Abstract
Analysis of N-acyl-l-homoserine lactones (AHLs) produced by Rhizobium leguminosarum bv. viciae indicated that there may be a network of quorum-sensing regulatory systems producing multiple AHLs in this species. Using a strain lacking a symbiosis plasmid, which carries some of the quorum-sensing genes, we isolated mutations in two genes (raiI and raiR) that are required for production of AHLs. The raiIR genes are located adjacent to dad genes (involved in d-alanine catabolism) on a large indigenous plasmid. RaiR is predicted to be a typical LuxR-type quorum-sensing regulator and is required for raiI expression. The raiR gene was expressed at a low level, possibly from a constitutive promoter, and its expression was increased under the influence of the upstream raiI promoter. Using gene fusions and analysis of AHLs produced, we showed that expression of raiI is strongly reduced in strains carrying mutations in cinI or cinR, genes which determine a higher-level quorum-sensing system that is required for normal expression of raiIR. The product of CinI, N-(3-hydroxy-7-cis tetradecenoyl) homoserine lactone, can induce raiR-dependent raiI expression, although higher levels of expression are induced by other AHLs. Expression of raiI in a strain of Agrobacterium that makes no AHLs resulted in the identification of N-(3-hydroxyoctanoyl)-l-homoserine lactone (3OH,C8-HSL) as the major product of RaiI, although other AHLs that comigrate with N-hexanoyl-, N-heptanoyl-, and N-octanoyl-homoserine lactones were also made at low levels. The raiI gene was strongly induced by 3OH,C8-HSL (the product of RaiI) but could also be induced by other AHLs, suggesting that the raiI promoter can be activated by other quorum-sensing systems within a network of regulation which also involves AHLs determined by genes on the symbiotic plasmid. Thus, the raiIR and cinIR genes are part of a complex regulatory network that influences AHL biosynthesis in R. leguminosarum.
Many plant-associated bacteria regulate gene expression in a cell density-dependent manner by using quorum sensing via N-acyl homoserine lactones (AHLs) (7). These AHLs pass out of, and into, bacterial cells. As the population of bacteria increases, so does the concentration of AHLs (38). Once the AHLs reach a threshold concentration, they act as coinducers to induce gene expression. Three types of AHL synthases, corresponding to LuxI, LuxM and HtdS types, have been identified (see reference 32). Often, the enzyme that is involved in AHL production can be induced by the AHL it produces, thereby creating a positive feedback loop that induces yet higher levels of AHLs. Genes that are regulated via such quorum-sensing-based systems in plant-associated bacteria include genes involved in plasmid transfer, antibiotic production, secretion of enzymes involved in pathogenesis, and production of various secondary metabolites, such as pyocyanin and pyoverdin (38).
Species of legume-nodulating rhizobia are included in this diverse group of bacteria that use AHL-based quorum-sensing systems. In a survey of AHLs produced by diverse soil bacteria, it was noted that some Rhizobium spp. produced the greatest diversity of quorum-sensing signaling molecules that were detected following thin-layer chromatography (TLC) (7). In Rhizobium leguminosarum bv. viciae many different AHLs are produced, and it was predicted that this diverse range of AHLs could be due to at least four separate loci involved in AHL production (17). In the Sinorhizobium meliloti database (http://sequence.toulouse.inra.fr/meliloti.html), one AHL synthase gene (encoding a LuxI-like protein) and multiple luxR-like genes, two of which regulate motility genes, are predicted (30). In the Mesorhizobium loti database (http://www.kazusa.or.jp/rhizobase/index.html), there are three predicted LuxI-like proteins and multiple LuxR-like regulators, but their roles are not known. In Rhizobium etli at least two separate AHL production genes have been predicted to be present, one of which, raiI, has been shown to determine the production of several undefined AHLs (25). In R. leguminosarum bv. viciae, two AHL production loci are thought to be located on the symbiotic plasmid pRL1JI. One of these (traI) produces multiple undefined AHLs and is involved with plasmid transfer (17, 37), while the other (rhiI) is involved in the production of AHLs that induce the rhizosphere-expressed genes rhiABC, in association with the regulator RhiR (24). The rhiABC genes play an undefined role in nodulation; in some genetic backgrounds, mutation of the rhi genes can decrease nodulation (9). Expression of rhiI is rhiR dependent and is positively autoregulated by the AHLs N-hexanoyl-l-homoserine lactone (C6-HSL), N-heptanoyl-l-homoserine lactone (C7-HSL), and N-octanoyl-l-homoserine lactone (C8-HSL), all of which are produced by RhiI (24).
Besides rhiI and traI, there is evidence for two other AHL synthases, neither of which is on the symbiosis plasmid (17). One of these is encoded by cinI, which is on the chromosome, and is regulated by the product of the adjacent gene, cinR (17). Mutations in cinI or cinR reduce, but do not block, rhiI expression, and there is a net decrease in levels of RhiI-made AHLs (17). Therefore, the cinRI locus imposes a higher level of control of rhiI and rhiABC expression. CinI produces N-(3-hydroxy-7-cis-tetradecenoyl)-l-homoserine lactone (3OH,C14:1-HSL), which does not directly induce rhiI; the effect of this AHL on rhiI gene expression appears to be indirect.
