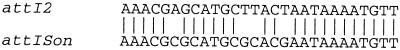Abstract
We have found an integron-like integrase gene and an attI site in Shewanella oneidensis as part of a small chromosomal integron. We have cloned this gene and tested the ability of the integrase to excise cassettes from various integrons. Most cassettes flanked by two attC sites are readily excised, while cassettes in the “first” position, with an attI1 or attI3 site on one end, are not excised. An exception is a cassette with attI2 on one end. The attI2 site, from Tn7, has greater similarity to the attI site adjacent to the integrase of S. oneidensis than do attI1 or attI3. We cloned the attI site of S. oneidensis and observed the integration of two different cassettes. We have, therefore, demonstrated the function of this integron-like integrase.
Integrons are gene capture systems that can acquire and disseminate elements known as gene cassettes. These systems are implicated in the dissemination of antibiotic resistance genes. The essential components of the integron are found within the 5′-conserved segment and include an integrase gene, intI, an adjacent recombination site, attI, and a promoter region from which integrated cassettes are expressed (4). The gene cassettes are located within the variable region and are integrated in tandem at the attI site. These cassettes contain a structural gene and an imperfect palindromic integrase-specific recombination site known as an attC site or 59-base element. The cassettes are mobile, nonreplicating, and generally lack a promoter region (10).
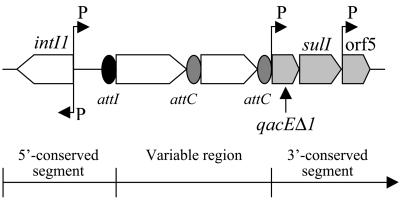
Several classes of integrons have been reported, all of which contain distinct but related integrase genes (12). Class 1 integrons are the most widespread, and most contain a 3′-conserved segment that contains a qacEΔ1 gene encoding resistance to quaternary ammonium compounds and a sulI gene encoding resistance to sulfonamides (Fig. 1) (13, 14). Classes 2 and 3, which are rarer, are, like class 1, plasmid borne (9). The class 4 integron is a first example of a chromosomal superintegron and is located in the genome of Vibrio cholerae (5). Several other classes have been identified, but they are not fully characterized yet (7, 12).
FIG. 1.
General structure of class 1 integrons. Cassettes are inserted in the variable region by the integrase using a site-specific recombination mechanism. The attI and attC sites are shown by a black and a grey oval, respectively, and promoters are denoted by P. Genes are as follows: intI1, integrase gene; qacEΔ1, antiseptic resistance gene; sulI, sulfonamide resistance gene; orf5, gene of unknown function.
The integron integrase is a member of the tyrosine recombinase family, a superfamily of site-specific recombination proteins (3). Among this family, there are also the well-known XerC/XerD, Cre, Flp, and λ integrase (2, 8). However, there is an extra domain, composed of an additional 36 amino acids near Patch III that distinguishes integron integrases from other tyrosine recombinases and which is implicated in the recognition of the attI and attC sites (6).
Sequence analysis.
