Abstract
d-(−)-3-Hydroxybutyrate (DHB), the immediate depolymerization product of the intracellular carbon store poly-3-hydroxybutyrate (PHB), is oxidized by the enzyme 3-hydroxybutyrate dehydrogenase to acetoacetate (AA) in the PHB degradation pathway. Externally supplied DHB can serve as a sole source of carbon and energy to support the growth of Sinorhizobium meliloti. In contrast, wild-type S. meliloti is not able to utilize the l-(+) isomer of 3-hydroxybutyrate (LHB) as a sole source of carbon and energy. In this study, we show that overexpression of the S. meliloti acsA2 gene, encoding acetoacetyl coenzyme A (acetoacetyl-CoA) synthetase, confers LHB utilization ability, and this is accompanied by novel LHB-CoA synthetase activity. Kinetics studies with the purified AcsA2 protein confirmed its ability to utilize both AA and LHB as substrates and showed that the affinity of the enzyme for LHB was clearly lower than that for AA. These results thus provide direct evidence for the LHB-CoA synthetase activity of the AcsA2 protein and demonstrate that the LHB utilization pathway in S. meliloti is AcsA2 dependent.
Poly-3-hydroxybutyrate (PHB), a bacterial intracellular reserve of carbon and reducing energy, accumulates when a nutrient other than carbon is limiting for growth, as reviewed by Madison and Huisman (18). Based on biochemical evidence, the metabolism of PHB has been proposed to be a cyclical process, comprising the pathways for synthesis and degradation of PHB. Synthesis of PHB occurs when excess carbon, in the form of acetyl coenzyme A (acetyl-CoA), is condensed via a ketothiolase (EC 2.3.1.9; encoded by the phbA gene) enzyme to generate acetoacetyl-CoA. Reduction of acetoacetyl-CoA to d-3-hydroxybutyryl-CoA is carried out by an NADP-dependent acetoacetyl-CoA reductase (EC 1.1.1.36; encoded by the phbB gene) (21). The PHB synthase enzyme (encoded by the phbC gene) catalyzes the final polymerization of d-3-hydroxybutyryl-CoA (21, 22).
Degradation of PHB is initiated by the depolymerization of the PHB granules, catalyzed by PHB depolymerase. A central enzyme in the PHB cycle is d-(−)-3-hydroxybutyrate dehydrogenase (BDH; EC 1.1.1.30; encoded by bdhA), which catalyzes the oxidation of the immediate PHB depolymerization product d-(−)-3-hydroxybutyrate (DHB) to acetoacetate (AA). The AA product of this reaction is activated to acetoacetyl-CoA by the enzyme acetoacetyl-CoA synthetase (AACS; EC 6.2.1.16), encoded by acsA2 in Sinorhizobium meliloti (5, 7, 15). The final step in the degradation portion of the PHB cycle yields two molecules of acetyl-CoA from one molecule of acetoacetyl-CoA via ketothiolase (EC 2.3.1.9). S. meliloti acsA2 mutants are able to grow with acetate as a carbon source but not with AA; acsA1 (7, 15) mutants, which do not produce acetyl-CoA synthetase (EC 6.2.1.1), are unable to grow on acetate but are able to grow on AA (20). While wild-type S. meliloti is able to grow using either DHB or AA, it is unable to use l-(+)-3-hydroxybutyrate (LHB) as a sole source of carbon.
Although there is a considerable body of research on the biochemistry and genetics of bacterial PHB synthesis, apart from our recent studies on the PHB degradation pathway of S. meliloti (1, 5, 8), not much is known about the genetics of the bacterial PHB degradation pathway(s) or assimilation of 3-hydroxybutyrate (3-HB). Our studies of the PHB cycle have included the isolation and characterization of transposon Tn5 insertion mutants that show altered growth on minimal media containing dl-3-hydroxybutyrate (DLHB) and/or AA as a sole carbon source (8). To ensure that these mutants had lesions specific to the PHB degradation pathway, we screened for mutants that were unaffected in the ability to utilize acetate and glucose (8). This screening protocol resulted in the initial isolation and characterization of bdhA and acsA2 mutants.
In addition to the mutants that were unable to grow on 3-HB and AA, we also isolated a mutant that exhibited an enhanced growth rate on these carbon sources (8). The Tn5 insertion in this mutant was found to be closely linked in φM12 transduction to the acsA2 gene. The Age (for AA growth-enhanced) phenotype was postulated to be due to a regulatory mutation that specifically alters growth on the PHB degradation pathway intermediates 3-HB and AA, since growth on glucose or acetate remained unaffected. In this report, we show that spontaneous mutations that restore growth of the bdhA mutant on DLHB minimal medium, without restoration of BDH activity, are tightly linked to the age mutation previously reported. We also offer evidence that the apparent suppression phenotype is a consequence of elevated AcsA2 levels, is specific for utilization of LHB but not DHB, and is in fact due to appearance of an l-(+)-3-hydroxybutyryl-CoA synthetase (LHBCS)-dependent pathway for utilization of LHB.
MATERIALS AND METHODS
Bacterial strains, plasmids, and transposons.