In addition to decreasing the levels of RhiI-made AHLs, mutation of cinR or cinI decreased the levels of various other AHLs made by R. leguminosarum bv. viciae. In a strain lacking a symbiotic plasmid (and hence rhiI and traI), multiple AHLs are made in addition to 3OH,C14:1-HSL, the only detected product of CinI (17, 24). The fact that mutation of cinI in a strain lacking a symbiotic plasmid abolished the production of 3OH,C14:1-HSL but only reduced the production of the other AHLs (17) implies that there is another locus involved in the production of these AHLs.
AHLs in R. leguminosarum strains have been proposed to be involved in stationary-phase adaptation and maintenance of viability in stationary-phase cultures. Thus, the product of CinI (3OH,C14:1-HSL) inhibited the growth of some strains of R. leguminosarum bv. viciae and in fact was previously known as “small bacteriocin” because of its growth-inhibiting properties (13). Gray et al. (12) showed that this was due to growth arrest and proposed that the growth inhibition may be due to a conversion of exponential-phase cells to nongrowing stationary cells. Subsequently, added 3OH,C14:1-HSL was shown to confer long-term viability on cultures of R. leguminosarum that had not adapted to stationary phase (33).
In this work we have characterized a nonsymbiotic-plasmid-borne locus (raiIR) involved in production of AHLs, and we show that it is part of a quorum-sensing network.
MATERIALS AND METHODS
Microbiological techniques.
Rhizobium and Agrobacterium strains were grown at 28°C in TY medium (4), and Escherichia coli was grown at 37°C in L medium (26). Antibiotics were added as appropriate to maintain selection for plasmids. Bacterial growth was monitored at 600 nm using an MSE Spectroplus spectrophotometer. β-Galactosidase activities were measured (21) using a Titertek Multiscan Plus spectrophotometer. When added, AHLs were added at the start of growth to a final concentration of 20 nM or 1 μM. The AHLs C6-HSL, C7-HSL, C8-HSL, N-(3-hydroxyoctanoyl)-l-homoserine lactone (3OH,C8-HSL), 3OH,C14:1-HSL, N-(3-oxooctanoyl)-l-homoserine lactone (3O,C8-HSL), and N-(3-oxohexanoyl)-l-homoserine lactone (3O,C6-HSL) were synthesized essentially as described previously (8).
Nodulation tests were done using peas (Pisum sativum L.) of the Wisconsin Perfection variety as described previously (5), using a minimum of 16 matched plants per test; at least two separate tests were carried out with similar results.
Bacterial strains and plasmids.
The strains and plasmids used in this study are listed in Table 1. R. leguminosarum strain 8401 lacks a symbiotic plasmid, and all Rhizobium strains used are derived from 8401. A34 is a derivative of 8401 carrying the symbiotic plasmid pRL1JI. Plasmids were mobilized into Rhizobium and Agrobacterium spp. by triparental matings using a helper plasmid. For genetic complementation studies, a cosmid library of R. leguminosarum A34 DNA cloned in pLAFR1 (15) was transferred into mutants by filter mating and selection of tetracycline-resistant colonies.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| R. leguminosarum | ||
| 8401 | Strain lacking a symbiotic plasmid; Strr | 15 |
| A34 | Like 8401 but containing pRL1JI (pSym) | 10 |
| A643 | 8401 carrying cinI3::Spc | This work |
| A644 | A34 carrying cinI3::Spc | This work |
| A700 | Mutant of 8401; probably raiI::IS50 | This work |
| A789 | 8401 carrying raiI7::Tn5 | This work |
| A793 | A34 carrying raiI7::Tn5 | This work |
| A797 | 8401 carrying raiI7::Tn5 and cinI3::Spc | This work |
| A798 | A34 carrying raiI7::Tn5 and cinI3::Spc | This work |
| A802 | 8401 carrying raiR8::Tn5 | This work |
| A803 | A34 carrying raiR8::Tn5 | This work |
| Agrobacterium sp. | ||
| C58.00 | Lacks AT and Ti plasmids; AHL negative | 35 |
| NT1/pZLR4 | traG-lacZ-based AHL detection strain | 7 |
| C. violaceum CV026 | AHL detection strain | 20 |
| Plasmids | ||
| pIJ9001 | Cosmid carrying raiIR region | This work |
| pIJ9161 | pIJ9001 carrying raiR5::Tn5 | This work |
| pIJ9163 | pIJ9001 carrying raiR3::Tn5 | This work |
| pIJ9164 | pIJ9001 carrying raiR4::Tn5 | This work |
| pIJ9165 | pIJ9001 carrying raiR6::Tn5 | This work |
| pIJ9220 | raiIR on 8.5-kb EcoRI fragment from pIJ9001 in Bluescript(KS) | This work |
| pIJ9222 | 8.5-kb fragment from pIJ9222 in pBBR1MCS-5 | This work |
| pIJ9225 | raiIR on 2.3-kb BamHI fragment in Bluescript(KS) | This work |
| pIJ9228 | raiIR on 2.3-kb BamHI fragment in pBBR1MCS-5 | This work |
| pIJ9231 | pIJ9228 carrying raiI7::Tn5 | This work |
| pIJ9234 | pIJ9228 carrying raiR8::Tn5 | This work |
| pIJ9271 | raiIR-lacZ in pMP220 | This work |
| pIJ9272 | raiR-lacZ in pMP220 | This work |
| pIJ9276 | raiR cloned in pBBR1MCS-5 | This work |
| pIJ9280 | raiI-lacZ in pMP220 | This work |
| pBBR1MCS-5 | Broad-host-range cloning vector | 14 |
| pMP220 | Broad-host-range lacZ expression vector | 31 |
Strain 8401 was mutagenized with Tn5-gus by using E. coli strain MM294/pRK600::Tn5-gusA1 as a donor of the suicide plasmid pRK600::Tn5-gusA1 essentially as described previously (27). A population of about 8,000 colonies was screened for impaired AHL production by picking colonies onto a lawn of the AHL biosensor strain Chromobacterium violaceum CV026 (20) to identify mutants that did not induce the purple pigment violacein. Strain A700 was one such mutant, and small-bacteriocin tests revealed that A700 retained the ability to produce small bacteriocin (3-OH,C14:1-HSL). Unfortunately, transduction tests revealed that the Tn5 in A700 was not linked to the defect in AHL production, so the mutant phenotype was complemented by using a cosmid library, leading to the identification of pIJ9001 and subsequently pIJ9228, containing the subcloned raiIR region that complemented the mutation.
