Abstract
Most of the exoproteins secreted by Pseudomonas aeruginosa are transported via the type II secretion system. This machinery, which is widely conserved in gram-negative bacteria, consists of 12 Xcp proteins organized as a multiprotein complex, also called the secreton. We previously reported that the mutual stabilization of XcpZ and XcpY plays an important role in the assembly of the secreton. In this study, we engineered variant XcpZ proteins by using linker insertion mutagenesis. We identified three distinct regions of XcpZ required for both the stabilization of XcpY and the functionality of the secreton. Interestingly, we also demonstrated that another component of the machinery, XcpP, can modulate the stabilizing activity of XcpZ on XcpY.
Many gram-negative bacteria have evolved several secretory pathways to release proteins into the surrounding environment. In the opportunistic pathogen Pseudomonas aeruginosa, most of the exoproteins are transported by the type II secretory system (9, 19, 22). This system has been found to be widely conserved in gram-negative bacteria, such as in Aeromonas hydrophila (15), Vibrio cholerae (23), Erwinia species (13, 21), Klebsiella oxytoca (5), and Pseudomonas species (6, 7, 8, 10). Exoproteins that use the type II secretory pathway are Sec dependent and are secreted into the extracellular medium by a two-step process involving a transient periplasmic intermediate (19). Transport of these exoproteins across the outer membrane of P. aeruginosa requires a specialized machinery (the secreton), which is composed of 12 proteins designated XcpA and XcpP to -Z (9). The Xcp apparatus is most probably organized as a multiprotein complex spanning the periplasm (9). Little is known about the function of the various components of the type II machinery. XcpA (also called PilD) is involved in the maturation of both type IV pilins (17); Xcp T, -U, -V, -W, and -X are known as pseudopilins (1, 3, 4); XcpR is thought to play a role in the energization of the secretory process (9, 23); and XcpQ might function as a specialized pore (2). XcpP, -Y, and -Z span the inner membrane once with their N-terminal part in the cytoplasm (3). The XcpP and XcpZ proteins are essentially exposed to the periplasm. In contrast, the periplasmic domain is short in XcpY, and most of the protein extends into the cytoplasm (3).
Previous results obtained in our laboratory have demonstrated an interaction of XcpY and XcpZ within the P. aeruginosa secreton by showing a mutual stabilization of these two proteins (16). This interaction has been since demonstrated in homologous systems of other microorganisms, such as K. oxytoca (18), Erwinia chrysanthemi (20), and V. cholerae (24), by using different approaches and has been confirmed in P. aeruginosa by coimmunoprecipitation and copurification studies (unpublished results). In this study, we investigated domains of XcpZ involved in the stabilization of XcpY by engineering mutated proteins with linker insertions.
Linker insertion mutagenesis of XcpZ.
We constructed a series of xcpZ gene derivatives either by the insertion of a 12-bp linker in the gene (semirandom linker insertion mutagenesis [SRLIM]) as described by Bitter et al. (2) or by using the pentapeptide scanning mutagenesis (PSM) technique (11, 12). The mutant xcpZ library was completed by using a two-step PCR strategy as described by Higuchi (14). This technique was designed to mimic PSM and led to the insertion of 15 nucleotide pairs at specific locations in xcpZ.