Bacterial strains, plasmids, and transposons used in this study are listed in Table 1. Construction of new strains is described in the text or summarized in the table.
TABLE 1.
Strains, plasmids, and transposons used in this study
| Strain, plasmid, or transposon | Relevant characteristicsa | Reference, source, and/or construction |
|---|---|---|
| Strains | ||
| S. meliloti | ||
| RCR2011 | SU47 wild type | Rothamsted Experimental Station |
| Rm1021 | SU47 str-21 | 19 |
| Rm1021 derivatives | ||
| Rm11104 | age-1::Tn5 | 8 |
| Rm11107 | bdhA1::Tn5 | 8 |
| Rm11143 | age-1::Tn5-233 | 8 |
| Rm11159 | bdhA2::Tn5 | 1 |
| Rm11172 | age-1::Tn5-Tp | This study; Tn5-Tp replacement of Tn5-233 insertion in Rm11143 |
| Rm11175 | age-1::Tn5-Tp bdhA2::Tn5 | This study; φRm11159→Rm11172 |
| Rm11192 | bdhA2::Tn5 shb-1 | This study; spontaneous growth on DLHB |
| Rm11194 | bdhA2::Tn5 shb-2 | This study; spontaneous growth on DLHB |
| Rm11196 | bdhA2::Tn5 shb-3 | This study; spontaneous growth on DLHB |
| Rm11198 | bdhA2::Tn5 shb-4 | This study; spontaneous growth on DLHB |
| Rm11218 | bdhA2::Tn5 Ω218::Tn5-233 Hbu− | φTn5-233 bank→Rm11192 |
| Rm11219 | bdhA2::Tn5 Ω219::Tn5-233 Hbu− | φTn5-233 bank→Rm11196 |
| Rm11223 | bdhA2::Tn5 Ω223::Tn5-233 Hbu− | φTn5-233 bank→Rm11198 |
| Rm11228 | bdhA2::Tn5 Ω218::Tn5-233 shb-1 Hbu+ | φRm11218→Rm11192 |
| Rm11229 | bdhA2::Tn5 Ω219::Tn5-233 shb-3 Hbu+ | φRm11219→Rm11196 |
| Rm11232 | bdhA2::Tn5 Ω223::Tn5-233 shb-4 Hbu+ | φRm11223→Rm11198 |
| Rm11281 | bdhA1::Tn5age-1::Tn5-Tp | φRm11107→Rm11172 |
| Rm11364 | acsA27::Tn5 acsA115::Tn5-233; deficient in AACS and acetyl-CoA synthetase activities | 20 |
| E. coli | ||
| NovaBlue | endA1 hsdR17 (rK-12− mK-12+) supE44 thi-1 recA1 gyrA96 relA1 lac[F′ proA+B+ lacIqZΔM15::Tn10] Tcr | Novagen |
| BL21(DE3)pLysS | F−ompT hsdSB (rB− mB−) gal dcm (DE3) pLysS (Cmr) | Novagen |
| Plasmids | ||
| pSP329 | IncP cloning vector; Tcr | 6; S. Porter |
| pGQ105 | pSP329 carrying the S. meliloti acsA2 gene on a 4.0-kb KpnI fragment | 5 |
| pET30 Xa/LIC | N-terminal His tag expression vector; Kanr | Novagen |
| pRD112 | pET30 Xa/LIC expressing His-tagged acsA2 | This study |
| Transposons | ||
| Tn5 | Nmr Smr | 3 |
| Tn5-233 | Gmr-Spr | 11 |
| Tn5-Tp | Tpr | 16; S. Klein |
Abbreviations for antibiotic resistance phenotypes: Cm, chloramphenicol; Gm, gentamicin; Kan, kanamycin; Nm, neomycin; Sm, streptomycin; Sp, spectinomycin; Tc, tetracycline; Tp, trimethoprim. Abbreviations for other phenotypes: Hbu+, growth on M9 containing DLHB; Hbu−, no growth on M9 containing DLHB.
Media, antibiotics, and culture conditions.
Bacterial cultures in Luria-Bertani (LB), tryptone-yeast extract (TY), and modified M9 minimal medium with different carbon sources and antibiotic concentrations were as previously described (8, 9). Sodium salts of DHB and LHB were purchased from Sigma-Aldrich Chemical Co. (Oakville, Canada). Growth kinetics in liquid medium were determined as described previously (8).
Genetics and molecular biology.
Bacterial conjugation, φM12 transductions, construction of a Tn5-233 random insertion bank in Rm1021, homogenotizations, and transposon replacements were carried out as described previously (10, 12, 13). Standard methods were used for DNA manipulations (2).
Enzyme assays.