Mutagenesis of plasmids pIJ9001 and pIJ9228 was carried out using phage λ carrying Tn5 (29). The Tn5-containing derivatives of pIJ9001 were mated into a rifampin-resistant derivative of 8401, and the transconjugants were screened for low levels of AHL production. Four mutant cosmids, pIJ9161, pIJ9163, pIJ9164, and pIJ9165, were isolated; from each of these, part of Tn5 and the flanking DNA were subcloned with BamHI into pBluescript, and the precise sites of Tn5 insertions were identified by DNA sequencing using a Tn5-specific primer. The mutated derivatives of pIJ9228 were mated into a rifampin-resistant derivative of Agrobacterium strain C58.00, and the transconjugants were screened for inability to activate pigment production by C. violaceum CV026. Two of the resulting plasmids, pIJ9231 and pIJ9234, carrying Tn5 in raiI and raiR, respectively, were selected, and the sites of Tn5 insertion were identified by DNA sequencing.
Tn5-containing fragments from pIJ9231 and pIJ9234 were subcloned with ApaI-XbaI into the sacB suicide vector pJQ200KS (23). The sacB gene confers lethal susceptibility to sucrose, allowing for selection of recombinants. The raiI7::Tn5 and raiR8::Tn5 mutations were recombined from the plasmids into strain 8401 to form A789 and A802, obtained by selecting for kanamycin-resistant, sucrose-resistant colonies (23). The mutations in A789 (raiI7::Tn5) and A802 (raiR8::Tn5) were then transduced into A34 by using phage RL38 (6) to produce A793 and A803, respectively. To generate a derivative of 8401 defective in both cinI and raiI, the raiI7::Tn5 mutation was transduced into the cinI mutant A643 to generate A797. To create a double mutant in a strain carrying pRL1JI, the raiI7::Tn5 mutation was transduced into A664 to generate A798, which is a derivative of A34 carrying mutations in both cinI and raiI.
The raiIR gene region was subcloned from pIJ9001 into pBBR1MCS-5 (14) on an 8.5-kb EcoRI fragment to make pIJ9222. A 2.3-kb BamHI fragment carrying raiIR was subcloned in pBBR1MCS-5 to form pIJ9228. Plasmid pIJ9276, containing only raiR, was obtained by deleting a SacI fragment from pIJ9228 by using a SacI site at the end of raiI and a SacI site in the vector.
To construct pIJ9280 (raiI-lacZ), a 1.35-kb EcoRI-MfeI fragment from pIJ9228 was cloned into pMP220 (31) cut with EcoRI. To make pIJ9271 and pIJ9272 (raiIR-lacZ and raiR-lacZ), a 1.8-kb EcoRI-ClaI fragment was first subcloned from pIJ9228 into pBluescript and then subcloned as a 1.8-kb EcoRI-KpnI fragment (still carrying raiI) or a 0.5-kb MfeI-KpnI fragment (not carrying raiI) into pMP220 cut with EcoRI and KpnI.
Molecular biology techniques.
DNA cloning, ligation, transformation, restriction enzyme mapping, and DNA hybridization were performed by standard methods (26). Sequencing reactions were carried out by using the Amersham “Big Dye” kits and an Applied Biosystems automated sequencer (ABI 377). DNA sequencing of the raiIR genes was carried out on both strands by using primer walking on pIJ9225, which is a derivative of pBluescript carrying the 2.3-kb BamHI fragment. Other DNA sequences were determined by using pIJ9220 (which carries the 8.5-kb EcoRI fragment in pBluescript) or various derivatives of pIJ9220 carrying subcloned fragments. Database searches of the predicted protein sequences were carried out by using the BLAST and FASTA (2) programs to find related sequences in the EMBL and SwissProt protein sequence databases.
Assay of AHLs.
Rhizobium and Agrobacterium cultures were grown for 62 and 48 h, respectively, in TY medium to optical density at 600 nm (OD600) readings of approximately 0.9. Cells were removed by centrifugation, and AHLs were extracted from culture supernatants, as described previously (39). AHLs were analyzed by TLC using Agrobacterium tumefaciens NT1/pZLR4 (7, 28) or C. violaceum CV026 as the AHL indicator organism (20). Extracts of culture supernatants were spotted onto aluminum-backed RP18 reverse-phase TLC plates (Merck) and dried in a stream of air. Samples were separated by using 60% (vol/vol) methanol in water as the mobile phase. Once the solvent front had migrated to within 2 cm of the top of the chromatogram, the plate was removed from the chromatography tank and dried in air. Because Rhizobium cultures produce several closely related AHLs, the separation process was repeated so as to achieve adequate separation of AHLs. After being dried for the second time, the chromatogram was overlaid with a thin film of either soft L agar (0.7% [wt/vol]) seeded with C. violaceum CV026 or soft AB agar (28) containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) and seeded with A. tumefaciens NT1/pZLR4.