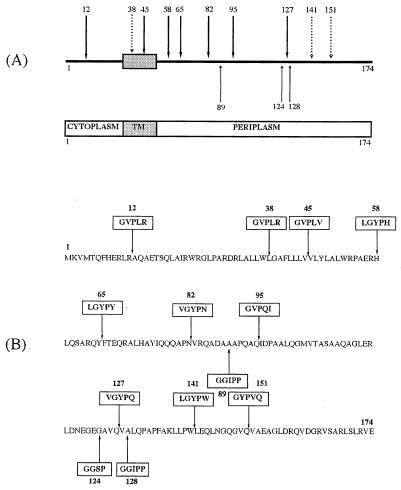
Thirteen independent mutants whose predicted protein sequences differed from that of the wild-type XcpZ only by the presence of a peptide insertion were isolated. The characteristics of the various mutants obtained are summarized in Fig. 1. Briefly, three insertions were obtained by SRLIM (insertions 89, 124, and 128), seven were obtained by PSM (insertions 12, 45, 58, 65, 82, 95, and 127), and three were obtained by PCR mutagenesis (insertions 38, 141, and 151). The mutations were randomly distributed in the protein sequence, with the majority being in the periplasmic domain of XcpZ (Fig. 1A). One mutation was in the cytoplasmic domain of XcpZ (XcpZ12), and two were within the transmembrane domain (XcpZ38 and XcpZ45) (Fig. 1B). Importantly, none of the 13 insertions was found to change the localization of XcpZ to the inner membrane or the growth rate of the variant strains (data not shown).
FIG. 1.
Mapping and amino acid sequences of peptide linker insertions in XcpZ. (A) Peptide insertions are named by the amino acid residue to the N-terminal side of the insertion. Residues are numbered from 1 (N terminus) to 174 (C-terminal end). TM, transmembrane domain, represented as a filled box. Heavy arrows, insertions obtained by PSM; light arrows, insertions obtained by SRLIM; dashed arrows, insertions obtained by double PCR mutagenesis. (B) Amino acid sequences of peptide insertions. Note that SRLIM insertions 89 and 128 were introduced within a codon leading to the change of an A residue to GGIPP (indicated by an arrow).
Stabilization of XcpY by the mutated XcpZ proteins in P. aeruginosa.
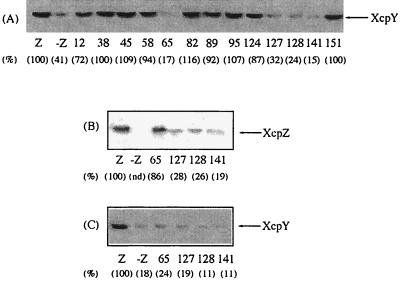
In order to characterize the interaction of XcpZ and XcpY in more molecular detail, XcpZ variants were expressed in an xcpZ-defective strain of P. aeruginosa (strain KS902) obtained by ethyl methanesulfonate mutagenesis. The xcpZ mutation in strain KS902 is a substitution of a thymine for a cytosine (C16T) introducing a stop codon near the 5′ end of the gene (16). All variant XcpZ proteins were clearly detected after immunoblotting (data not shown), except for XcpZ65, -127, -128, and -141, which were observed in detectable amounts only following induction with IPTG (isopropyl-β-d-thiogalactopyranoside) (Fig. 2B). Mutated proteins XcpZ12, -38, -45, -58, -82, -89, -95, -124, and -151 efficiently stabilized XcpY (which accounted for 72 to 116% of the control amount) and pinpointed permissive regions of XcpZ not directly involved in interaction with XcpY (Fig. 2A). We observed that XcpZ levels as low as those found for the permissive variant XcpZ124 (35% of the control value) and for the XcpZ127, -128, and -141 variants after IPTG induction were sufficient to promote stabilization of XcpY. Nevertheless, in the presence of IPTG, XcpZ65, -127, -128, and -141 (Fig. 2B) were unable to stabilize XcpY, showing a lack of interaction (Fig. 2C).
FIG. 2.
Influence of mutated XcpZ proteins on the stability of XcpY in P. aeruginosa. (A) The variant xcpZ genes were subcloned in pMMB67HE and expressed in the xcpZ mutant strain KS902, and the levels of XcpY were analyzed by immunoblotting. Only the relevant portions of the blots are shown. Lane Z, KS902 expressing wild-type xcpZ gene; lane -Z, KS902 bearing pMMB67HE alone. Lane numbers indicate the insertion position in the variant XcpZ. Six independent experiments were performed, and results were found to be reproducible. Protein amounts estimated by scanning densitometry were expressed as percentages of the control value (lane Z), and results are indicated in parentheses. (B) Effect of 175 μM IPTG on the production of variant XcpZ65, -127, -128, and -141. The immunoblot was probed with XcpZ antiserum. (C) Stability of XcpY in the presence of the XcpZ variants used in panel B. The immunoblot was probed with XcpY antiserum.