Preparation of cell extracts, protein determination, and assays for BDH activity (NADH formation) and for AACS and acetyl-CoA synthetase activities (acetyl-CoA formation coupled to reduction of NAD+ via malate dehydrogenase and citrate synthase) were carried out as described previously (5, 8). NADH formation was measured using an Ultrospec 2000 spectrophotometer (Amersham Pharmacia Biotech, Baie d'Urfé, Canada). For an assay of AACS activity of purified protein samples, a sonicated cell extract (20 μl of an 11-mg/ml sample) from a culture of TY-grown strain Rm11364 (an acsA1 acsA2 double mutant) was added as a source of thiolase to convert acetoacetyl-CoA to acetyl-CoA; the amount of acetyl-CoA production was then determined in the assay. LHBCS activity was assayed by coupling the reaction to l-3-hydroxyacyl-CoA dehydrogenase. The reaction mixture (1 ml) contained LHB (varying amounts for kinetic studies with purified protein; 22 μmol for assay of crude extracts), Tris-Cl (200 μmol; pH 8.4), MgCl2 (10 μmol), NAD (10 μmol), CoA (0.2 μmol), l-3-hydroxyacyl-CoA dehydrogenase (8 U, from bovine liver [Sigma-Aldrich]), KCl (100 μmol), ATP (20 μmol), and cell extract or purified protein. The reaction was initiated by addition of ATP. The rate of NADH formation was monitored at 340 nm.
Overexpression of acsA2 and purification of AcsA2.
The Novagen (Madison, Wis.) pET30 Xa/LIC (ligation-independent cloning) kit was used. Primers contained vector-compatible overhangs (underlined) and were designed according to the coding sequence of acsA2 (acsA2licfor, 5′-GGTATTGAGGGTCGCCAAGCAGAACGACCTTTGT-3′; acsA2licrev, 5′-AGAGGAGAGTTAGAGCCAGCCGGCACTACGACA-3′) and synthesized by Sigma-Aldrich. The PCR mixture contained 3 μl of 25 mM MgCl2, 5 μl of 10× Tli DNA polymerase reaction buffer (Promega, Madison, Wis.), 1 μl of 10 mM deoxynucleoside triphosphate mix, 2.5 μl of each primer (10 pmol/μl), 2.5 U of Tli proofreading polymerase (Promega), 0.2 μg of RCR2011 genomic DNA as the template, and deionized H2O to make up the final volume to 50 μl. Reactions were carried out in an Eppendorf Mastercycler Gradient thermocycler (Brinkmann Instruments, Mississauga, Canada) using a hot-start amplification protocol (94°C for 2 min; 30 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 150 s, followed by a final extension at 72°C for 10 min). The single PCR product obtained was purified using the QIAquick PCR purification kit (Qiagen, Mississauga, Canada), followed by extraction with 1 volume of chloroform-isoamyl alcohol (24:1). The purified PCR product was cloned into pET-30 Xa/LIC using T4 DNA polymerase according to the manufacturer's instructions. Escherichia coli NovaBlue competent cells were transformed with the reaction mixture, and kanamycin-resistant cells containing the desired recombinant plasmid, pRD112, were thus obtained.
Plasmid pRD112 was transferred into expression strain E. coli BL21(DE3)pLysS, and a 5-ml volume of a saturated LB-chloramphenicol-kanamycin culture was used to inoculate a 2.8-liter Fernbach flask containing 500 ml of LB-chloramphenicol-kanamycin. Growth under noninducing conditions was carried out at 37°C with shaking at 200 rpm for 2 h, after which isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 1 mM, and cells were incubated for another 3 h. Cells were lysed using Bugbuster plus Benzonase (Novagen). Insoluble cell debris was removed by centrifugation, the resulting extract (ca. 8 ml) was applied to a His·Bind resin column (Novagen) with a 1-ml column bed, and 1-ml eluted fractions were collected. Purified protein was visualized as a single band of ca. 72 kDa, and the remaining protein preparation was stored at −70°C in elution buffer containing 50% glycerol.
RESULTS
The age-1 insertion mutation allows the bdhA mutant to grow on DLHB without restoration of BDH activity.
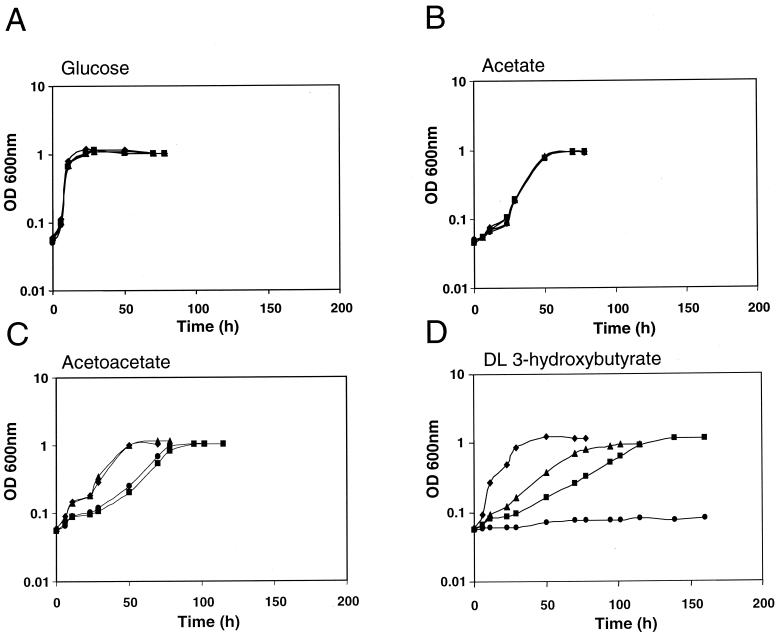
In an earlier report (8) we had hypothesized that the Age phenotype of the age-1 insertion mutant could provide clues to the mechanisms that regulate PHB degradation in S. meliloti, since the age-1 mutant exhibits aberrant growth on the PHB degradation intermediates HB and AA but not on glucose or acetate. To investigate whether the age-1 insertion mutation confers DLHB utilization on a bdhA mutant, we generated a bdhA age-1 double mutant. The bdhA1::Tn5 insertion in strain Rm11107 was transduced into strain Rm11172, which bears the age-1::Tn5-Tp insertion. The resulting double mutant, strain Rm11281, exhibited growth on DLHB that was intermediate between the growth rates of the wild type and the age-1 mutant (Fig. 1). None of the strains exhibited altered growth on glucose or acetate as a sole carbon source. To investigate the possibility that this phenotypic suppression was mediated by activation of an unidentified, secondary BDH-encoding gene, cell extracts of DLHB-grown Rm11175, a similar bdhA age-1 double mutant, were assayed for BDH activity (Table 2) by using DLHB as a substrate. The lack of BDH activity in this strain indicated that the suppression of the DLHB growth defect is not due to restoration of BDH activity.
