Abstract
Fruiting body formation in Myxococcus xanthus involves three morphologic stages—rippling, aggregation, and sporulation—all of which are induced by the cell surface-associated C-signal. We analyzed the function of the DevT protein, a novel component in the C-signal response pathway. A mutant carrying an in-frame deletion in the devT gene displays delayed aggregation and a cell autonomous sporulation defect, whereas it remains rippling proficient. To further define the function of DevT, the methylation pattern of FrzCD, a cytoplasmic methyl-accepting chemotaxis protein homologue, was examined in the ΔdevT mutant, and we found that DevT is required for methylation of FrzCD during development. Specifically, DevT was found to be required for the C-signal-dependent methylation of FrzCD. The ΔdevT mutant produced wild-type levels of C-signal. However, accumulation of the FruA response regulator protein, which is essential for the execution of the C-signal-dependent responses, was reduced in the ΔdevT mutant. The DevT protein was found to stimulate the developmentally activated transcription of the fruA gene. Epistasis analyses indicate that DevT acts independently of the A- and E-signals to stimulate fruA transcription. These findings suggest that the developmental defects of the ΔdevT mutant are associated with a lack of FruA to ensure a proper response to the C-signal during the aggregation and sporulation stages of development.
In response to starvation, Myxoxoccus xanthus cells initiate a developmental program in which multicellular morphogenesis and cell differentiation are highly coordinated (4, 32). The program results in the formation of multicellular fruiting bodies, within which the rod-shaped cells differentiate into spherical spores. Fruiting body formation depends on the starvation of cells at a high cell density on a solid surface and involves three morphologic stages: rippling, aggregation, and sporulation. Aggregation initiates 4 to 8 h after the initiation of starvation and culminates after 24 h with the formation of mound-shaped fruiting bodies, each of which is built from 105 cells. Sporulation initiates when the organized cell movements have resulted in the accretion of cells in the developing fruiting bodies. Rippling precedes and accompanies the earliest stages of aggregation (34). During rippling, cells accumulate in ridge-like structures that move coordinately and synchronously over the substrate like ripples on a water surface. Key questions in understanding fruiting body formation are how the correct temporal order of these morphologic stages is brought about and how the developmental program is propelled from one stage to the next.
At least five intercellular signals, known as the A-, B-, C-, D-, and E-signals, have important functions for the progression of the developmental program (2, 18). Mutants defective in the production of either of these signals are arrested in development at a particular stage. Examination of gene expression and fruiting body morphogenesis in these mutants have shown that the A- and B-signals are required early during development (7, 19, 23) and that the D- and E-signals are required for development after 3 to 5 h (1-3). The C-signal becomes important for development after ca. 6 h (19) and is the latest acting of the known signals.
The C-signal is encoded by the csgA gene (33) and induces rippling, aggregation, sporulation, and expression of genes that are induced after 6 h of starvation (15, 19, 21, 25, 34). The defects in aggregation, sporulation, and C-signal-dependent gene expression in csgA mutants are corrected by codevelopment with wild-type (wt) cells (9). csgA encodes two proteins (21), one with a size of 25 kDa, corresponding to full-length CsgA protein, and one with a size of 17 kDa, which is similar in size to the C-factor protein (16). Kaiser and coworkers have proposed that C-factor protein is identical to the C-signal based on the observation that addition of C-factor protein, which was purified from starving wt cells, to csgA cells rescues development of these cells (16). However, exogenous full-length CsgA protein also rescues development of csgA cells (24). Full-length CsgA protein shares homology to short chain alcohol dehydrogenases (24). The molecular difference between the full-length CsgA protein and the C-factor protein has yet to be elucidated. Thus, it remains to be resolved whether the csgA proteins act as enzymes to produce the C signal or whether one of the csgA proteins is the actual C signal.
Recent studies have revealed several components in the C-signal response pathway. The DNA-binding response regulator protein FruA is the earliest known component in the pathway and is required for all four C-signal-dependent responses (5, 28). FruA synthesis is developmentally regulated at the transcriptional level, and FruA protein is not detectable until 3 h of starvation (5, 28). Since the C-signal is not required for FruA synthesis it has been suggested that C-signal transmission results in activation of FruA posttranslationally to interact with its downstream targets (5). The aspartate residue, which becomes phosphorylated in other response regulators, is conserved in FruA and is essential for FruA activity (5). Therefore, it has been hypothesized that C-signaling activates FruA by inducing phosphorylation of the protein (5). Downstream from FruA the C-signal response pathway branches, with one branch controlling rippling and aggregation. The proteins in the cytoplasmic Frz signal transduction system are components in this branch (36). The Frz proteins share homology with bacterial chemotaxis proteins and are essential for rippling and aggregation (29, 40) and control specific gliding motility parameters in response to the C-signal during development (11). The C-signal acts as a FruA-dependent input signal to the Frz system and induces methylation of the FrzCD protein, a methyl-accepting chemotaxis protein homologue, after 6 to 9 h of starvation (35). The second branch in the pathway controls sporulation. In this branch, FruA and the C-signal interact to induce transcription of the essential sporulation operon devRS after 6 to 9 h of development (5, 19).
