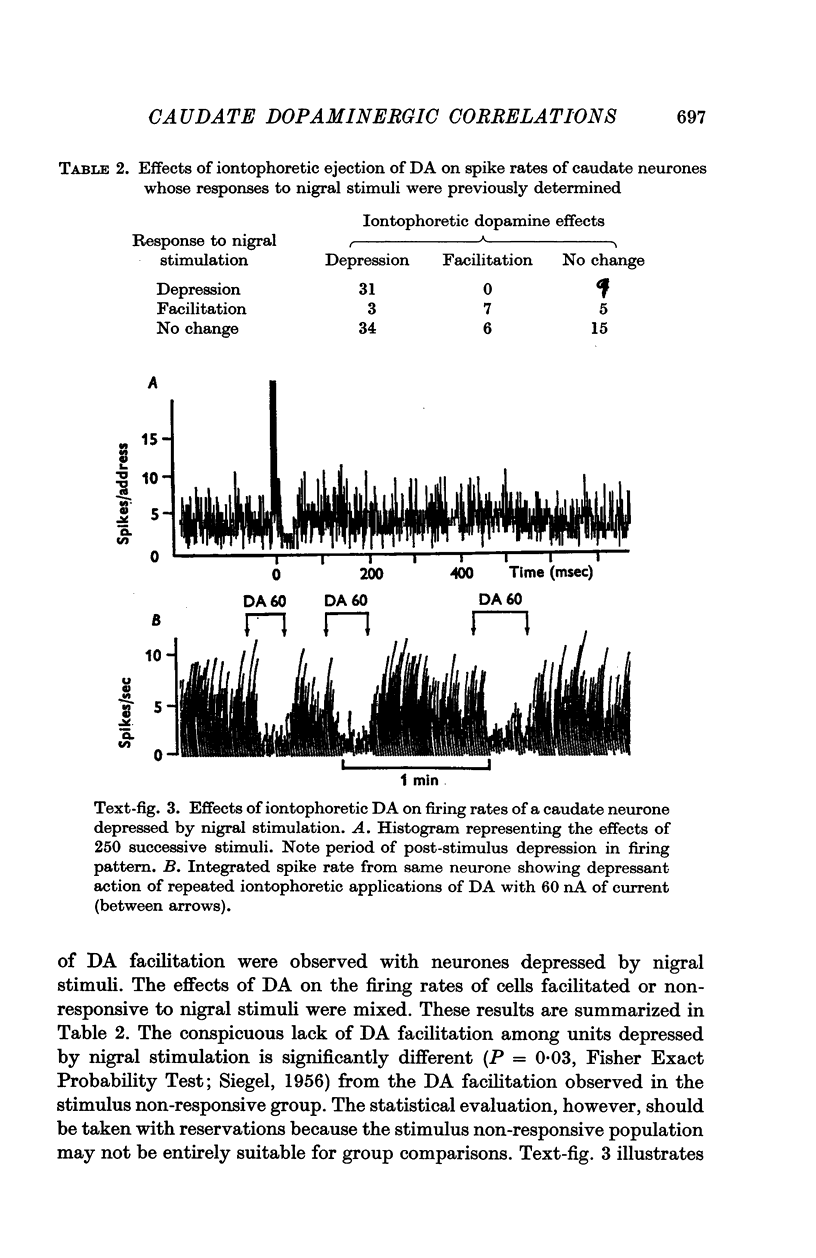
3. Neurones depressed by nigral stimuli were consistently depressed by micro-iontophoretic dopamine.
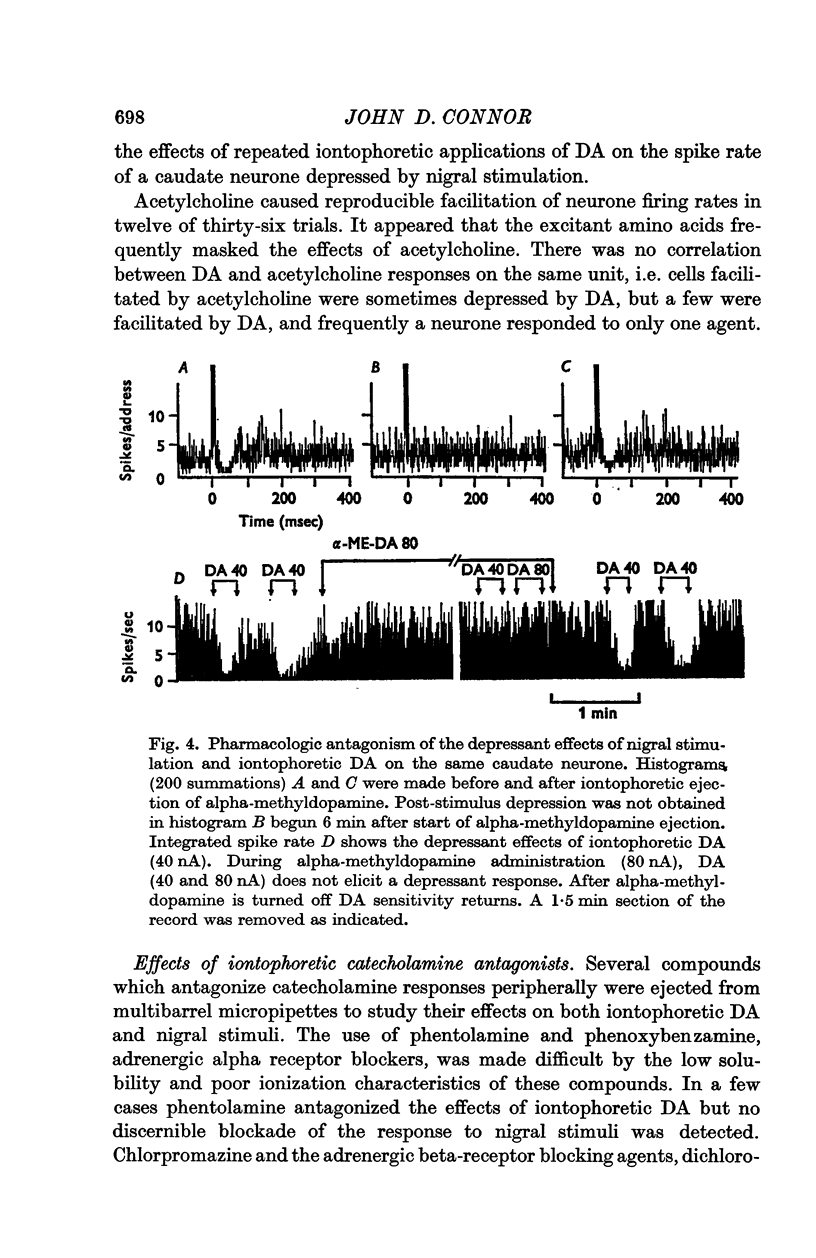
4. Alpha-methyldopamine, administered iontophoretically, antagonized the depressant responses induced by both nigral stimulation and iontophoretic dopamine.
5. The data are compatible with the concept that the caudate neuronal depressant responses produced by nigral stimulation are mediated by a direct dopaminergic nigro-neostriatal pathway. The possibility of mediation via polysynaptic pathways cannot, however, be completely excluded.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AFIFI A., KAELBER W. W. EFFERENT CONNECTIONS OF THE SUBSTANTIA NIGRA IN THE CAT. Exp Neurol. 1965 Apr;11:474–482. doi: 10.1016/0014-4886(65)90061-0. [DOI] [PubMed] [Google Scholar]
- ANDEN N. E., CARLSSON A., DAHLSTROEM A., FUXE K., HILLARP N. A., LARSSON K. DEMONSTRATION AND MAPPING OUT OF NIGRO-NEOSTRIATAL DOPAMINE NEURONS. Life Sci. 1964 Jun;3:523–530. doi: 10.1016/0024-3205(64)90161-4. [DOI] [PubMed] [Google Scholar]
- ANDEN N. E., DAHLSTROEM A., FUXE K., LARSSON K. FURTHER EVIDENCE FOR THE PRESENCE OF NIGRO-NEOSTRIATAL DOPAMINE NEURONS IN THE RAT. Am J Anat. 1965 Jan;116:329–333. doi: 10.1002/aja.1001160117. [DOI] [PubMed] [Google Scholar]
- Albe-Fessard D., Raieva S., Santiago W. Sur les relations entre substance noire et noyau caudé. J Physiol (Paris) 1967;59(4 Suppl):324–325. [PubMed] [Google Scholar]
- Andén N. E., Hfuxe K., Hamberger B., Hökfelt T. A quantitative study on the nigro-neostriatal dopamine neuron system in the rat. Acta Physiol Scand. 1966 Jul-Aug;67(3):306–312. doi: 10.1111/j.1748-1716.1966.tb03317.x. [DOI] [PubMed] [Google Scholar]
- Ariëns E. J. The structure-activity relationships of beta adrenergic drugs and beta adrenergic blocking drugs. Ann N Y Acad Sci. 1967 Feb 10;139(3):606–631. doi: 10.1111/j.1749-6632.1967.tb41232.x. [DOI] [PubMed] [Google Scholar]
- Bloom F. E., Costa E., Salmoiraghi G. C. Anesthesia and the responsiveness of individual neurons of the caudate nucleus of the cat to acetylcholine, norepinephrine and dopamine administered by microelectrophoresis. J Pharmacol Exp Ther. 1965 Nov;150(2):244–252. [PubMed] [Google Scholar]