Therefore, it appears that two distinct periplasmic domains of XcpZ, one located in the proximity of the transmembrane domain in the vicinity of residue 65 (domain I) and the other located towards the C-terminal part of the protein in the vicinity of residues 127, 128, and 141 (domain II), are both involved in the interaction with XcpY (Fig. 3).
FIG. 3.
Mapping of XcpZ domains required for interaction with XcpY. Hatched, black, and white blocks represent potential interaction domains with XcpY referred to as I, II, and III, respectively. TM, transmembrane domain. Numbers correspond to amino acid position in the sequence.
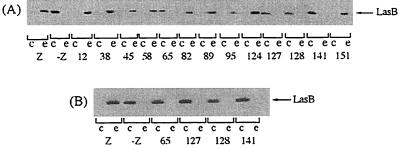
In other respects, it should be emphasized that there is a perfect correlation between XcpY stabilization and the functionality of the secretory apparatus as shown by secretion studies of elastase, the most abundant exoenzyme secreted by the Xcp system of P. aeruginosa. Indeed, only the XcpZ variants which stabilize XcpY were able to restore LasB secretion in strain KS902, while the nonpermissive variants XcpZ65, -127, -128, and -141 did not provide complementation of the xcpZ mutation, even in the presence of IPTG (Fig. 4). Stabilization of the Xcp components is thus a key factor for efficient functioning of the secretion machinery.
FIG. 4.
Effect of peptide insertions in XcpZ on the functionality of the Xcp machinery of P. aeruginosa. (A) The variant xcpZ genes were subcloned in pMMB67HE and expressed in strain KS902. Elastase LasB was analyzed by immunoblotting in cellular (c) and extracellular (e) fractions obtained from cells grown in the absence of IPTG. (B) Effect of 175 μM IPTG on the functionality of variant XcpZ65, -127, -128, and -141. LasB from cellular and extracellular extracts was analyzed by immunoblotting. At least two independent experiments were carried out, and the results were found to be reproducible. Other legend details are as indicated for Fig. 2.
Effect of the XcpZ variants on the stability of XcpY in Escherichia coli.
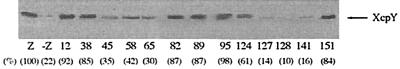
Experiments were also carried out with E. coli in order to study the stabilization of XcpY by XcpZ in the absence of any other component of the secretion machinery of P. aeruginosa. Under these conditions, all of the XcpZ variants were clearly detected by immunoblotting analysis. Strikingly, in contrast with the results obtained in the P. aeruginosa background, mutated proteins XcpZ45 and XcpZ58 poorly stabilized XcpY in E. coli, most probably because conformational changes in XcpZ altered interaction between the Xcp components (Fig. 5). These results suggested that a third partner, present in P. aeruginosa but absent in E. coli might be involved in the stabilizing activity of XcpZ. Indeed, the stabilizing activity of a third region (domain III), spanning from the end of the transmembrane domain (residue 45) to the beginning of the periplasmic part of XcpZ (residue 58), is clearly modulated by another component present in P. aeruginosa (Fig. 3).
FIG. 5.
Influence of variant XcpZ proteins on the stability of XcpY in E. coli. The variant xcpZ genes were cloned in pMMB67HE and expressed in E. coli TG1 bearing the xcpY gene subcloned in pLAFR3, and the levels of XcpY were analyzed by immunoblotting. Only the relevant portions of the blots are shown. Lane Z, TG1 expressing both the xcpY and xcpZ genes; lane -Z, TG1 expressing only the xcpY gene. Lane numbers indicate the insertion position in the variant XcpZ. Six independent experiments were performed, and results were found to be reproducible. Protein amounts estimated by scanning densitometry were expressed as percentages of the control value (lane Z), and the results are indicated in parentheses.