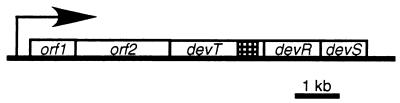
Recently, it was shown that the devRS genes are part of the larger dev operon, which consists of five genes (B. Julien, A. Garza, and D. Kaiser, unpublished data) (Fig. 1). The devRS genes are the two distal genes in the dev operon. The devT gene is part of this operon and is located immediately upstream from the devRS genes. devT is predicted to encode a protein with a size of ca. 58 kDa and which does not share homology to any known protein. Upstream from devT two additional reading frames, designated orf1 and orf2, with unknown functions are located. An in-frame deletion in devT causes delayed aggregation and a reduction in the sporulation efficiency (Julien et al., unpublished). To investigate the function of DevT at the molecular level, we carried out a detailed characterization of the ΔdevT mutant. We show here that DevT stimulates FruA synthesis.
FIG. 1.
Physical map of the dev operon. White boxes indicate the genes. The hatched box within devT indicate the portion of the gene that was removed to construct the in-frame deletion mutation. The arrow indicate the direction of transcription of the dev operon.
MATERIALS AND METHODS
Bacterial strains.
All M. xanthus strains used in this work were derived from wt strain DK1622. The M. xanthus strains used in this work are listed in Table 1. DK11207 carries a 279-bp in-frame deletion in devT, corresponding to a deletion between codon 408 and codon 502 in devT (Julien et al., unpublished) (Fig. 1). Strains SA1300, SA1301, SA1303, and SA1304 were constructed by generalized transduction into DK11207 by using Mx8 clp2 ts3 (38) propagated on DK11063, DK5208, DK9013, and JD300, respectively. DK11207/pSWU30 and SA1300/pEE244 were constructed by integration of the plasmids pSWU30 and pEE244, respectively, into the phage Mx8 attB site after electroporation (14). All strains constructed by generalized transductions or after electroporation of plasmids were tested by Southern blot analysis as described previously (30).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsb | Source or reference |
|---|---|---|
| Strainsa | ||
| DK1622 | wt | 13 |
| DK5208 | csgA::Tn5-132 ΩLS205 | 19 |
| DK11207 | ΔdevT | B. Julien et al., unpublished data |
| DK9013 | asgB480 Tn5-132 Ω4679 | 5 |
| DK11063 | fruA::Tn5 lacΩ7540 | 36 |
| DK11207/pSWU30 | ΔdevT attB::pSWU30 | This study |
| JD300 | esg::Tn5 Ω258 | 39 |
| SA1300 | ΔdevT fruA::Tn5 lacΩ7540 | This study |
| SA1301 | ΔdevT csgA::Tn5-132 ΩLS205 | This study |
| SA1303 | ΔdevT asgB480 Tn5-132 Ω4679 | This study |
| SA1304 | ΔdevT esg::Tn5 Ω258 | This study |
| DK11063/pEE244 | fruA::Tn5 lacΩ7540 attB::pEE244 | 5 |
| SA1300/pEE244 | ΔdevT fruA::Tn5 lacΩ7540 attB::pEE244 | This study |
| Plasmids | ||
| pSWU30 | Tetr; derivative of pBGS18 containing the Mx8 attP region | 37; S. Wu, unpublished data |
| pEE244 | Tetr; SWU30 derivative containg the entire fruA | 5 |
All M. xanthus strains are derivatives of DK1622.
Tetr, tetracycline resistance.
Growth, development, and measurement of β-galactosidase activity.
M. xanthus cells were grown in CTT medium in liquid cultures or on CTT agar plates as described previously (10). Kanamycin or oxytetracycline was used for selective growth at concentrations of 40 or 10 μg/ml, respectively. Aggregation and rippling were monitored on CF agar (34) as described previously (36). Briefly, cells were grown to a density of 5 × 108 cells/ml in CTT, harvested and resuspended in TPM buffer (10 mM Tris-HCl [pH 7.5], 1 mM KH2PO4, 8 mM MgSO4) at a calculated density of 5 × 109 cells/ml. Then, 20-μl aliquots of concentrated cells were spotted on CF agar and incubated at 32°C. Aggregation and rippling were monitored visually by using a Leica IMB/E inverted microscope or a Leica MZ8 stereomicroscope. Cells were photographed by using a Sony 3CCD color video camera. The levels of sporulation were determined after development for 72 and 120 h on CF agar. Unless otherwise mentioned, spore titers were determined as the number of sonication and heat-resistant CFU (36). To develop cells under submerged culture conditions, cells were treated as described for development on CF agar except that they were resuspended in MC7 buffer (10 mM morpholinepropanesulfonic acid [pH 7.0], 1 mM CaCl2). Next, 25 μl of concentrated cells was mixed with 375 μl of MC7 buffer, transferred to a well with a diameter of 15 mm in a microtiter dish, and incubated in a humid chamber at 32°C for the indicated periods of time. To measure specific activities of β-galactosidase during development, cells were induced to develop on CF agar as described above. Cells were harvested at the indicated time points and treated as described previously (36). Quantification of the specific activities of β-galactosidase was performed as described previously (20).
Immunoblot analysis.