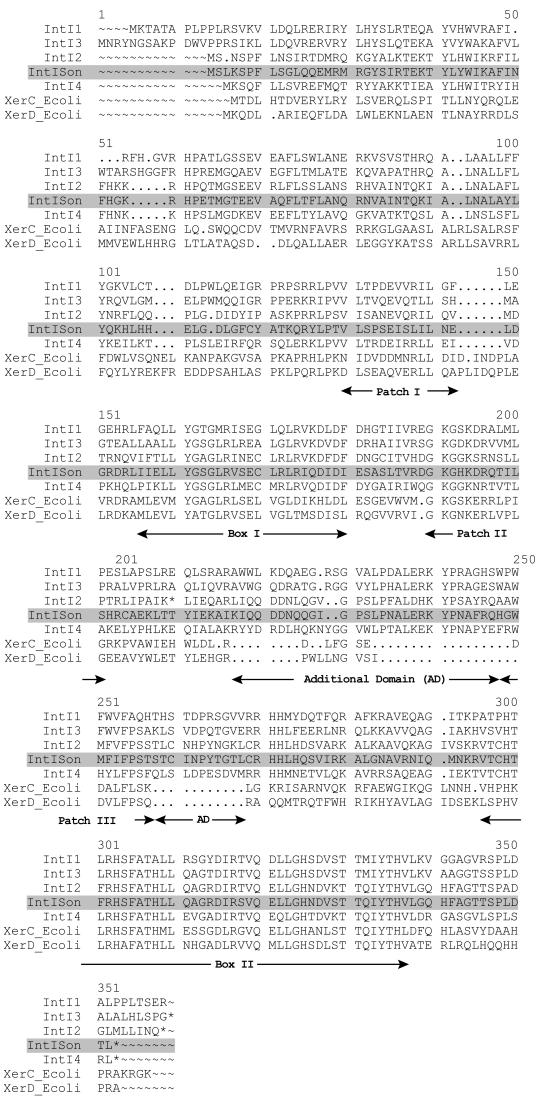
Shewanella oneidensis MR-1 partial genomic data was from The Institute for Genomic Research (TIGR). Sequence analysis was done using Sequencher (version 3.1) and Genetics Computer Group (version 10.1) software. By screening the partial genomic DNA sequences from TIGR, we found in contig 7833 of S. oneidensis MR-1 a sequence encoding a protein 45% identical and 54% similar to IntI1. The translated sequence also contained the extra domain of integron integrases. This bacterium was isolated from lake sediment and was previously named Shewanella putrefaciens (15). Figure 2 shows the alignment of some integron integrases with the tyrosine recombinase of S. oneidensis and with XerC/XerD from Escherichia coli.
FIG. 2.
Alignment of various integron integrases with XerC/XerD from E. coli. IntI1, class 1 integron integrase from plasmid pVS1; IntI3, class 3 integron integrase from a Serratia marcescens plasmid; IntI2, class 2 integron integrase from Tn7; IntISon, integron integrase from S. oneidensis; IntI4, class 4 integron integrase from the V. cholerae superintegron; XerC, recombinase from E. coli; XerD, recombinase from E. coli.
We found a putative attI site adjacent to the tyrosine recombinase gene. The attI site is generally located upstream of the intI gene, at the right-hand end of the 5′-conserved segment of class 1 integrons. It is around 70 bp long, nonpalindromic, and ends with the consensus sequence GTTRRRY. The crossover site is between the G and the first T (11). Figure 3 shows the alignment of the partial attI site of S. oneidensis with its closest homologue, attI2. In this study, we characterized the ability of the tyrosine recombinase of S. oneidensis to excise cassettes at the attC sites and to integrate some cassettes at the attI site.
FIG. 3.
Alignment of the 3′ extremities of the attI2 site from class 2 integrons with the attI site of S. oneidensis. The GTT is the crossover site.