XcpP restores the stabilizing properties of the variants XcpZ45 and XcpZ58.
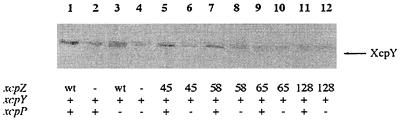
Previous studies carried out in our laboratory which showed that the XcpZ level was drastically reduced in an xcpP mutant of P. aeruginosa (G. Ball, unpublished results) suggested that XcpP might be the expected partner associated with the XcpYZ subcomplex. In order to test this hypothesis, XcpY and XcpP were expressed independently or simultaneously with wild-type or XcpZ variants in E. coli and the proteins were analyzed by immunoblotting (Fig. 6). Wild-type XcpZ alone was sufficient to stabilize XcpY (Fig. 6, lanes 1 and 3), while XcpP alone did not exhibit such a stabilizing effect on this protein (Fig. 6, lane 2). As mentioned above, XcpZ45 and XcpZ58 did not stabilize XcpY, which was decreased by 80 and 60%, respectively, in comparison to the control (Fig. 6, lanes 6 and 8). However, when these proteins were expressed together with XcpP, XcpY was efficiently stabilized (Fig. 6, lanes 5 and 7), suggesting that XcpP could trigger XcpY stabilization by these XcpZ variants either via a direct interaction or indirectly by acting through XcpY. Importantly, the stabilizing effect of XcpP seems to be specifically related to the XcpZ45 and XcpZ58 variants, since XcpP was not shown to influence the level of XcpY in the presence of either XcpZ12 (permissive insertion) (data not shown) or XcpZ65 and XcpZ128 (nonpermissive insertions) (Fig. 6, lanes 9 and 11).
FIG. 6.
Influence of XcpP on the mutual stabilization of XcpY and XcpZ. E. coli TG1 cells expressing independently or simultaneously genes xcpZ or xcpZ derivatives, xcpY, and xcpP (subcloned in pYZ4) were grown to the end of the exponential growth phase, and proteins were analyzed by immunoblotting using antisera directed to XcpY. wt, wild-type XcpZ; 45, 58, 65, and 128, XcpZ variants. Two independent experiments were performed, and the results were found to be reproducible.
Thus, it seems clear that XcpP is the third partner required by the variants XcpZ45 and XcpZ58 to promote the stabilization of XcpY.
Conclusions.
On the one hand, the results obtained in this study are in agreement with those reported on by Py et al. (20) for E. chrysanthemi, who showed, using a two-hybrid system expressed in yeast, an interaction between OutL and OutM (homologous to XcpY and XcpZ, respectively) via their periplasmic domains. On the other hand, consistent with the results of Sandkvist et al. (25) showing that EpsM and EpsL (homologous to XcpZ and XcpY, respectively) interact with each other in the cytoplasmic membrane of V. cholerae, we found a stabilizing domain of XcpZ in P. aeruginosa that was located between the end of the transmembrane domain (residue 45) and the beginning of the periplasmic domain (residue 58) (Fig. 3).
Therefore, our findings provide new insights into the structural organization of XcpZ by showing two distinct periplasmic domains of XcpZ (domains I and II) that are required for XcpY stabilization. In addition, our approach allowed us to clearly identify the positions of these domains which are crucial for interactions with XcpY. Very interestingly, we also identified a third region of XcpZ (domain III) which required the presence of XcpP to promote XcpY stabilization. The identification of this domain, located between the end of the transmembrane domain and the beginning of the periplasmic domain of XcpZ, led us to suggest the existence of an XcpPYZ subcomplex within the secreton. Such a multiprotein complex is also supported by the results of Possot et al. (18), who showed that the XcpP homologue of the Pul system (PulC) coimmunoprecipitates with PulE, -L, and -M (homologous to XcpR, -Y, and -Z, respectively).