FIG. 1.
Growth kinetics of the wild-type strain and representative mutants on different carbon sources. Strains used were Rm1021 (wild type) (▪), Rm11172 (age-1::Tn5-Tp) (⧫), Rm11107 (bdhA1::Tn5) (•), and Rm11281 (bdhA1::Tn5 age-1::Tn5-Tp) (▴). Carbon sources used were 10 mM glucose(A), 30 mM acetate (B), 15 mM AA (C), and 15 mM DLHB (D).
TABLE 2.
Enzyme activities of cell extracts of DLHB-grown culturesa
| Strain | Relevant characteristic(s)b | Activity (nmol/min/mg of protein)c of:
|
||
|---|---|---|---|---|
| 3-Hydroxybutyrate dehydrogenase | AACS | LHBCS | ||
| Rm1021 | wt | 44.4 ± 0.8 | 9.8 ± 3.6 | 0 |
| Rm11172 | age-1::Tn5-Tp | 52.7 ± 2.0 | 27.5 ± 2.2 | 40.7 ± 8.5 |
| Rm11175 | bdhA2::Tn5 age-1::Tn5-Tp | 0 | 42.7 ± 7.3 | 83.3 ± 19.3 |
| Rm11192 | bdhA2::Tn5 shb-1 | 0 | 206.8 ± 23.7 | 98.9 ± 5.2 |
| Rm1021(pSP329) | wt (vector) | 52.0 ± 0.9 | 5.9 ± 1.7 | 0 |
| Rm1021(pGQ105) | wt (acsA2) | 42.1 ± 6.8 | 73.9 ± 6.5 | 51.6 ± 19.4 |
| Rm11107(pGQ105) | bdhA1::Tn5 (acsA2) | 0 | 115.2 ± 27.5 | 65.2 ± 12.7 |
Cell extracts were prepared from cultures grown in minimal medium supplemented with 15 mM DLHB. Note that bdhA mutant controls could not be included because such strains are unable to grow in DLHB.
wt, wild type.
Values are averages from triplicate assays ± standard deviations.
Isolation of spontaneous mutations that suppress the DLHB growth defect phenotype of the bdhA mutant.
By isolating spontaneous mutants that restore the growth of the bdhA mutant on DLHB, we investigated whether S. meliloti is capable of employing alternative mechanisms for utilization of DLHB. Five independent, TY-grown cultures of Rm11159, carrying the bdhA2::Tn5 allele, were prepared, and ca. 109 cells from each were spread on M9 DLHB plates. Following incubation at 30°C for 10 days, colonies had appeared on most of the plates. Of the five independent cultures plated, one gave rise to no colonies, even after an extended period of incubation, while the other four cultures gave rise to 1 to 24 colonies each. One colony from each plate was isolated and streak purified twice on DLHB minimal medium; these mutants were designated Rm11192 (shb-1), Rm11194 (shb-2), Rm11196 (shb-3), and Rm11198 (shb-4), respectively. The bdhA2::Tn5 insertion was confirmed to be still present in each spontaneous mutant by transducing the Nmr marker out of the suppressing background into the Rm1021 wild-type background. The resulting transductants were unable to grow on DLHB, confirming that these strains carried true second-site mutations.