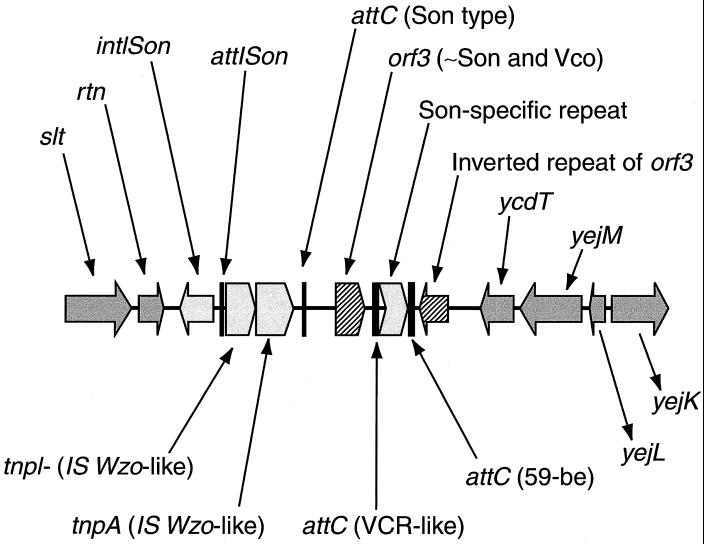
We found that the tyrosine recombinase and the attI site were in fact part of an organized small chromosomal superintegron, shown in Fig. 4. On the left-hand side of intI, there are genes homologous to chromosomal genes of E. coli, namely slt (soluble lytic transglycosylase) and rtn (hypothetical protein). To the right of the attI site, there is a cassette containing an insertion sequence (IS). This IS is composed of a tyrosine recombinase, probably functioning as a resolvase, and a transposase. These two genes are homologous with those of the IS found linked to the AccI restriction modification system of Weeksella zoohelcum (1). This cassette is also found at two other chromosomal locations in S. oneidensis MR-1. Downstream of this first cassette, there is a second cassette composed of an open reading frame (ORF) that shares some similarity to ORFs from S. oneidensis and V. cholerae. Its attC site resembles a VCR repeat (2). The third cassette is a short noncoding region followed by another attC site. This site is like aadA/aadB attC sites (≈60 bp) (10). After this cassette there is, on the bottom strand, an inverted repetition of the ORF located in the second cassette. The sequence afterwards returns to typical chromosomal genes with homologs in E. coli. These are ycdT, yejM, yejL, and yejK. The yej genes are in the same order in E. coli. Therefore, there appears to be a superintegron of about 6 kb in length that is flanked on either side by chromosomal genes.
FIG. 4.
Representation of the chromosomal superintegron of S. oneidensis. The chromosomal genes are in dark grey, the integrase and cassettes are in light grey, and attC sites are in solid black. The integron does not include the six chromosomal genes shown, which are identified by their similarities with E. coli chromosomal genes.
Construction of the IntISon overproduction vector.
PCR amplification was done on the genomic DNA of S. oneidensis MR-1 with primer sonint-BspHI (5′-GGCGCGTTTTCCAAACCTATTATC-3′) and mutagenic primer sonint-BamHI (5′-CAGTATTTCCCGGATCCCTATGGA-3′). Thereafter, the amplicon was digested with BspHI and BamHI and it was then cloned into pTRC99a (Amersham-Pharmacia) digested with NcoI and BamHI. Genes cloned using these sites are expressed from the trc promoter and use a vector-provided ribosome binding site. The clone, pFD03, was then transformed into E. coli JM109. This clone was used to overexpress the integrase in excision assays. The attI site of S. oneidensis was cloned into pFD03 by amplifying it with the mutagenic primers attIson-BamHI (5′-ATTCCCGGATCCCAAGGAAAAT-3′) and attIson-HindIII (5′-TAGGAGTAAGCTTGGAGCAGCG-3′). The amplicon was then digested with BamHI and HindIII and then cloned into pFD03 digested with BamHI and HindIII to yield pFD04. This clone was used in the excision-integration assays.
Excision assays.
We tried to excise several cassettes with different flanking sequences. The cells harboring the clone pFD03 were transformed by various clones harboring gene cassettes cloned into pACYC184. The double-transformants were then incubated overnight in 5 ml of Luria-Bertani medium with 0.2% glucose (wt/vol), subcultured (0.1 ml into 15 ml), and grown to an optical density at 600 nm of 0.6. Then, isopropyl-β-d-thiogalactopyranoside was added to a final concentration of 0.4 mM and the culture was incubated at 37°C overnight; 5 ml was harvested and a miniprep (Qiagen) was done. PCR was done using primers pACYC184-5′ (5′-TGTAGCACCTGAAGTCAGCC-3′) and pACYC184-3′ (5′-ATACCCACGCCGAAACAAG-3′) with the following cycles: 10 min at 94°C, 30 cycles of 1 min at 94°C, 1 min at 55°C, and 5 min at 72°C, and a final step of 10 min at 72°C. The length of the resulting amplicon indicated the presence or absence of excision.