For electrospray mass spectrometry (ES-MS) of AHLs, Agrobacterium strain A58.00 carrying pIJ9228 (raiIR) was grown in 2 liters of AB medium (28) and the cells were pelleted by centrifugation. The spent culture supernatant was pumped through a C18 reverse-phase column (Sep Pak; Waters Corporation). The column was washed with 20 ml of H2O, and the AHLs were eluted with 3 ml of acetonitrile, which was then evaporated off under a stream of N2 gas. The sample was redissolved in 100 μl of acetonitrile and separated by TLC as described above. Part of the chromatograph was developed, and bands corresponding to the detected components were scraped off the remaining part of the TLC. The silica was extracted twice with 3 ml of acetone, which was then evaporated off under N2. Samples were redissolved in 100 μl of a 1:1 mixture of acetonitrile and water to which was added formic acid to a final concentration of 0.5% (vol/vol). The sample (20 μl) was introduced into the electrospray source (Platform VG Instruments) at a flow rate of 10 μl min−1. Spectra were recorded in the positive mode at a speed of 10 s for m/z 100 to 400 with a core voltage of 40 V. Spectra were processed by using Masslynx, version 2.0, software and compared with chemically synthesized standards for C6-HSL, 3O,C8-HSL, and 3OH,C8-HSL.
Plasmid hybridization.
Rhizobium cells were cultured, prepared for in-gel lysis, and loaded in a horizontal back-trap agarose gel (0.8%) to separate high-molecular-weight plasmids (36). After 16 h at 100 V, the gel was transferred to a Hybond N membrane (Amersham). To probe the raiIR region, a 1.35-kb gel-purified EcoRI-MfeI fragment carrying part of raiI was labeled with [α-32P]dCTP by using the Rediprime II kit (Amersham). The membrane was hybridized overnight at 60°C and then washed twice for 15 min each time with 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)-0.5% sodium dodecyl sulfate (SDS) and twice for 10 min each time with 2× SSC-0.2% SDS.
Nucleotide sequence accession number. The sequence of the raiIR gene region has been deposited in the EMBL database under accession no. AJ427969.
RESULTS AND DISCUSSION
Identification of the raiIR genes involved in AHL production.
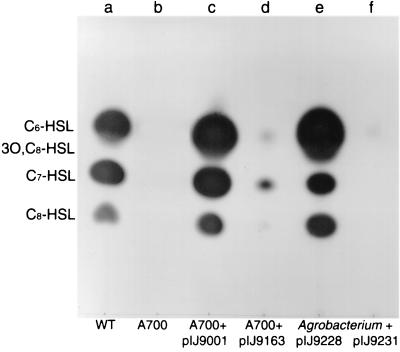
R. leguminosarum strain 8401, which lacks a symbiosis plasmid, produces several AHLs (Fig. 1 and 2). The C. violaceum CV026 detection system reveals three strongly reacting spots (Fig. 1, lane a), whereas the Agrobacterium traG-lacZ detection system reveals at least five components (Fig. 2, lane d). C. violaceum CV026 does not detect 3OH,C14:1-HSL (the product of CinI) (Fig. 1), and this enabled us to screen for mutants defective in the production of AHLs determined by genes other than cinI. One mutant, A700, lacked CV026-detectable AHLs (Fig. 1, lane b) but produced normal levels of 3OH,C14:1-HSL based on a bacteriocin type bioassay (data not shown), and therefore the mutation did not affect the expression of cinI or cinR.
FIG. 1.
Identification of AHLs produced by raiI. Rhizobium and Agrobacterium cultures were grown in 100 ml of TY broth for 62 and 48 h, respectively. AHLs extracted from cell-free culture supernatants were separated by TLC and detected by using an overlay of agar seeded with C. violaceum CV026. Each lane was loaded with a volume of extract equivalent to 25 ml of culture. Lanes a and b, extracts from R. leguminosarum strain 8401 and mutant A700, respectively; lanes c and d, extracts from mutant A700 complemented with cosmids pIJ9001 and pIJ9163 (raiR3::Tn5), respectively; lanes e and f, extracts from A. tumefaciens C58.00 containing pIJ9228 and pIJ9231 (raiI7::Tn5), respectively. The migration positions of standards (not shown) are indicated to the left of the chromatogram.
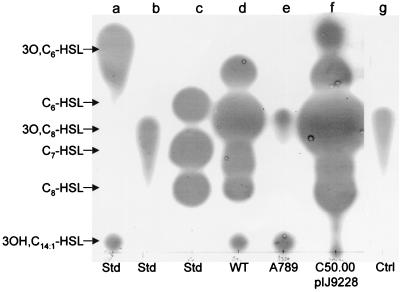
FIG. 2.