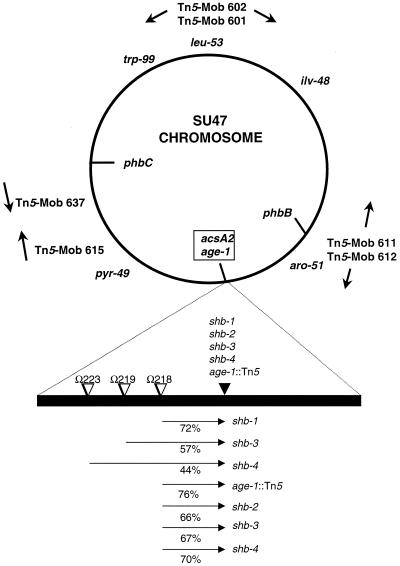
To facilitate further characterization of the spontaneous mutations, a selectable genetic marker was linked to them. A φM12 transducing lysate was prepared on a pool of Tn5-233 (Gmr-Spr) insertion mutants. The Tn5-233 insertions were transduced into the second-site mutants Rm11192, Rm11196, and Rm11198, and the resulting Gmr-Spr transductants were screened by replica plating for inability to grow on DLHB, indicating cotransduction of the Tn5-233 insertion with the wild-type allele counterpart of the second-site mutation. Tn5-233 insertions linked to the second-site alleles were thus isolated. Strains Rm11218, Rm11219, and Rm11223 each carry a Tn5-233 insertion linked to the second-site allele in Rm11192 (shb-1), Rm11196 (shb-3), and Rm11198 (shb-4), respectively.
The cotransduction linkage of the Tn5-233 insertions and the second-site alleles was determined by two-factor transduction analysis. In separate backcrosses, each of the linked Tn5-233 insertions was transduced into the corresponding original suppressor strain. The resulting Gmr-Spr transductants were tested for inability to utilize DLHB. This provided a measurement of the cotransduction frequency of the wild-type counterpart of the suppressor alleles and each linked Tn5-233 insertion (Fig. 2). One of the linked Tn5-233 insertions, Ω218::Tn5-233, was transduced into each of the independently isolated suppressor strains, and similar linkages to each suppressor allele were found, suggesting that all of the suppressor alleles map to the same locus. The Ω218::Tn5-233 insertion was also found to have a similar linkage to the age-1::Tn5 insertion, suggesting that the spontaneous suppressor alleles are located near the age-1 locus on the S. meliloti chromosome and that the mechanisms of DLHB suppression in the spontaneous suppressor strains and the age-1 strain are likely similar. Furthermore, the cell extracts of the suppressor strain Rm11192 did not possess BDH activity (Table 2).
FIG. 2.
Genetic map of the S. meliloti chromosome, showing the locations of the acsA2 gene, required for utilization of AA, and age-1, an insertion in which results in an increased growth rate on AA and HB. Arrows indicate positions and orientations of Tn5-mob insertions. The positions of the auxotrophic markers are from the genetic map of the chromosome (17). The positions of the mapped loci are approximate and are based on conjugation and transduction linkage data (8). The relative order of the Tn5-233 insertions and the suppressor alleles depicted is arbitrary. Cotransduction frequencies are given as percentages below the arrows. The tail of each arrow indicates the donor marker and the head represents the recipient in transduction.
Plasmid-encoded synthesis of AcsA2 restores growth of the bdhA mutant on DLHB.
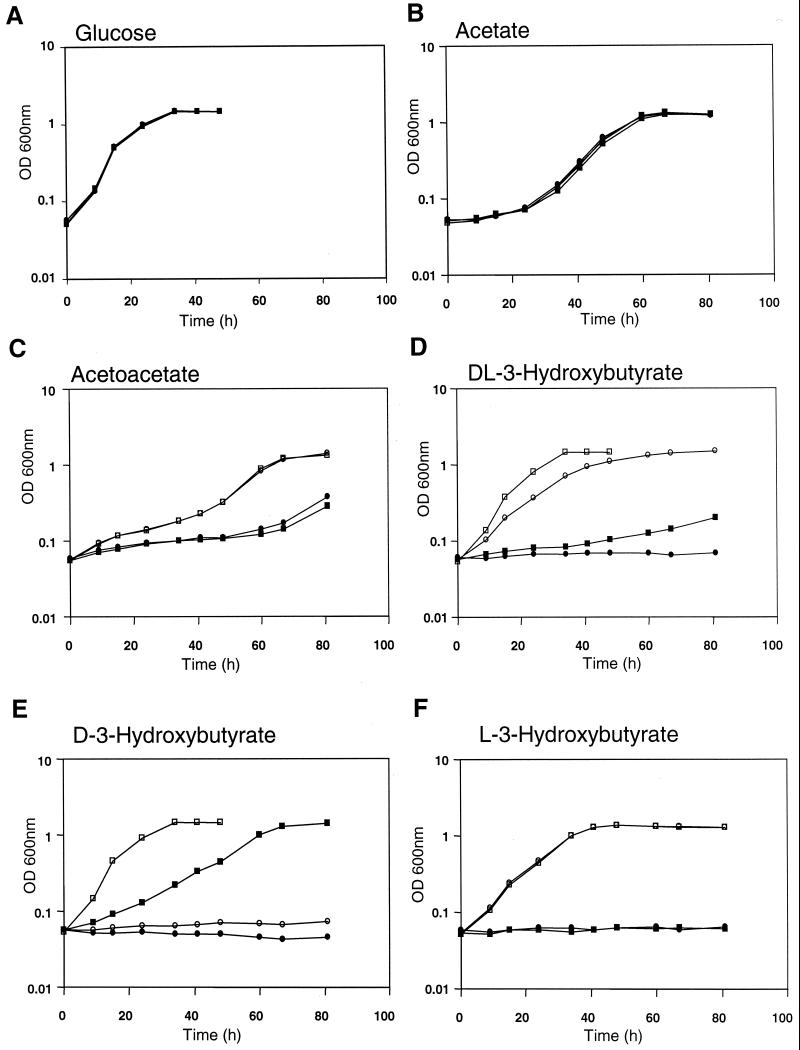
Since the age-1::Tn5 insertion,which bestows DLHB utilization ability on the bdhA mutant (Fig. 1), is tightly linked in transduction to the acsA2 gene, encoding AcsA2 (8), and also causes elevated expression of the acsA2 transcript (4), we wished to test whether introduction of acsA2 on a multicopy plasmid would affect the bdhA mutant phenotype. Plasmid pGQ105, carrying acsA2 as the only predicted functional gene (7, 15) on a 4-kb KpnI fragment, was introduced into the bdhA mutant Rm11107, and the transconjugant was tested for the ability to utilize DLHB. Growth kinetics of the wild-type and bdhA strains carrying pGQ105 or the vector control plasmid pSP329 confirmed that only strains containing multiple acsA2 copies exhibit enhanced growth rates on both AA and DLHB minimal media (Fig. 3). Although the bdhA mutant is unable to grow on