Cells were exposed to starvation as described above. At the indicated times, cells were harvested. Cells were pelleted by centrifugation and immediately frozen and stored at −80°C until all samples were collected. Cells were lysed in 100 μl of sodium dodecyl sulfate (SDS) lysis buffer (30) containing protease inhibitors as described previously (27), boiled for 5 min, and loaded on Tris-glycine-SDS-12.5% polyacrylamide gels (prepared from a 29:1 acrylamide-bisacrylamide stock solution) for detection of FruA or on 11.56% acrylamide-0.08% bisacrylamide Tris-glycine-SDS gels for detection of FrzCD. For CsgA immunoblots, cells were lysed in 100 μl of Tris-Tricine-SDS lysis buffer (31) containing protease inhibitors as described previously (27), boiled for 5 min, and loaded onto Tris-Tricine-SDS-10% polyacrylamide gels (31) (prepared from a 40:2 acrylamide-bisacrylamide stock solution). Subsequently, proteins were transferred to Immobilon P membranes (Millipore) with a semidry blotting apparatus (Pharmacia). Immunoblots were prepared by standard procedures (30). The blots were probed with rabbit anti-FruA serum (28), rabbit anti-CsgA serum (21) or rabbit anti-FrzCD serum (27), followed by treatment with peroxidase-conjugated goat anti-rabbit immunoglobulin G (Boehringer-Mannheim). The blots were developed with Renaissance Plus chemiluminescence reagent (NEN Life Science Products). Protein concentrations were determined by using the Bio-Rad protein assay according to the manufacturer's suggestions, with bovine immunoglobulin G as a standard.
Purification of MalE-CsgA protein and FrzCD methylation assay.
The MalE-CsgA protein was purified from Escherichia coli as described previously (24). Protein concentrations were determined by using the Bio-Rad protein assay. The C-factor activity of the protein was determined in the C-factor bioassay (17). The specific activity of the protein was 65 C-factor U/mg of protein. One C-factor unit is the amount of protein that restores wt fruiting body formation and sporulation to 2.5 × 105 csgA cells (17). The effect of the fusion protein on FrzCD methylation was analyzed as described previously (35). Briefly, 2.5 × 108 of the relevant cells were developed as described for submerged culture development except that the cells were developed in A50 buffer (10 mM morpholinepropanesulfonic acid, 1 mM CaCl2, 4 mM MgCl2, 50 mM NaCl; pH 7.2). After 6 h of starvation, overlying buffer was removed and replaced with prewarmed A50; cells were harvested by gently scraping them off the bottom of the well, and then they were transferred to a 12-ml polypropylene tube. Cells were shaken vigorously to break clumps, the fusion protein was added to give a final volume of 400 μl per 2.5 × 108 cells, and the cells were transferred to a 32°C water bath and incubated for 30 min with shaking. Subsequently, cells were harvested and FrzCD immunoblots prepared as described above to determine the level of FrzCD methylation.
RESULTS
Phenotype of a ΔdevT mutant.
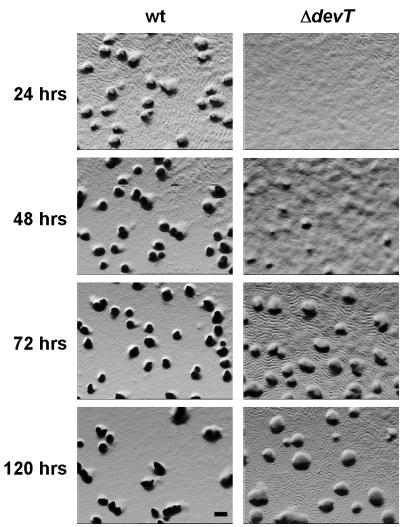
To analyze the role of the DevT protein in rippling and aggregation, the isogenic strains DK1622 (wt) and DK11207 (ΔdevT) were exposed to starvation on CF starvation agar. DK11207 carries an in-frame deletion in the devT gene (see Materials and Methods) (Fig. 1). After 24 h of starvation, wt cells had aggregated to form translucent mounds, and cells were rippling between the mounds (Fig. 2). After 48 h, rippling was no longer observed and the mounds had darkened. In agreement with the observation of Julien et al. (unpublished data), the ΔdevT cells were delayed in aggregation and translucent mounds were not detectable until after 48 to 72 h (Fig. 2). The mounds formed by the ΔdevT cells remained translucent until at least 120 h (Fig. 2). Interestingly, the ΔdevT cells were rippling after 24 h, and rippling was still observed even after 120 h (Fig. 2). Thus, DevT is important for timely aggregation.
FIG. 2.
Rippling and aggregation phenotypes of wt and ΔdevT mutant strains. DK1622 (wt) and DK11207 (ΔdevT) cells were exposed to starvation on CF agar for the indicated period of times. Bar, 0.1 mm. Cells were viewed with a Leica MZ8 stereomicroscope.
The effect of the ΔdevT mutation on sporulation was assessed after starvation of DK1622 and DK11207 on CF agar for 72 and 120 h. After 72 and 120 h, 6 and 10% of wt cells, respectively, had differentiated into spores. In agreement with the observation of Julien et al. (unpublished data), we observed that the ΔdevT mutation caused a sporulation defect and, after 72 and 120 h, the sporulation efficiencies of the ΔdevT cells were 105- and 104-fold lower, respectively, than that of wt cells. This sporulation efficiency was calculated from the number of heat- and sonication-resistant, germinating spores (see Materials and Methods). To distinguish whether the ΔdevT mutation interfered with spore formation or spore germination, ΔdevT cells that had been starved for 72 and 120 h were examined microscopically. This analysis showed that the number of spores formed by the ΔdevT cells is similar to the number of heat- and sonication-resistant, germinating spores. Thus, the DevT protein is important for spore formation.