Moreover, our results strongly suggest that XcpP could modulate, directly or indirectly via XcpY, conformational changes in XcpZ to promote interaction between these components. The modulating action of XcpP sheds light on dynamic aspects of protein interaction within the type II secretory system of gram-negative bacteria.
Acknowledgments
We are grateful to Sophie Bleves for providing us with plasmid pSB14 and XcpY antiserum, to Manon Gérard-Vincent for the gift of XcpP antiserum, and to Alain Filloux and Claude Lazdunski for reading the manuscript and for helpful discussions. Geneviève Ball is acknowledged for her excellent technical assistance.
Work in the laboratory of Finbarr Hayes was supported by Biotechnology and Biological Sciences Research Council grants G11604 and G13032. Work in the Laboratoire d'Ingénierie des Systèmes Macromoléculaires was supported by the French cystic fibrosis foundation, AFLM (Association pour la Lutte contre La Mucoviscidose). Viviane Robert is the recipient of a fellowship from the Ministère de l'Education Nationale, de la Recherche et de la Technologie.
REFERENCES
- 1.Bally, M., A. Filloux, M. Akrim, G. Ball, A. Lazdunski, and J. Tommassen. 1992. Protein secretion in Pseudomonas aeruginosa: characterization of seven xcp genes and processing of secretory apparatus components by prepilin peptidase. Mol. Microbiol. 6:1121-1131. [DOI] [PubMed] [Google Scholar]
- 2.Bitter, W., M. Koster, M. Latijnhouwers, H. de Cock, and J. Tommassen. 1998. Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol. Microbiol. 27:209-219. [DOI] [PubMed] [Google Scholar]
- 3.Bleves, S., A. Lazdunski, and A. Filloux. 1996. Membrane topology of three Xcp proteins involved in exoprotein transport by Pseudomonas aeruginosa. J. Bacteriol. 178:4297-4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleves, S., R. Voulhoux, G. Michel, A. Lazdunski, J. Tommassen, and A. Filloux. 1998. The secretion apparatus of Pseudomonas aeruginosa: identification of a fifth pilin, XcpX (GspK family). Mol. Microbiol. 27:31-40. [DOI] [PubMed] [Google Scholar]
- 5.d'Enfert, C., A. Ryter, and A. P. Pugsley. 1987. Cloning and expression in Escherichia coli of the Klebsiella pneumoniae genes for production, surface localization and secretion of the lipoprotein pullulanase. EMBO J. 6:3531-3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Groot, A., A. Filloux, and J. Tommassen. 1991. Conservation of xcp genes, involved in the two-step protein secretion process, in different Pseudomonas species and other gram-negative bacteria. Mol. Gen. Genet. 229:278-284. [DOI] [PubMed] [Google Scholar]
- 7.de Groot, A., J. J. Krijger, A. Filloux, and J. Tommassen. 1996. Characterization of type II protein secretion (xcp) genes in the plant growth-stimulating Pseudomonas putida, strain WCS358. Mol. Gen. Genet. 250:491-504. [DOI] [PubMed] [Google Scholar]
- 8.Filloux, A., M. Bally, G. Ball, J. Tommassen, and A. Lazdunski. 1990. Protein secretion in gram-negative bacteria: transport across the outer membrane involves common mechanisms in different bacteria. EMBO J. 9:4323-4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filloux, A., G. Michel, and M. Bally. 1998. GSP-dependent protein secretion in gram-negative bacteria: the Xcp system of Pseudomonas aeruginosa. FEMS Microbiol. Rev. 22:177-198. [DOI] [PubMed] [Google Scholar]
- 10.Gerritse, G., R. Ure, F. Bizoullier, and W. J. Quaax. 1998. The phenotype enhancement method identifies the Xcp outer membrane secretion machinery from Pseudomonas alcaligenes as a bottleneck for lipase production. J. Biotechnol. 64:23-38. [DOI] [PubMed] [Google Scholar]
- 11.Hayes, F., C. Cayanan, D. Barillà, and A. N. A. Monteiro. 2000. Functional assay for BRCA1: mutagenesis of the COOH-terminal region reveals critical residues for transcription activation. Cancer Res. 60:2411-2418. [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes, F., and B. Hallet. 2000. Pentapeptide scanning mutagenesis: encouraging old proteins to execute unusual tricks. Trends Microbiol. 8:571-577. [DOI] [PubMed] [Google Scholar]
- 13.He, S. Y., M. Lindeberg, A. Chatterjee, and A. Collmer. 1991. Cloned Erwinia chrysanthemi out genes enable Escherichia coli to selectively secrete a diverse family of heterologous proteins to its milieu. Proc. Natl. Acad. Sci. USA 88:1079-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higuchi, R. 1990. Recombinant PCR, p. 177-183. In M. A. Innis, D. H. Gelfand, J. J. Sinsky, and T. J. White (ed.), PCR protocols. Academic Press, Inc., New York, N.Y.