- CARPENTER M. B., MCMASTERS R. E. LESIONS OF THE SUBSTANTIA NIGRA IN THE RHESUS MONKEY. EFFERENT FIBER DEGENERATION AND BEHAVIORAL OBSERVATIONS. Am J Anat. 1964 Mar;114:293–319. doi: 10.1002/aja.1001140209. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES R. M. The excitation of Renshaw cells by pharmacological agents applied electrophoretically. J Physiol. 1958 May 28;141(3):435–445. doi: 10.1113/jphysiol.1958.sp005987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. D. Caudate unit responses to nigral stimuli: evidence for a possible nigro-neostriatal pathway. Science. 1968 May 24;160(3830):899–900. doi: 10.1126/science.160.3830.899. [DOI] [PubMed] [Google Scholar]
- Connor J. D., Crawford I. L. Hyperthermia in midpontine lesioned cats. Brain Res. 1969 Oct;15(2):590–593. doi: 10.1016/0006-8993(69)90192-9. [DOI] [PubMed] [Google Scholar]
- FUXE K., HOEKFELT T., NILSSON O. OBSERVATIONS ON THE CELLULAR LOCALIZATION OF DOPAMINE IN THE CAUDATE NUCLEUS OF THE RAT. Z Zellforsch Mikrosk Anat. 1964 Aug 18;63:701–706. doi: 10.1007/BF00339917. [DOI] [PubMed] [Google Scholar]
- Feltz P., MacKenzie J. S. Properties of caudate unitary responses to repetitive nigral stimulation. Brain Res. 1969 May;13(3):612–616. doi: 10.1016/0006-8993(69)90273-x. [DOI] [PubMed] [Google Scholar]
- Frigyesi T. L., Purpura D. P. Electrophysiological analysis of reciprocal caudato-nigral relations. Brain Res. 1967 Nov;6(3):440–456. doi: 10.1016/0006-8993(67)90057-1. [DOI] [PubMed] [Google Scholar]
- GERSTEIN G. L., KIANG N. Y. An approach to the quantitative analysis of electrophysiological data from single neurons. Biophys J. 1960 Sep;1:15–28. doi: 10.1016/s0006-3495(60)86872-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz A., Zieglgänsberger W. Synaptic excitation in the corpus striatum inhibited by microelectrophoretically administered dopamine. Experientia. 1966 Dec 15;22(12):839–840. doi: 10.1007/BF01897452. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev. 1966 Jun;18(2):925–964. [PubMed] [Google Scholar]