To investigate whether the sporulation defect in the ΔdevT mutant was cell autonomous, ΔdevT cells were codeveloped with an equal number of wt cells, and then the numbers of spores formed by the ΔdevT cells were measured after 72 and 120 h of starvation. To distinguish spores formed by the ΔdevT cells from those formed by wt cells, the ΔdevT derivative DK11207/pSWU30, which carries the plasmid pSWU30 integrated at the phage Mx8 attB site, was used instead of DK11207. pSWU30 confers resistance to oxytetracycline and, thus, colonies formed from the ΔdevT spores could be identified on the basis of their resistance to oxytetracycline. The sporulation defect in the ΔdevT mutant remained unaffected by codevelopment with wt cells (data not shown). Thus, in the ΔdevT mutant rippling is observed for an extended period of time, aggregation is delayed, and the sporulation efficiency is reduced. The ΔdevT mutation being cell autonomous implies that the developmental defects are not caused by the lack of an intercellular signal.
The DevT protein acts upstream of the Frz signal transduction.
The FrzCD protein shows modulated methylation during development (26). Thus, the pattern of methylation of FrzCD may serve as a molecular marker to determine the time of action of components required for fruiting body formation (6). To further define the role of DevT and to determine whether DevT acts upstream or downstream of the events that lead to FrzCD methylation, the FrzCD methylation pattern was analyzed in DK1622 (wt) and in DK11207 (ΔdevT) cells during starvation.
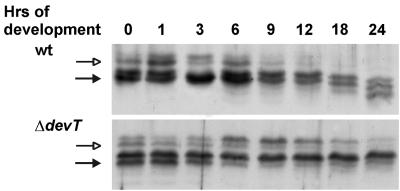
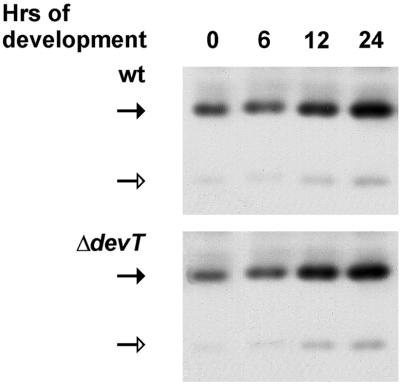
As previously reported (26), nonmethylated and methylated FrzCD is present during the first 6 h of development in wt cells (Fig. 3). From 9 h on, the ratio between nonmethylated FrzCD and methylated FrzCD decreased in wt cells and, after 18 h, FrzCD was only present in the methylated form. In the ΔdevT cells the pattern of FrzCD methylation was similar to that in wt cells until 6 h (Fig. 3). However, the ratio between nonmethylated FrzCD and methylated FrzCD did not decrease significantly in the ΔdevT cells until 18 to 24 h. Moreover, the nonmethylated form of FrzCD was still present after 24 h. The different patterns of FrzCD methylation in wt and ΔdevT cells suggest that DevT acts upstream from the events leading to methylation of FrzCD during development and is required either for the production of input signals to the Frz system or for the reception of the signals.
FIG. 3.
Immunoblot analyses of the FrzCD methylation patterns in wt and ΔdevT cells. Total cell lysates were prepared from cells after exposure to starvation in submerged culture for the indicated periods of time and reacted with anti-FrzCD antibody. (Top) Proteins isolated from DK1622 (wt) cells; (bottom) proteins isolated from DK11207(ΔdevT) cells. The two upper bands represent nonmethylated FrzCD (indicated by the open arrows), and the four lower bands represent methylated FrzCD (indicated by the solid arrows). Protein from 5 × 107 cells was added to each lane.
The DevT protein is required for C-signal-dependent methylation of FrzCD.
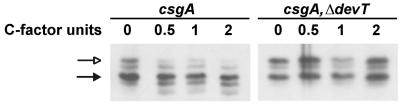
C-signal induces the methylation of FrzCD after 6 h of starvation (37). To investigate whether DevT is required for the C-signal-dependent FrzCD methylation, we analyzed C-signal-dependent methylation of FrzCD in a ΔdevT mutant by using a previously described experimental setup (35). Specifically, csgA cells (DK5208) and ΔdevT csgA cells (SA1301) were starved at a high cell density on a solid surface. At 6 h the cells were gently harvested and then exposed to various amounts of a MalE-CsgA fusion protein for 30 min while in suspension (see Materials and Methods). The MalE-CsgA protein has C-signal activity and rescues aggregation and sporulation in csgA cells (24) and induces FrzCD methylation in this assay (35). In this assay, cells are exposed to the MalE-CsgA protein while in suspension and under these conditions they do not aggregate (35). Therefore, any effect of the fusion protein on FrzCD methylation is not secondary to a primary effect on aggregation. In response to the addition of 0.5, 1.0, and 2.0 C-factor units of MalE-CsgA protein to the csgA cells, the level of FrzCD methylation increased (Fig. 4). However, in the ΔdevT csgA cells the same amounts of MalE-CsgA protein did not have any effect on FrzCD methylation (Fig. 4). Thus, DevT is required for the C-signal to act as an input signal to the Frz system.