DLHB, introduction of the acsA2 plasmid resulted in a DLHB growth rate considerably higher than that of the wild-type strain that expressed BDH activity. The wild-type strain containing the acsA2 plasmid had a slightly higher DLHB growth rate, reflecting the presence of BDH activity. On AA, the acsA2 plasmid enhanced the growth rates of the bdhA mutant and the wild-type strains to about the same level. Thus, multiple acsA2 copies not only restore the ability to utilize DLHB to the bdhA mutant but also confer on the wild type the enhanced-growth phenotype that is associated with the age-1 mutation. All strains tested had similar growth rates on glucose and acetate.
FIG. 3.
Growth kinetics of Rm1021 (wild type) and Rm11107 (bdhA1::Tn5) containing multiple copies of acsA2 (pGQ105) or parent vector plasmid (pSP329) on different carbon sources. Strains used were Rm1021(pSP329) (▪), Rm1021(pGQ105) (□), Rm11107(pSP329) (•), and Rm11107(pGQ105) (○). Carbon sources were 10 mM glucose (A), 30 mM acetate (B), 15 mM AA (C), 15 mM DLHB (D), 15 mM DHB (E), and 15 mM LHB (F).
Suppression is specific for utilization of the l(+) isomer of 3-HB.
The observed differences between the extents of enhanced growth of the age-1 and bdhA age-1 strains on DLHB point toward the possibility of novel LHB utilization ability conferred by the age-1 mutation. It seemed reasonable to propose that in the age-1 mutant, the d(−) and l(+) isomers of HB would be assimilated by BDH-dependent and BDH-independent pathways, respectively. In the absence of BDH activity, however, as in the bdhA age-1 double mutant, only the l(+) isomer would serve as a potential source of carbon and energy. The growth rate would thus be lower than that of the age-1 single mutant on DLHB. In order to test this hypothesis, we compared the growth profiles of the wild-type and bdhA mutant carrying either the parental vector plasmid pSP329 or the acsA2 clone pGQ105 on DHB and LHB (Fig. 3). While neither the wild-type nor the bdhA mutant control strain was able to grow on LHB, both of these strains had similar growth rates on LHB when they harbored pGQ105. The bdhA mutant was unable to utilize DHB even when it carried pGQ105. Therefore, assimilation of LHB in S. meliloti occurs only when acsA2 is overexpressed, and utilization of DHB occurs solely via a BDH-dependent pathway. The suppression of the bdhA mutant growth phenotype on DLHB is therefore due to acquisition of the ability to assimilate LHB and does not involve restoration of DHB utilization ability. Interestingly, it was also noted that the growth rate of the wild-type strain Rm1021 on DHB is significantly greater than that observed on DLHB, suggesting that LHB may be an inhibitor of acsA2 expression or AACS activity.
Ability to grow on LHB upon provision of multiple acsA2 copies suggested that the AcsA2 enzyme either is directly involved in assimilation of LHB by utilizing LHB as an alternative substrate or may act indirectly by influencing the expression of a latent LHBCS-encoding gene or enzyme activity. To test this, we developed an assay for LHBCS activity based on the coupling of LHB activation to the enzyme l-3-hydroxyacyl-CoA dehydrogenase (see Materials and Methods) and used the assay to measure the levels of LHBCS-specific activity in the mutant and acsA2-overexpressing strains (Table 2). While LHBCS was not detectable in the wild-type strain, it was clearly present in extracts from strains that were able to utilize LHB and exhibited enhanced AACS activity. The LHB utilization property is therefore directly related to the introduction of LHBCS enzyme activity resulting from increased expression of acsA2.
Substrate specificity of purified AcsA2 protein.