Identification of AHLs produced by RaiI. Cultures were grown in 100 ml of TY broth. AHLs extracted from cell-free culture supernatants were separated by TLC and detected by using an overlay of agar seeded with A. tumefaciens carrying traG-lacZ (NT1/pZLR4) as a detection system. Lane a, standards 3O,C6-HSL and 3OH,C14:1-HSL; lane b, standard 3O,C8-HSL; lane c, standards C6-HSL, C7-HSL, and C8-HSL; lanes d and e, extracts (equivalent to 5 ml of culture) from R. leguminosarum 8401 and A789 (raiI7::Tn5), respectively; lane f, extract (equivalent to 2.5 ml of culture) from A. tumefaciens C58.00 containing pIJ9228 (raiIR); lane g, extract (equivalent to 5 ml of culture) of sterile growth medium in which bacteria had not been grown, used as a control.
Plasmids that restored AHL production were isolated by conjugating cosmids into A700 and screening for transconjugants that restored activation of pigment production by C. violaceum CV026. Two classes of complementing clones were isolated, one corresponding to the previously identified rhiI gene region from pRL1JI and one novel plasmid that was called pIJ9001. As shown in Fig. 1, lane c, pIJ9001 restored A700 to a wild-type pattern of AHL production, based on activation of purple pigment in CV026. DNA hybridization experiments confirmed that pIJ9001 did not contain DNA from the symbiosis plasmid pRL1JI (data not shown).
Plasmid pIJ9001 was mutagenized with Tn5, and several mutant derivatives (e.g., pIJ9163) in which AHL production was affected were identified (Fig. 1, lane d). The Tn5 insertions were located by mapping and DNA sequencing at different positions in an 8.5-kb EcoRI fragment and a 2.3-kb BamHI fragment (Fig. 3). The subcloned EcoRI and BamHI fragments (pIJ9222 and pIJ9228, respectively) complemented A700 for AHL production. Plasmid pIJ9228 also enabled a non-AHL-producing strain (C58.00) of Agrobacterium to make AHLs detected by CV026 (Fig. 1, lane e) or by A. tumefaciens carrying traG-lacZ as a detection system (Fig. 2, lane f). The pattern of AHLs produced by C58.00/pIJ9228 was similar to that produced by 8401 (Fig. 1 and 2) except that no 3OH,C14:1-HSL was detected and one additional fast-migrating spot was detected (Fig. 2, lane f). Occasionally, we have detected such a fast-migrating component in the growth medium supernatant of strain 8401 (data not shown).
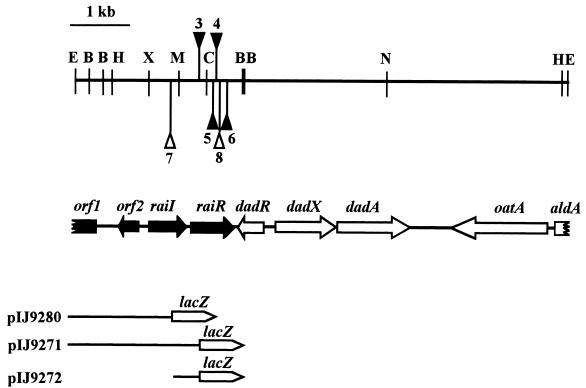
FIG. 3.
Map of the rai gene region. Open reading frames corresponding to raiI, raiR, dadR, dadX, and dadA are shown as thick arrows. Filled triangles, locations of the four Tn5 insertions obtained after mutagenesis of cosmid pIJ9001 (pIJ9161 raiR5::Tn5, pIJ9163 raiR3::Tn5, pIJ9164 raiR4::Tn5, and pIJ9165 raiR6::Tn5). Open triangles, locations of the raiI7::Tn5 insertion (in plasmids pIJ9231 and pIJ9237 and in mutants A789, A793, A797, and A798) and the raiR8::Tn5 insertion (in plasmids pIJ9234 and pIJ9238 and in mutants A802 and A803). The DNA fragments used to create the raiI-lacZ plasmid (pIJ9280), the raiR-lacZ plasmid carrying raiI (pIJ9271), and the raiR-lacZ plasmid that does not carry raiI (pIJ9272) are shown. Some restriction enzyme sites are shown, abbreviated as follows: B, BamHI; E, EcoRI; C, ClaI; H, HindIII; M, MfeI; N, NotI; X, XhoI.
The DNA sequence of the 2.3-kb BamHI fragment revealed two genes strongly homologous to raiI and raiR from R. etli (25). Sequencing of various end fragments revealed identities to parts of dadR, dadX, and dadA from R. leguminosarum strain 3841 (1) (accession number AJ249196). The deduced map of the region based on whole and partial sequence alignments is shown in Fig. 3. Thus, raiI and raiR are adjacent to a gene (dadR) encoding a regulator in the LRP family, which regulates adjacent genes that encode enzymes involved in alanine metabolism (1). Also shown in Fig. 3 are orf1, an incomplete open reading frame (136 amino acids) that shows 37% identity over a 60-amino-acid region with a glucoamylase (accession number P26989) from Schizosaccharomyces pombe; a partial open reading frame that shows 81% identity with a predicted (34) aldehyde dehydrogenase (AldA) from Agrobacterium radiobacter (accession number X95394); and an open reading frame which shows 52% identity to β-alanine pyruvate transaminase from Pseudomonas putida (A42800) and is very similar (72 and 68% identities, respectively) to predicted omega amino transferases (oatA) from S. meliloti (CAC47872) and Agrobacterium (AAK90062). Although the dadR-oatA region is highly conserved at the DNA sequence level (about 98% identity) between two isolates (strains 8401 and 3841) of R. leguminosarum, the sequence similarity ends abruptly downstream of dadR. No sequence corresponding to raiR was found in the region downstream of dadR sequenced previously from strain 3841 (1). The difference is not due to a cloning artifact, because DNA hybridization failed to detect raiR in strain 3841 (data not shown). This is in accord with the previous conclusion that strain 3841 does not produce the AHLs defined here as being made by RaiI (18). This suggests that the raiIR gene region has recently been lost by 3841 or has recently been acquired by 8401. The observation that the context of the raiIR genes in R. etli is similar to that in strain 8401 (25) may imply that a loss from strain 3841 is more likely. Such events may partially explain the diversity of AHLs found in different strains (7, 18).