FIG. 4.
Immunoblot analyses of the FrzCD methylation patterns in csgA and ΔdevT csgA cells in response to exogenous MalE-CsgA protein. Total cell lysates were prepared from cells that had been starved for 6 h in submerged culture, brought into suspension, and exposed to the indicated amounts of MalE-CsgA fusion protein for 30 min while in suspension. Subsequently, proteins were reacted with anti-FrzCD antibody. (Left panel) Proteins isolated from DK5208 (csgA) cells; (right panel) proteins isolated from SA1301(ΔdevT csgA) cells. Protein from 5 × 107 cells were added to each lane. The two upper bands represent nonmethylated FrzCD (indicated by the open arrows), and the three lower bands represent methylated FrzCD (indicated by the solid arrows). One C-factor unit is the amount of protein that restores wt fruiting body formation and sporulation to 2.5 × 108 csgA cells (17).
DevT is not required for accumulation of the csgA proteins.
The previous analyses showed that DevT acts between the C-signaling event and the Frz system. To determine whether DevT is required for the accumulation of the two csgA encoded proteins during development, quantitative CsgA immunoblots were performed. Total protein was isolated from DK1622 (wt) and DK11207 (ΔdevT) cells that had been starved for different periods of time on CF agar, separated by SDS-polyacrylamide gel electrophoresis, and reacted with anti-CsgA serum (see Materials and Methods). The accumulation profiles of the two csgA-encoded proteins were similar in wt and ΔdevT cells (Fig. 5): the 25-kDa csgA protein was detected in vegetatively growing cells and reached a maximum after 24 h. Low levels of the 17-kDa csgA protein were detected in vegetatively growing cells and reached a maximum after 24 h. Thus, the DevT protein is not required for accumulation of the csgA proteins during development.
FIG. 5.
Immunoblot analyses of the accumulation of the csgA proteins in wt and ΔdevT cells. Total cell lysates were prepared from cells after exposure to starvation on CF agar for the indicated periods of time and reacted with anti-CsgA serum. (Top) Proteins isolated from DK1622 (wt) cells; (bottom) proteins isolated from DK11207 (ΔdevT) cells. Solid and open arrows indicate the 25-kDa full-length csgA protein and the 17-kDa csgA protein, which is similar in size to the C-factor protein, respectively. A total of 7.5 μg of total protein was added per lane.
DevT stimulates transcription of the fruA gene.
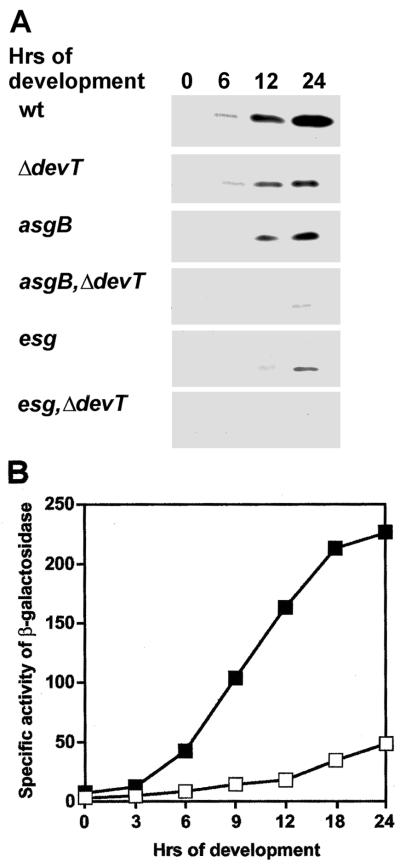
FruA protein is required for the C-signal to act as an input signal to the Frz system (35). To determine whether DevT is required for accumulation of FruA during development, we carried out quantitative FruA immunoblot analyzes of total protein isolated from developing DK1622 (wt) and DK11207 (ΔdevT) cells with anti-FruA serum. In wt cells FruA protein was detected by 6 h and reached a maximum after 24 h (Fig. 6A). In the ΔdevT mutant, the level of FruA protein was lower than in wt cells at all time points (Fig. 6A), and at 24 h the level of FruA protein was approximately fivefold lower in the ΔdevT cells than in wt cells. Thus, DevT stimulates accumulation of FruA during development.
FIG. 6.
Synthesis of the FruA protein during development. (A) Immunoblot analyses of the accumulation of FruA protein. Total cell lysates were prepared from cells after exposure to starvation on CF agar for the indicated periods of time and reacted with anti-FruA serum. Top panel, proteins isolated from DK1622 (wt) cells; second panel, proteins isolated from DK11207 (ΔdevT) cells; third panel, proteins isolated from DK9013 (asgB) cells; fourth panel, proteins isolated from SA1303 (asgB ΔdevT) cells; fifth panel, proteins isolated from JD300 (esg) cells; bottom panel, proteins isolated from SA1304 (esg ΔdevT) cells. A total of 5.0 μg of total protein was added per lane. (B) Specific activity of β-galactosidase expressed from the fruA::Tn5 lacΩ7540 transcriptional fusion. Specific activities of β-galactosidase were determined in cells after exposure to starvation on CF agar for the indicated periods of time. Symbols: ▪, DK11063/pEE244 (fruA::Tn5 lacΩ7540 attB::pEE244); □, SA1300/pEE244 (ΔdevT fruA::Tn5 lacΩ7540 attB::pEE244). The specific activities of β-galactosidase are given in nanomoles of o-nitrophenyl phosphate per minute per milligram. Each value is the average of two determinations from two independent experiments.