To test whether the LHBCS enzyme activity detected above was directly due to AcsA2, we carried out enzyme assays with a purified AcsA2 protein preparation. The purified His-tagged AcsA2 protein was active with either AA (i.e., AACS activity) or LHB (i.e., LHBCS activity) as the substrate, but no activity was detected with acetate as the substrate. No AACS activity was detected when the assay was carried out in the absence of the thiolase activity provided by addition of the Rm11364 cell extract. The apparent Km and Vmax for AA were 0.30 ± 0.05 mM and 479 ± 12 nmol/min/mg, respectively, compared to 20 ± 3 mM and 259 ± 12 nmol/min/mg for LHB. This further confirms that the LHBCS activity in S. meliloti cells that overexpress acsA2 is directly due to the increased levels of AcsA2.
DISCUSSION
S. meliloti isolate SU47 and its derivatives are naturally unable to use LHB as a sole source of carbon and energy. We have shown, however, that this organism does have the metabolic capacity to utilize LHB. Transposon-generated and spontaneously occurring mutations, as well as plasmid-mediated overproduction of the AcsA2 protein, are able to confer LHB utilization ability. Our growth kinetics and enzyme assay data suggest that S. meliloti can use LHB as a growth substrate, but only when the AcsA2 protein is overproduced as a result of genetic alterations. We note that the ratio of AACS activity to LHBCS activity in individual strains varies; for example, strain Rm11192 has an AACS/LHBCS ratio of ca. 2, while strain Rm11175 has an AACS/LHBCS ratio of only ca. 0.5 (see Table 2). This is almost certainly due to differences in the affinities of the two different substrates for AcsA2. We have clearly demonstrated that the purified AcsA2 protein does in fact possess LHBCS activity. The higher Km and lower Vmax for LHB than for AA suggest that LHB is not a natural substrate for the enzyme. A similar enzyme with AACS-activating activity was previously isolated from Zoogloea ramigera I-16-M, and it is also capable of activating LHB, but with lower efficiency than its activation of AA (14).
There are several possible metabolic fates for LHB following activation to LHB-CoA. It could be further metabolized by 3-hydroxyacyl-CoA dehydrogenase, yielding acetoacetyl-CoA, or by 3-hydroxybutyryl-CoA epimerase, yielding DHB-CoA. Both of these enzyme activities are predicted to be encoded by the fadB gene (23) that is present in the S. meliloti genome (7, 15). The genome sequence also predicts at least three additional genes encoding 3-hydroxybutyryl-CoA dehydrogenase. Whether either or both of these possible pathways are used during growth on LHB could be tested by mutational analysis of these genes in S. meliloti.
Is LHB a naturally utilized carbon source for wild-type S. meliloti? We have clearly demonstrated that the organism has the genetic capacity to utilize LHB as a carbon source. Simple up-regulation of the gene expression, without alteration of the coding sequence, was sufficient to confer LHB activation ability on the organism. We have also shown that presence of LHB inhibits the ability to utilize DHB as a carbon source (Fig. 3D and E). The inability of the wild-type cell to utilize LHB is perhaps due to the lack of acsA2 gene expression induction, or even its inhibition, by exogenous LHB. When the cell is utilizing AA generated from the oxidation of DHB under carbon-limiting conditions, the ability to utilize exogenous LHB could perhaps be deleterious to the maintenance of the PHB degradation process. This might explain why wild-type S. meliloti preferentially utilizes DHB over LHB. It is conceivable, however, that under certain environmental and/or physiological conditions, expression of the acsA2 gene is sufficiently induced by intracellular metabolites and/or by other extracellular environmental signals to result in significant levels of LHB activation.
Acknowledgments
This work was supported by a Natural Sciences and Engineering Research Council operating grant to T.C.C.
Tli polymerase was a generous gift of VWR Canlab.
REFERENCES
- 1.Aneja, P., and T. C. Charles. 1999. Poly-3-hydroxybutyrate degradation in Rhizobium (Sinorhizobium) meliloti: isolation and characterization of a gene encoding 3-hydroxybutyrate dehydrogenase. J. Bacteriol. 181:849-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1997. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 3.Berg, C. M., and D. E. Berg. 1987. Uses of transposable elements and maps of known insertions, p. 1071-1107. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
- 4.Cai, G.-Q. 2001. Molecular genetic analysis of acetoacetate metabolism in Sinorhizobium meliloti. Ph.D. thesis. McGill University, Montreal, Quebec, Canada.