- Libet B., Kobayashi H. Generation of adrenergic and cholinergic potentials in sympathetic ganglion cells. Science. 1969 Jun 27;164(3887):1530–1532. doi: 10.1126/science.164.3887.1530. [DOI] [PubMed] [Google Scholar]
- McLennan H., York D. H. The action of dopamine on neurones of the caudate nucleus. J Physiol. 1967 Apr;189(3):393–402. doi: 10.1113/jphysiol.1967.sp008175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POIRIER L. J., SOURKES T. L. INFLUENCE OF THE SUBSTANTIA NIGRA ON THE CATECHOLAMINE CONTENT OF THE STRIATUM. Brain. 1965 Mar;88:181–192. doi: 10.1093/brain/88.1.181. [DOI] [PubMed] [Google Scholar]
- Portig P. J., Vogt M. Activation of a dopaminergic nigro-striatal pathway. J Physiol. 1968 Jul;197(1):20P–21P. [PubMed] [Google Scholar]
- SALMOIRAGHI G. C., STEINER F. A. Acetylcholine sensitivity of cat's medullary neurons. J Neurophysiol. 1963 Jul;26:581–597. doi: 10.1152/jn.1963.26.4.581. [DOI] [PubMed] [Google Scholar]
- Salmoiraghi G. C., Weight F. Micromethods in neuropharmacology: an approach to the study of anesthetics. Anesthesiology. 1967 Jan-Feb;28(1):54–64. [PubMed] [Google Scholar]
- Thoenen H., Haefely W., Gey K. F., Hürlmann A. Liberation of alpha-methyldopamine as a "false" sympathetic transmitter after pretreatment of cats with alpha-methyldopa and disulfiram. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1967;258(2):181–196. doi: 10.1007/BF00535791. [DOI] [PubMed] [Google Scholar]
- VONEIDA T. J. An experimental study of the course and destination of fibers arising in the head of the caudate nucleus in the cat and monkey. J Comp Neurol. 1960 Aug;115:75–87. doi: 10.1002/cne.901150107. [DOI] [PubMed] [Google Scholar]
- Werman R. Criteria for identification of a central nervous system transmitter. Comp Biochem Physiol. 1966 Aug;18(4):745–766. doi: 10.1016/0010-406x(66)90209-x. [DOI] [PubMed] [Google Scholar]
- Woodruff G. N., Walker R. J. The effect of dopamine and other compounds on the activity of neurones of Helix aspersa; structure-activity relationships. Int J Neuropharmacol. 1969 May;8(3):279–289. doi: 10.1016/0028-3908(69)90049-5. [DOI] [PubMed] [Google Scholar]