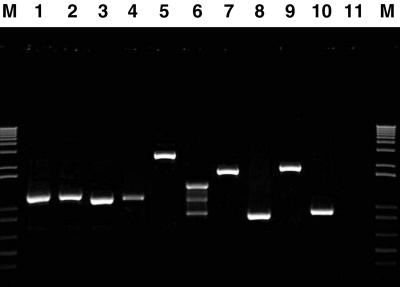
Figure 5 shows an example of results obtained in excision assays. Table 1 shows a summary of the results. Some cassettes, like aacA1a-orfG and orfH, are always strongly excised and other cassettes, like dfrA1, are never excised by the integron integrase of S. oneidensis. This protein is able, like IntI1 but not IntI2 and IntI3 (F. Gagnon and P. H. Roy, unpublished results), to excise cassettes with a heterologous attI site as its left-hand neighbor, but this specificity is limited to attI2, with which attISpu shares sequence similarity (Fig. 3). The left-hand neighbor site seems to play a role in the ability of the tyrosine recombinase IntISon to excise cassettes, as the aadA1 cassette is excised weakly to strongly, depending on the left-hand site of the cassette. The attC site of aadA2 seems to be a very good left-hand neighbor site, since when this element is present, there is always a strong excision of the following cassette, with the exception of dfrA1. The integron integrase of S. oneidensis is in fact able to excise several cassettes associated with various attC elements, except for dfrA1. These results indicate that this integrase should be able to excise cassettes from various classes of integrons.
FIG. 5.
Example of the results obtained for an excision test. Lane M, 1 Kb Plus (Gibco BRL) molecular weight marker; lane 1, attI2-dfr1_attC (pLQ 424) IntISon not induced; lane 2, attI2-dfr1_attC (pLQ 424) IntISon induced; lane 3, attI1-dfrI_attC (pLQ427) not induced; lane 4, attI1-dfrI_attC (pLQ427) not induced; lane 5, aadA2_attC-aacA1a-orfG_attC (pLQ428) not induced; lane 6, aadA2_attC-aacA1a-orfG_attC (pLQ428) induced; lane 7, aadA2_attC-blaimp_attC (pLQ438) not induced; lane 8, aadA2_attCblaimp_attC (pLQ438) induced; lane 9, aadA2_attC-aadA1_attC (pLQ443) not induced; lane 10, aadA2_attC-aadA1_attC (pLQ443) induced; lane 11, PCR negative control.
TABLE 1.
Results obtained from the excision testsa
| Clone name (pLQ) | Left-hand neighbor site | Crossover site sequence | Gene cassette with its attC site | Crossover site sequence | Right-hand neighbor site | Excisionb |
|---|---|---|---|---|---|---|
| 423 | attI1 | GTTAAAC | aadA1 | GTTAGAT | qacEΔ1 | − |
| 424 | attI2 | GTTAACC | dfrA1 | GTTAGGC | orf1 | − |
| 425 | attI3 | GTTAGAA | blaimp | GTTAGGC | aacA4 | − |
| 427 | attI1 | GTTAACC | dfrA1 | GTTAAAC | aadA1 | − |
| 429 | attI1 | GTTAGAC | aadA2 | GTTAGGG | aacA1a | − |
| 439 | attI1 | GTTAGAA | blaimp | GTTAGGC | aacA4 | − |
| 440 | attI1 | GTTAGGG | aacA1a-orfG + orfH | GTTAGGC | orfI | − |
| 441 | attI2 | GTTAGGG | aacA1a-orfG + orfH | GTTAGGC | orfI | + |
| 442 | attI3 | GTTAGGG | aacA1a-orfG + orfH | GTTAGGC | orfI | − |
| 443 | aadA2_attC | GTTAAAC | aadA1 | GTTAGAT | qacEΔ1 | +++ |
| 428 | aadA2_attC | GTTAGGG | aacA1a-orfG + orfH | GTTAGGC | orfI | +++ |
| 437 | aadA2_attC | GTTAACC | dfrA1 | GTTAAAC | aadA1 | − |
| 438 | aadA2_attC | GTTAGAA | blaimp | GTTAGGC | orfI | +++ |
| 426 | dfrA1_attC | GTTAAAC | aadA1 | GTTAGAT | qacEΔ1 | + |
| 444 | dfrA1_attC | GTTAGGG | aacA1a-orfG + orfH | GTTAGGC | orfI | +++ |
| 430 | dfrA1_attC | GTTAGGC | sat | GTTAAAC | aadA1 | ++ |
| 445 | aacA1_attC | GTTAAAC | aadA1 | GTTAGAT | qacEΔ1 | ++ |
| 446 | blaimp_attC | GTTAGGG | aacA1a-orfG + orfH | GTTAGGC | orfI | +++ |
| 431 | aacA4_attC | GTTAGCC | pse1 | GTTAGAC | aadA2 | ++ |
The cassette aacA1-orfG is always cloned in tandem with orfH. This is why there are two attC sites in clones with aacA1a-orfG cassette.