To examine whether DevT is required for the developmentally regulated transcription of the fruA gene, transcription of fruA was measured by using the transcriptional fruA::Tn5 lacΩ7540 fusion. Tn5 lacΩ7540 is inserted 167 bp downstream from the transcriptional start site of fruA, and the lac genes are transcribed from the fruA promoter (5). Tn5 lacΩ7540 has a polar effect on the downstream fruA gene, and a strain carrying Tn5 lacΩ7540 does not synthesize FruA protein (E. Ellehauge, unpublished data). To measure fruA transcription in a ΔdevT strain, Tn5 lac Ω7540 was introduced into the ΔdevT strain DK11207 by Mx8-dependent generalized transduction (see Materials and Methods), yielding SA1300. Subsequently, the plasmid pEE244, which carries the entire fruA gene including the promoter, was introduced into SA1300 giving rise to SA1300/pEE244. pEE244 carries the Mx8 attP site and integrates by site-specific recombination at attB on the M. xanthus chromosome. Likewise, pEE244 was introduced into DK11063, which carries Tn5 lacΩ7540, giving rise to DK11063/pEE244.
Expression of the fruA::Tn5 lacΩ7540 fusion was low at the onset of starvation in DK11063/pEE244; expression increased after 3 h and reached a maximum after 24 h of starvation (Fig. 6B). In SA1300/pEE244 the level of expression of the fruA::Tn5 lacΩ7540 fusion was strongly reduced compared to the level in DK11063/pEE244 at all time points (Fig. 6B), and at 24 h the specific activity of β-galactosidase in SA1300/pEE244 was approximately fivefold lower than in DK11063/pEE244. Thus, DevT stimulates the developmentally regulated activation of fruA transcription.
The intercellular A and E signals are required for transcriptional activation of the fruA gene (5, 28). To determine whether DevT acts in the A- or in the E-signaling pathway to promote fruA transcription, we performed epistasis experiments in which the accumulation of FruA protein was analyzed in asgB, esg, and ΔdevT single mutants and compared to the accumulation in asgB ΔdevT and esg ΔdevT double mutants. A strain carrying an asgB mutation is unable to synthesize the A- signal (22), and a strain carrying an esg mutation is unable to synthesize the E signal (2). As shown in Fig. 6A, the FruA accumulation profile in the asgB mutant was similar to that observed in the ΔdevT mutant. In the asgB ΔdevT double mutant, however, the FruA accumulation profile was different from those observed in the strains carrying either of the single mutations and FruA was not detected until after 24 h of starvation. In the esg mutant, FruA accumulated at low levels and accumulation was not detected until 12 and 24 h. In the esg ΔdevT double mutant, accumulation of the FruA protein was not detected. These data suggest that DevT acts independently of the A-signaling and E-signaling pathways to promote fruA transcription.
DISCUSSION
devT is part of the dev operon, which is transcribed after 6 to 9 h of development in a C-signal- and FruA-dependent manner (5, 19; Julien et al., unpublished). A ΔdevT mutant displays delayed aggregation and a cell autonomous sporulation defect, whereas it remains rippling proficient. Using the pattern of FrzCD methylation as a molecular marker to further define the function of DevT, it was found that DevT is required for methylation of FrzCD during development. This deficiency was further narrowed down to an inability of the ΔdevT mutant to respond properly to the C-signal, suggesting that DevT acts between the C-signaling event on the cell surface and the cytoplasmic Frz signal transduction system. The ΔdevT mutant produced normal levels of the csgA proteins, indicating that the defect in methylation of FrzCD is associated with a failure to respond properly to the C-signal rather than in the production of the signal. Consistent with this observation, DevT was found to be required for normal levels of accumulation of the FruA protein, which is indispensable for the C-signal to act as an input signal to the Frz signal transduction system (35). Specifically, DevT was found to stimulate fruA transcription. This effect of the ΔdevT mutation is not caused by a polar effect on the downstream devRS genes in the dev operon, because a devR devS double mutant accumulates wt levels of FruA protein (5). Nor is this effect of the ΔdevT mutation on fruA expression an indirect effect caused by blocking sporulation because other mutants that are deficient in sporulation have normal levels of FruA accumulation (5).
In addition to DevT, the early acting intercellular A- and E- signals are required for transcriptional activation of the fruA gene during development (5, 28). Interestingly, epistasis analyses in which the accumulation of FruA in ΔdevT, asgB, and esg single mutants was compared to the accumulation of FruA in ΔdevT asgB and ΔdevT esg double mutants strongly indicate that DevT acts independently of these two signaling pathways to promote fruA transcription. Thus, regulation of the fruA promoter is highly complex. Since DevT does not share significant sequence homology with known proteins (Julien et al., unpublished), the molecular mechanism underlying DevT-stimulated transcription of fruA remains unknown. Likewise, it remains to be shown whether the effect of DevT on fruA transcription is direct or indirect.