- 5.Cai, G.-Q., B. T. Driscoll, and T. C. Charles. 2000. Requirement for the enzymes acetoacetyl coenzyme A synthetase and poly-3-hydroxybutyrate (PHB) synthase for growth of Sinorhizobium meliloti on PHB cycle intermediates. J. Bacteriol. 182:2113-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cangelosi, G. A., E. A. Best, G. Martinetti, and E. W. Nester. 1991. Genetic analysis of Agrobacterium. Methods Enzymol. 204:384-397. [DOI] [PubMed] [Google Scholar]
- 7.Capela, D., F. Barloy-Hubler, J. Gouzy, G. Bothe, F. Ampe, J. Batut, P. Boistard, A. Becker, M. Boutry, E. Cadieu, S. Dreano, S. Gloux, T. Godrie, A. Goffeau, D. Kahn, E. Kiss, V. Lelaure, D. Masuy, T. Pohl, D. Portetelle, A. Puhler, B. Purnelle, U. Ramsperger, C. Renard, P. Thebault, M. Vandenbol, S. Weidner, and F. Galibert. 2001. Analysis of the chromosome sequence of the legume symbiont Sinorhizobium meliloti strain 1021. Proc. Natl. Acad. Sci. USA 98:9877-9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charles, T. C., G.-Q. Cai, and P. Aneja. 1997. Megaplasmid and chromosomal loci for the PHB degradation pathway in Rhizobium (Sinorhizobium) meliloti. Genetics 146:1211-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charles, T. C., and T. M. Finan. 1991. Analysis of a 1600-kilobase Rhizobium meliloti megaplasmid using defined deletions generated in vivo. Genetics 127:5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charles, T. C., W. Newcomb, and T. M. Finan. 1991. ndvF, a novel locus located on megaplasmid pRmeSU47b (pEXO) of Rhizobium meliloti, is required for normal nodule development. J. Bacteriol. 173:3981-3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Vos, G. F., G. C. Walker, and E. R. Signer. 1986. Genetic manipulations in Rhizobium meliloti utilizing two new transposon Tn5 derivatives. Mol. Gen. Genet. 204:485-491. [DOI] [PubMed] [Google Scholar]
- 12.Finan, T. M., E. K. Hartwieg, K. LeMieux, K. Bergman, G. C. Walker, and E. R. Signer. 1984. General transduction in Rhizobium meliloti. J. Bacteriol. 159:120-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finan, T. M., B. Kunkel, G. F. DeVos, and E. R. Signer. 1986. Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J. Bacteriol. 167:66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukui, T., M. Ito, and K. Tomita. 1982. Purification and characterization of acetoacetyl-CoA synthetase from Zoogloea ramigera I-16-M. Eur. J. Biochem. 127:423-428. [DOI] [PubMed] [Google Scholar]
- 15.Galibert, F., T. M. Finan, S. R. Long, A. Puhler, P. Abola, F. Ampe, F. Barloy-Hubler, M. J. Barnett, A. Becker, P. Boistard, G. Bothe, M. Boutry, L. Bowser, J. Buhrmester, E. Cadieu, D. Capela, P. Chain, A. Cowie, R. W. Davis, S. Dreano, N. A. Federspiel, R. F. Fisher, S. Gloux, T. Godrie, A. Goffeau, B. Golding, J. Gouzy, M. Gurjal, I. Hernandez-Lucas, A. Hong, L. Huizar, R. W. Hyman, T. Jones, D. Kahn, M. L. Kahn, S. Kalman, D. H. Keating, E. Kiss, C. Komp, V. Lelaure, D. Masuy, C. Palm, M. C. Peck, T. M. Pohl, D. Portetelle, B. Purnelle, U. Ramsperger, R. Surzycki, P. Thebault, M. Vandenbol, F. J. Vorholter, S. Weidner, D. H. Wells, K. Wong, K. C. Yeh, and J. Batut. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293:668-672. [DOI] [PubMed] [Google Scholar]
- 16.Glazebrook, J., and G. C. Walker. 1991. Genetic techniques in Rhizobium meliloti. Methods Enzymol. 204:398-418. [DOI] [PubMed] [Google Scholar]
- 17.Klein, S., K. Lohmann, R. Clover, G. C. Walker, and E. R. Signer. 1992. A directional, high-frequency chromosomal localization system for genetic mapping in Rhizobium meliloti. J. Bacteriol. 174:324-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madison, L. L., and G. W. Huisman. 1999. Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol. Mol. Biol. Rev. 63:21-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meade, H. M., S. R. Long, G. B. Ruvkun, S. E. Brown, and F. M. Ausubel. 1982. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon mutagenesis. J. Bacteriol. 149:114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thaha, F. Z. 2000. Characterization of acetate metabolism genes in Sinorhizobium (Rhizobium) meliloti. M.Sc. thesis. McGill University, Montreal, Quebec, Canada.
- 21.Tombolini, R., S. Povolo, A. Buson, A. Squartini, and M. P. Nuti. 1995. Poly-β-hydroxybutyrate (PHB) biosynthetic genes in Rhizobium meliloti 41. Microbiology 141:2553-2559. [DOI] [PubMed] [Google Scholar]
- 22.Willis, L. B., and G. C. Walker. 1998. The phbC (poly-β-hydroxybutyrate synthase) gene of Rhizobium (Sinorhizobium) meliloti and characterization of phbC mutants. Can. J. Microbiol. 44:554-564. [DOI] [PubMed] [Google Scholar]
- 23.Yang, S. Y., J. M. Li, X. Y. He, S. D. Cosloy, and H. Schulz. 1988. Evidence that the fadB gene of the fadAB operon of Escherichia coli encodes 3-hydroxyacyl-coenzyme A (CoA) epimerase, Δ3-cis-Δ2-trans-enoyl-CoA isomerase, and enoyl-CoA hydratase in addition to 3-hydroxyacyl-CoA dehydrogenase. J. Bacteriol. 170:2543-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]