−, no excision; +, weak excision (<20%); ++, medium excision (20 to 75%); +++, strong excision (>75%).
Excision-integration assays.
Using two clones for which excision was particularly strong, namely pLQ428 and pLQ443, coupled excision-integration assays were done. The protocol was the same as for the excision assays, except that the integrase clone was pFD04, which also contained the attI site. We tried two cassettes known to be efficiently excised, namely aacA1a and aadA1. For the detection of integration, PCR was done using primers for the cassette tested. For the aacA1a cassette, the primers sonint1143 (5′-TTCACATCTGCTCCATAGGATC-3′) and aacA1a-NH2 (5′-TAATTGCTGCATTCCGC-3′) were used. For the aadA1 cassette, primers sonint1143 and aadA-Tn21 (5′-TCGATGACGCCAACTAC-3′) were used.
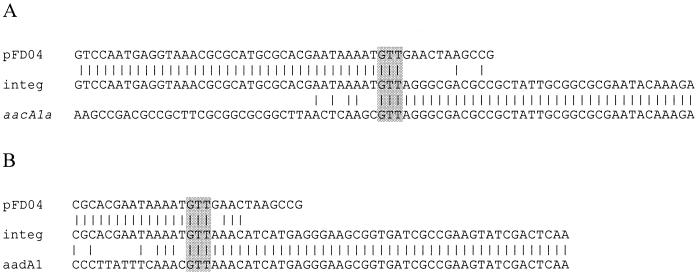
Figures 6A and B show the integration results obtained for pLQ428 and pLQ443, respectively. The upper sequence (pFD04) is that of the clone pFD04. The middle sequence (integ) is that obtained from the amplicon generated by sonint1143 and aacA1a-NH2 or aadA-Tn21 primers. The lower sequence (pLQ428 or pLQ443) is that of the pLQ clone from which the excised cassette originates. These results show that integration has occurred at the predicted crossover point, namely G/TT (11) and, thus, that the tyrosine recombinase of S. oneidensis is a fully active integron integrase. The fact that this integrase can recognize any type of attC site explains why there are three types of attC sites in the chromosomal superintegron of this strain. Class 1, 2, and 3 integrons are antibiotic resistance integrons (13). Although antibiotic resistance has emerged in the middle of the last century, its mechanisms must have evolved from ancestral forms found in antibiotic-producing organisms before the introduction of the antibiotics (12). By being able to excise and integrate antibiotic resistance gene cassettes, S. oneidensis has a potential for evolution of antibiotic resistance integrons similar to that seen for Class 1 integrons.
FIG. 6.
Sequence obtained from the amplicon generated with excision-integration assays. (A) Integration of aacA1a cassette; (B) integration of aadA1 cassette. Sequences are compared to the clone containing the attI site (pFD04, upper line) and to the aacA1a and aadA1 cassettes in the context of the donor plasmids pLQ428 and pLQ443, respectively (lower line).