The inability of the C-signal to induce FrzCD methylation shows that the C-signal response pathway does not function normally in the ΔdevT mutant. Consistently, the ΔdevT mutant accumulates reduced amounts of FruA. FruA is a central component in the C-signal response pathway and is required for rippling, aggregation, sporulation, and C-signal-dependent gene expression (5, 35, 36). Downstream from FruA the C-signal response pathway branches, with one branch leading to rippling and aggregation and the other to gene expression and sporulation. The observation that a defect in devT causes delayed aggregation and reduced sporulation and does not interfere with rippling indicates that the defect caused by the devT mutation precedes the separation between aggregation and sporulation in the response pathway. The lack of FruA could explain the differential defects in the C-signal-dependent response, i.e., sufficient amounts of FruA are synthesized to allow rippling while insufficient amounts are synthesized to allow timely aggregation and sporulation. It should be emphasized that we cannot rule out that the DevT protein may have functions in aggregation and sporulation other than stimulating FruA synthesis. Further experimentation will address this issue.
The ordered execution of the four C-signal-dependent responses relies on an ordered increase in the level of C signaling during development in combination with distinct activity thresholds (8, 15, 21, 25). Thus, low levels of C-signaling bring about rippling, intermediate levels bring about aggregation as well as the expression of early C-signal-dependent genes, and high levels result in sporulation and expression of late C-signal-dependent genes. The level of C-signaling is predicted to increase during development. There are two contributions to this increase. First, the two csgA proteins accumulate during development (16, 21). The proteins encoded by the act operon are involved in controlling the level and timing of csgA transcription (8). Second, C-signaling results in aggregation and, therefore, a higher cell density. Because C-signaling depends on direct cell-cell interactions (16), the prediction is that aggregation per se results in increased levels of C-signaling. The data described here provide evidence for an additional positive feedback loop in the C-signal response pathway. Activated FruA induces DevT accumulation by stimulating transcription of the dev operon (5, 19), and DevT is required for fruA transcription. The loop is closed by the C-signal-dependent activation of FruA (5). This positive feedback loop may constitute a regulatory device that ensures a burst in FruA and Dev protein synthesis once a certain level of C-signaling is reached. In this context, it is interesting that the dev operon is primarily expressed in cells within developing fruiting bodies (12). Therefore, the level of C-signaling that sets this positive feedback loop in motion may only be reached in cells within the fruiting bodies. Once the positive FruA/DevT feedback loop is initiated, it may ensure that the developmental program goes to completion.
Acknowledgments
A.B. and E.E. contributed equally to this study.
We thank Anthony Garza and Dale Kaiser for communicating unpublished results.
This study was supported by the FREJA-program.
REFERENCES
- 1.Cheng, Y., and D. Kaiser. 1989. dsg, a gene required for cell-cell interaction early in Myxococcus development. J. Bacteriol. 171:3719-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Downard, J., S. V. Ramaswamy, and K. S. Kil. 1993. Identification of esg, a genetic locus involved in cell-cell signaling during Myxococcus xanthus development. J. Bacteriol. 175:7762-7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Downard, J., and D. Toal. 1995. Branched-chain fatty acids: the case for a novel form of cell-cell signalling during Myxococcus xanthus development. Mol. Microbiol. 16:171-175. [DOI] [PubMed] [Google Scholar]
- 4.Dworkin, M. 1996. Recent advances in the social and developmental biology of the myxobacteria. Microbiol. Rev. 60:70-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellehauge, E., M. Nørregaard-Madsen, and M. Søgaard-Andersen. 1998. The FruA signal transduction protein provides a checkpoint for the temporal co-ordination of intercellular signals in Myxococcus xanthus development. Mol. Microbiol. 30:807-817. [DOI] [PubMed] [Google Scholar]
- 6.Geng, Y., Z. Yang, J. Downard, D. R. Zusman, and W. Shi. 1998. Methylation of FrzCD defines a discrete step in the developmental program of Myxococcus xanthus. J. Bacteriol. 180:5765-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill, R. E., and M. G. Cull. 1986. Control of developmental gene expression by cell-to-cell interactions in Myxococcus xanthus. J. Bacteriol. 168:341-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gronewold, T. M. A., and D. Kaiser. 2001. The act operon controls the level and time of C-signal production for Myxococcus xanthus development. Mol. Microbiol. 40:744-756. [DOI] [PubMed] [Google Scholar]
- 9.Hagen, D. C., A. P. Bretscher, and D. Kaiser. 1978. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev. Biol. 64:284-296. [DOI] [PubMed] [Google Scholar]