DNA hybridization revealed that strain 8401 contains a single copy of the raiIR locus (data not shown). To determine if the raiIR gene region is located on the chromosome or on one of the two large plasmids (of about 400 and 600 kb) in strain 8401, the plasmids were separated electrophoretically and hybridized with a raiIR probe. The probe hybridized strongly to the larger of the two plasmids (data not shown). On this basis we conclude that the raiIR genes are plasmid located.
The raiR gene is predicted to encode a protein that belongs to the LuxR family of transcriptional regulators. R. leguminosarum RaiR is 88% identical to RaiR from R. etli but showed no such strong similarity with any other LuxR-like gene products in the S. meliloti or M. loti database. RaiR is 30 and 21% identical, respectively, to CinR and RhiR, two other LuxR-type regulators from R. leguminosarum bv. viciae (17, 24). Similar levels of identity (25 to 30%) were found with a range of other LuxR-type regulators such as CerR from Rhodobacter sphaeroides (32%; accession number AF16298); TraR from Rhizobium sp. strain NGR234 (27%; P55407); RhlR (26%; P54292) and VsmR (25%; U15644), both from Pseudomonas aeruginosa; and VanR from Vibrio anguillarum (25%; U69677).
The predicted raiI gene product belongs to the LuxI family of AHL synthases. RaiI is 93% identical to RaiI from R. etli but only 33 and 25% identical, respectively, to CinI and RhiI, two other AHL synthases from R. leguminosarum bv. viciae. CinI produces 3OH,C14:1-HSL (17), while RhiI produces C6-HSL, C8-HSL (24), and another component now known to be C7-HSL (17). Similar levels of identity (27 to 35%) were seen with the LuxI-like proteins in the S. meliloti and M. loti databases.
Phenotypes of defined raiI and raiR mutants.
Following recombination and transduction, we transferred the raiI7::Tn5 and raiR8::Tn5 alleles (Fig. 3) into strain 8401 (lacking a symbiosis plasmid) to generate A789 (raiI7::Tn5) and A802 (raiR8::Tn5) and into A34 (carrying the symbiosis plasmid pRL1JI) to generate A793 (raiI7::Tn5) and A803 (raiR8::Tn5). A789 and A802 produced no AHLs detectable by C. violaceum CV026 (Fig. 4, lanes b and c). By use of A. tumefaciens carrying traG-lacZ as a detection system (Fig. 2), it is evident that the mutation in A789 abolished the production of most of the AHLs (Fig. 2, lane e), and a similar result was seen with A802 (data not shown). As expected, 3OH,C14:1-HSL is made normally by A789 (Fig. 2, lane e) and A802 (data not shown), and this was confirmed by bacteriocin type bioassays. A small spot of an unknown component was also detected in the culture supernatant of A789 (Fig. 2, lane e), but since an extract of an uninoculated growth medium also revealed a similar component (Fig. 2, lane g), we conclude that this is not a Rhizobium-specific AHL.
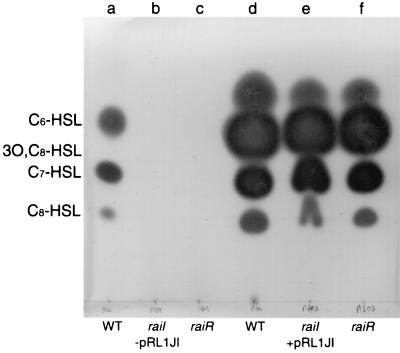
FIG. 4.
Mutation of raiI or raiR does not influence production of AHLs by pRL1JI. Cultures were grown in 100 ml of TY broth. AHLs extracted from cell-free culture supernatants were separated by TLC and detected by using an overlay of agar seeded with C. violaceum CV026. Each lane was loaded with a volume of extract equivalent to 25 ml of culture. Lanes a and d, extracts from wild-type R. leguminosarum 8401 and A34 (8401/pRL1JI), respectively; lanes b and e, extracts from raiI7::Tn5 mutants A789 (−pRL1JI) and A793 (+pRL1JI), respectively; lanes c and f, extracts from raiR8::Tn5 mutants A802 (−pRL1JI) and A803 (+pRL1JI), respectively. The migration positions of standards (not shown) are indicated to the left of the chromatogram.
To analyze the possible effects of raiI and raiR mutations on AHL production loci on pRL1JI, A793 (raiI) and A803 (raiR) were assayed for AHL production by using CV026, which detects various pRL1JI-determined AHLs (17, 24). No significant difference from A34 was seen (Fig. 4). Therefore, we conclude that raiI and raiR are not required for the production of pRL1JI-determined AHLs.
Strains A34 (wild type), A793 (raiI), and A803 (raiR) were inoculated onto peas for nodulation tests. The rate of nodulation and number of nodules formed were similar in each case. A793 and A803 consistently nodulated at a slightly higher level than the wild type (10 to 20% more), but this was not statistically significant at the 95% confidence level.