Acknowledgments
This work was supported by Canadian Institutes for Health Research grant MT-13564 to P.H.R.
We thank TIGR for the S. oneidensis MR-1 strain and for communication of preliminary sequence data. Sequencing of S. oneidensis at TIGR was accomplished with support from the Department of Energy.
REFERENCES
- 1.Brassard, S., H. Paquet, and P. H. Roy. 1995. A transposon-like sequence adjacent to the AccI restriction-modification operon. Gene 157:69-72. [DOI] [PubMed] [Google Scholar]
- 2.Clark, C. A., L. Purins, P. Kaewrakon, and P. A. Manning. 1997. VCR repetitive sequence elements in the Vibrio cholerae chromosome constitute a mega-integron. Mol. Microbiol. 26:1137-1138. [DOI] [PubMed] [Google Scholar]
- 3.Esposito, D., and J. J. Scocca. 1997. The integrase family of tyrosine recombinases: evolution of a conserved active site domain. Nucleic Acids Res. 25:3605-3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall, R. M., and C. M. Collis. 1995. Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol. Microbiol. 15:593-600. [DOI] [PubMed] [Google Scholar]
- 5.Mazel, D., B. Dychinco, V. A. Webb, and J. Davies. 1998. A distinctive class of integron in the Vibrio cholerae genome. Science 280:605-608. [DOI] [PubMed] [Google Scholar]
- 6.Messier, N., and P. H. Roy. 2001. Integron integrases possess a unique additional domain necessary for their activity. J. Bacteriol. 183:6699-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nield, B. S., A. J. Holmes, M. R. Gillings, G. D. Recchia, B. C. Mabbutt, K. M. Nevalainen, and H. W. Stokes. 2001. Recovery of new integron classes from environmental DNA. FEMS Microbiol. Lett. 195:59-65. [DOI] [PubMed] [Google Scholar]
- 8.Ouellette, M., and P. H. Roy. 1987. Homology of ORFs from Tn 2603 and from R46 to site-specific recombinases. Nucleic Acids Res. 15:10055.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Recchia, G. D., and R. M. Hall. 1995. Gene cassettes: a new class of mobile element. Microbiology 141(Pt. 12):3015-3027. [DOI] [PubMed] [Google Scholar]
- 10.Recchia, G. D., and R. M. Hall. 1997. Origins of the mobile gene cassettes found in integrons. Trends Microbiol. 5:389-394. [DOI] [PubMed] [Google Scholar]
- 11.Recchia, G. D., H. W. Stokes, and R. M. Hall. 1994. Characterisation of specific and secondary recombination sites recognised by the integron DNA integrase. Nucleic Acids Res. 22:2071-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowe-Magnus, D. A., A. M. Guerout, P. Ploncard, B. Dychinco, J. Davies, and D. Mazel. 2001. The evolutionary history of chromosomal super-integrons provides an ancestry for multiresistant integrons. Proc. Natl. Acad. Sci. USA 98:652-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sallen, B., A. Rajoharison, S. Desvarenne, and C. Mabilat. 1995. Molecular epidemiology of integron-associated antibiotic resistance genes in clinical isolates of enterobacteriaceae. Microb. Drug Resist. 1:195-202. [DOI] [PubMed] [Google Scholar]
- 14.Stokes, H. W., and R. M. Hall. 1989. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol. Microbiol. 3:1669-1683. [DOI] [PubMed] [Google Scholar]
- 15.Venkateswaran, K., D. P. Moser, M. E. Dollhopf, D. P. Lies, D. A. Saffarini, B. J. MacGregor, D. B. Ringelberg, D. C. White, M. Nishijima, H. Sano, J. Burghardt, E. Stackebrandt, and K. H. Nealson. 1999. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int. J. Syst. Bacteriol. 49(Pt. 2):705-724. [DOI] [PubMed] [Google Scholar]