- 10.Hodgkin, J., and D. Kaiser. 1977. Cell-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc. Natl. Acad. Sci. USA 74:2938-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jelsbak, L., and L. Søgaard-Andersen. 1999. The cell surface-associated intercellular C-signal induces behavioral changes in individual Myxococcus xanthus cells during fruiting body morphogenesis. Proc. Natl. Acad. Sci. USA 96:5031-5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Julien, B., A. D. Kaiser, and A. Garza. 2000. Spatial control of cell differentiation in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 97:9098-9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaiser, D. 1979. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 76:5952-5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kashefi, K., and P. L. Hartzell. 1995. Genetic suppression and phenotypic masking of a Myxococcus xanthus frzF defect. Mol. Microbiol. 15:483-494. [DOI] [PubMed] [Google Scholar]
- 15.Kim, S. K., and D. Kaiser. 1991. C-factor has distinct aggregation and sporulation thresholds during Myxococcus development. J. Bacteriol. 173:1722-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, S. K., and D. Kaiser. 1990. C-factor: a cell-cell signaling protein required for fruiting body morphogenesis of M. xanthus. Cell 61:19-26. [DOI] [PubMed] [Google Scholar]
- 17.Kim, S. K., and D. Kaiser. 1990. Purification and properties of Myxococcus xanthus C-factor, an intercellular signaling protein. Proc. Natl. Acad. Sci. USA 87:3635-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim, S. K., D. Kaiser, and A. Kuspa. 1992. Control of cell density and pattern by intercellular signaling in Myxococcus development. Annu. Rev. Microbiol. 46:117-139. [DOI] [PubMed] [Google Scholar]
- 19.Kroos, L., and D. Kaiser. 1987. Expression of many developmentally regulated genes in Myxococcus depends on a sequence of cell interactions. Genes Dev. 1:840-854. [DOI] [PubMed] [Google Scholar]
- 20.Kroos, L., A. Kuspa, and D. Kaiser. 1986. A global analysis of developmentally regulated genes in Myxococcus xanthus. Dev. Biol. 117:252-266. [DOI] [PubMed] [Google Scholar]
- 21.Kruse, T., L. Lobedanz, N. M. S. Berthelsen, and L. Søgaard-Andersen. 2001. C-signal: a cell surface-associated morphogen that induces and coordinates multicellular fruiting body morphogenesis and sporulation in M. xanthus. Mol. Microbiol. 40:156-168. [DOI] [PubMed] [Google Scholar]
- 22.Kuspa, A., and D. Kaiser. 1989. Genes required for developmental signalling in Myxococcus xanthus: three asg loci. J. Bacteriol. 171:2762-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuspa, A., L. Kroos, and D. Kaiser. 1986. Intercellular signaling is required for developmental gene expression in Myxococcus xanthus. Dev. Biol. 117:267-276. [DOI] [PubMed] [Google Scholar]
- 24.Lee, B.-U., K. Lee, J. Mendez, and L. J. Shimkets. 1995. A tactile sensory system of Myxococcus xanthus involves an extracellular NAD(P)+-containing protein. Genes Dev. 9:2964-2973. [DOI] [PubMed] [Google Scholar]
- 25.Li, S., B.-U. Lee, and L. J. Shimkets. 1992. csgA expression entrains Myxococcus xanthus development. Genes Dev. 6:401-410. [DOI] [PubMed] [Google Scholar]
- 26.McBride, M. J., and D. R. Zusman. 1993. FrzCD, a methyl-accepting taxis protein from Myxococcus xanthus, shows modulated methylation during fruiting body formation. J. Bacteriol. 175:4936-4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCleary, W. R., M. J. McBride, and D. R. Zusman. 1990. Developmental sensory transduction in Myxococcus xanthus involves methylation and demethylation of FrzCD. J. Bacteriol. 172:4877-4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogawa, M., S. Fujitani, X. Mao, S. Inouye, and T. Komano. 1996. FruA, a putative transcription factor essential for the development of Myxococcus xanthus. Mol. Microbiol. 22:757-767. [DOI] [PubMed] [Google Scholar]
- 29.Sager, B., and D. Kaiser. 1994. Intercellular C-signaling and the traveling waves of Myxococcus. Genes Dev. 8:2793-2804. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Schägger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 32.Shimkets, L. J. 1990. Social and developmental biology of the myxobacteria. Microbiol. Rev. 54:473-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimkets, L. J., R. E. Gill, and D. Kaiser. 1983. Developmental cell interactions in Myxococcus xanthus and the spoC locus. Proc. Natl. Acad. Sci. USA 80:1406-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimkets, L. J., and D. Kaiser. 1982. Induction of coordinated movement of Myxococcus xanthus cells. J. Bacteriol. 152:451-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Søgaard-Andersen, L., and D. Kaiser. 1996. C factor, a cell-surface-associated intercellular signaling protein, stimulates the Frz signal transduction system in Myxococcus xanthus. Proc. Natl. Acad. Sci. USA 93:2675-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Søgaard-Andersen, L., F. Slack, H. Kimsey, and D. Kaiser. 1996. Intercellular C-signaling in Myxococcus xanthus involves a branched signal transduction pathway. Genes Dev. 10:740-754. [DOI] [PubMed] [Google Scholar]
- 37.Spratt, B. G., P. J. Hedge, S. te Heesen, A. Edelman, and J. K. Broome-Smith. 1986. Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene 41:337-342. [DOI] [PubMed] [Google Scholar]
- 38.Stephens, K., and D. Kaiser. 1987. Genetics of gliding motility in Myxococcus xanthus: molecular cloning of the mgl locus. Mol. Gen. Genet. 207:256-266. [Google Scholar]
- 39.Toal, D. R., S. W. Clifton, B. A. Roe, and J. Downard. 1995. The esg locus of Myxococcus xanthus encodes the E1a and E1β subunits of a branched-chain keto acid dehydrogenase. Mol. Microbiol. 16:177-189. [DOI] [PubMed] [Google Scholar]
- 40.Zusman, D. R. 1982. “Frizzy” mutants: a new class of aggregation-defective developmental mutants of Myxococcus xanthus. J. Bacteriol. 150:1430-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]