To assess if nitrogen fixation was normal in the nodules formed, the levels of acetylene reduction by the nodulated root system were analyzed. Again, there was no significant difference between A34 and any of the mutants. The nodules formed by the mutants were a healthy pink color typical of nitrogen-fixing nodules, and no signs of nitrogen stress were visually observed in the plant.
Identification of AHLs produced by RaiI.
Strain 8401 was previously shown to produce C6-HSL, C7-HSL, and C8-HSL (17, 24), and it is now clear that mutation of raiI abolishes the production of these AHLs (Fig. 1). Furthermore, A. tumefaciens C58.00 (which produces no AHLs [17, 35]) containing raiI cloned in pIJ9228 produced an AHL profile similar to that of 8401 (Fig. 1 and 2), and mutation of raiI (pIJ9231) blocked the production of these AHLs (data not shown). Therefore, we conclude that RaiI produces C6-HSL, C7-HSL, and C8-HSL. However, RaiI also made other components (Fig. 2), one of which comigrated with 3O,C8-HSL (and with 3OH,C8-HSL [data not shown]). This component was not identified in earlier work, because it was not detected by the C. violaceum CV026 detection system used in that work (17, 24).
The band corresponding to this component made in C58.00/pIJ9228 was scraped off a preparative TLC plate. ES-MS revealed two strong peaks of m/z 244.2 and 266.2, which correspond to the predicted [M + H]+ and [M + Na]+ quasi-molecular ions of 3OH,C8-HSL, and a peak of 101.8, corresponding to the homoserine lactone moiety. In addition, a smaller peak of m/z 226.2 was observed, and this could correspond to the [M + H]+ of a dehydration product of 3OH,C8-HSL; such dehydration products are typically observed with hydroxy (but not oxo) homoserine lactones (16, 22, 28). 3OH,C8-HSL was chemically synthesized, essentially as described previously (8), and when this was used as a standard, peaks of m/z 244.4, 266.4, 226.2, and 102 were detected. We conclude that this AHL produced by RaiI is 3OH,C8-HSL, based on the mass spectrum; this is consistent with the migration position and the strong activation of the Agrobacterium detection system but weak activation of the C. violaceum detection system. ES-MS of fractions corresponding to the two fastest-migrating components detected (Fig. 2, lane f) revealed no major peaks corresponding to predicted m/z peaks for known AHLs.
To determine the relative levels of the different AHLs produced by RaiI, we separated AHLs produced by strain 8401 by TLC using the same conditions as those for the experiment for which results are shown in Fig. 2. Following chromatography, we spotted onto the dried plate a dilution series of chemically synthesized standards of C6-HSL, C7-HSL, C8-HSL, and 3OH,C8-HSL. This was done in duplicate, and the plates were then developed with A. tumefaciens NT1/pZLR4 or C. violaceum CV026. By comparing the relative staining intensities of the diluted standards with those of the AHLs produced by 8401, we estimated that a stationary-phase culture of strain 8401 contains about 0.1 to 0.2 μM 3OH,C8-HSL (assuming no losses during extraction). The estimated concentrations of C6-HSL and C7-HSL were about 1,000-fold lower, while that of C8-HSL was about 10-fold lower.
R. leguminosarum is rather unusual in that it makes two hydroxy-substituted AHLs, 3OH,C14:1-HSL from CinI and 3OH,C8-HSL from RaiI. Other strains that make hydroxy-substituted AHLs include Pseudomonas fluorescens (16, 28), Vibrio harveyi (3), and V. anguillarum (22). P. fluorescens has an HtdS-type AHL synthase (16), and the latter two bacteria use LuxM-type rather than LuxI-type AHL synthases (3, 22), so currently, RaiI and CinI are two of the very few described LuxI-type AHL synthases that make hydroxy-substituted AHLs.
Assay of AHLs that induce raiI-lacZ.
In order to assay which added AHLs induce raiI expression, a raiI-lacZ fusion (pIJ9280) was made (Fig. 3) and was introduced into A. tumefaciens C58.00, which makes no AHLs. In the absence of raiR, only background levels of activity were seen and the addition of various AHLs had no effect (Table 2). The results of an assay of raiI-lacZ expression with raiR present are also shown in Table 2. In the absence of added AHLs, raiR (on pIJ9276) had no effect on expression compared with the background level seen when raiR was absent. Various AHLs were tested for raiR-dependent induction of raiI-lacZ expression in A. tumefaciens C58.00/pIJ9280. The strongest induction was seen with 3OH,C8-HSL, and lower levels were seen with 3O,C8-HSL and 3OH,C14:1-HSL. No induction was seen with C6-HSL, C7-HSL, C8-HSL, or 3O,C6-HSL (Table 2). These results demonstrate that raiI is regulated by RaiR and that 3OH,C8-HSL, a product of RaiI, is the strongest inducer identified (Table 2). However, it is evident that some AHLs made at a low level by RaiI (C6-HSL, C7-HSL, and C8-HSL) do not induce much raiI-lacZ expression (Table 2). Paradoxically, the data also suggest that AHLs made by other gene products such as CinI can induce raiR-dependent raiI expression (Table 2).
TABLE 2.