- 15.Howard, S. P., J. Critch, and A. Bedi. 1993. Isolation and analysis of eight exe genes and their involvement in extracellular protein secretion and outer membrane assembly in Aeromonas hydrophila. J. Bacteriol. 175:6695-6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michel, G., S. Bleves, G. Ball, A. Lazdunski, and A. Filloux. 1998. Mutual stabilization of the XcpZ and XcpY components of the secretory apparatus in Pseudomonas aeruginosa. Microbiology 144:3379-3386. [DOI] [PubMed] [Google Scholar]
- 17.Nunn, D. N., and S. Lory. 1991. Product of the Pseudomonas aeruginosa gene pilD is a prepilin leader peptidase. Proc. Natl. Acad. Sci. USA 88:3281-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Possot, O. M., G. Vignon, N. Bomchil, F. Ebel, and A. P. Pugsley. 2000. Multiple interactions between pullulanase secreton components involved in stabilization and cytoplasmic membrane association of PulE. J. Bacteriol. 182:2142-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pugsley, A. P. 1993. The complete general secretion pathway in gram-negative bacteria. Microbiol. Rev. 57:50-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Py, B., L. Loiseau, and F. Barras. 2001. An inner membrane platform in the type II secretion machinery of Gram-negative bacteria. EMBO Rep. 21:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeves, P. J., D. Whitcombe, S. Wharam, M. Gibson, G. Allison, N. Bunce, R. Barallon, P. Douglas, V. Mulholland, S. Stevens, D. Walker, and G. P. C. Salmond. 1993. Molecular cloning and characterization of 13 out genes from Erwinia carotovora subspecies carotovora: genes encoding members of a general secretion pathway (GSP) widespread in gram-negative bacteria. Mol. Microbiol. 8:443-456. [DOI] [PubMed] [Google Scholar]
- 22.Salmond, G., and P. J. Reeves. 1993. Membrane traffic wardens and protein secretion in Gram-negative bacteria. Trends Biochem. Sci. 18:7-12. [DOI] [PubMed] [Google Scholar]
- 23.Sandkvist, M., M. Bagdasarian, S. P. Howard, and V. J. DiRita. 1995. Interaction between the autokinase EpsE and EpsL in the cytoplasmic membrane is required for extracellular secretion in Vibrio cholerae. EMBO J. 14:1664-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandkvist, M., L. P. Hough, M. M. Bagdasarian, and M. Bagdasarian. 1999. Direct interaction of the EpsL and EpsM proteins of the general secretion apparatus in Vibrio cholerae. J. Bacteriol. 181:3129-3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandkvist, M., J. M. Keith, M. Bagdasarian, and S. P. Howard. 2000. Two regions of EpsL involved in species-specific protein-protein interactions with EpsE and EpsM of the general secretion pathway in Vibrio cholerae. J. Bacteriol. 182:742-748. [DOI] [PMC free article] [PubMed] [Google Scholar]