Effects of added AHLs on raiI-lacZ expressiona
| AHL |
raiI-lacZ (pIJ9280) expression (Miller units)
|
||||||
|---|---|---|---|---|---|---|---|
|
A. tumefaciens C58.00
|
A789 (raiI7::Tn5)
|
A797 (cinI3::Spc raiI7::Tn5)
|
|||||
| Without raiR | With pIJ9276 (raiR) | Without raiR | With pIJ9276 (raiR) | Without raiR | With pIJ9276 (raiR) | ||
| None | 140 ± 12 | 150 ± 14 | 462 ± 25 | 1,695 ± 31 | 326 ± 19 | 364 ± 15 | |
| C6-HSL | 135 ± 11 | 121 ± 13 | 336 ± 21 | 856 ± 11 | 295 ± 12 | 353 ± 17 | |
| C7-HSL | 139 ± 14 | 161 ± 16 | 345 ± 22 | 1,113 ± 14 | 289 ± 14 | 335 ± 16 | |
| C8-HSL | 142 ± 15 | 278 ± 19 | 331 ± 15 | 1,542 ± 17 | 291 ± 17 | 359 ± 17 | |
| 3O,C6-HSL | 154 ± 13 | 297 ± 15 | 332 ± 12 | 687 ± 15 | 317 ± 15 | 471 ± 15 | |
| 3O,C8-HSL | 147 ± 11 | 4,135 ± 27 | 913 ± 54 | 7,032 ± 84 | 617 ± 16 | 4,550 ± 34 | |
| 3OH,C8-HSL | 141 ± 10 | 12,135 ± 119 | 3,854 ± 49 | 16,520 ± 164 | 1,338 ± 23 | 14,070 ± 157 | |
| 3OH,C14:1-HSL | 151 ± 13 | 1,272 ± 115 | 493 ± 17 | 2,094 ± 57 | 305 ± 21 | 895 ± 42 | |
AHLs were used at a final concentration of 1 μM, and β-galactosidase activity was assayed after 24 h for A. tumefaciens and after 48 h for R. leguminosarum.
The assay of raiI-lacZ (pIJ9280) in the R. leguminosarum raiI mutant (A789) is complicated by the observation (see below) that the Tn5 in raiI may affect the expression of raiR, so the assays were done in the absence and in the presence (on pIJ9276) of raiR. Only a low level of activity was seen in A789 (raiI) carrying raiI-lacZ on pIJ9280, and 3OH,C8-HSL induced the most expression, although 3O,C8-HSL also gave some induction (Table 2). Cloned raiR on pIJ9276 resulted in significant raiI-lacZ expression in A789, even though no AHLs were added. This suggested that either CinI-made 3OH,C14:1-HSL may induce raiI or multicopy raiR induced AHL-independent raiI expression. To test this, pIJ9280 (raiI-lacZ) was transferred to a raiI cinI double mutant of R. leguminosarum (A797). Mutation of cinI in addition to raiI greatly reduced raiI-lacZ expression in the presence of cloned raiR (Table 2). The decrease in expression of raiI-lacZ in the raiI cinI mutant compared with the raiI mutant indicates that CinI-made AHLs are responsible for the expression of raiI-lacZ in the raiI mutant A789 (Table 2). This is consistent with the observation that cloned raiR induced no expression of raiI-lacZ in the absence of added AHLs in A. tumefaciens C58.00 (Table 2). The effects of AHLs on raiI-lacZ expression in A797 (raiI cinI mutant) carrying cloned raiR are broadly similar to the results seen with AHLs added to A. tumefaciens carrying raiI-lacZ (Table 2). In the absence of cloned raiR, in A797 (raiI cinI mutant), 3OH,C8-HSL induced raiI-lacZ expression most strongly (Table 2); the level of expression was lower than that induced by 3OH,C8-HSL in A789 (raiI mutant), implying a role for CinI-made 3OH,C14:1-HSL in the higher expression seen in A789 (raiI mutant) than in A797 (rai cinI mutant).
The background level of raiI-lacZ expression seen in the raiI mutant (A789) carrying cloned raiR was decreased by addition of C6-HSL, C7-HSL, or 3O,C6-HSL (Table 2). This implies that these AHLs may compete with 3OH,C14-HSL for the binding site on RaiR. At lower levels (20 nM), 3OH,C8-HSL and 3O,C8-HSL induced raiI-lacZ expression in A. tumefaciens C58.00/pIJ9280 (9,324 ± 210 and 1,350 ± 33 Miller units, respectively) but 3OH,C14:1-HSL did not (160 ± 14 Miller units), suggesting that lower threshold concentrations of 3OH,C8-HSL and 3O,C8-HSL may be required for activation of raiI induction by RaiR.
Complementation tests with raiI and raiR mutants.
Genetic complementation tests were carried out with plasmids containing the cloned raiIR genes and derivatives of these plasmids carrying Tn5 insertions in raiI or raiR. These plasmids were introduced into A789 (raiI) and A802 (raiR), and AHLs were measured. Similar results were obtained using the C. violaceum CV026 and A. tumefaciens NT1/pZLR4 systems. Introduction of the cloned raiIR genes on pIJ9228 into 8401 resulted in enhanced AHL production (Table 3); we estimate that the overall level was about 5- to 10-fold higher, and mutation of raiI or raiR on the introduced plasmid (pIJ9231 or pIJ9234) reduced AHL production to wild-type levels. In the presence of pIJ9228, mutation of the genomic copy of raiI (A789) or raiR (A802) had relatively little effect on AHL production.
TABLE 3.
Genetic complementation of raiI and raiR mutantsa
| Plasmid | AHL productionb detected by CV026 in
|
|||
|---|---|---|---|---|
| 8401 | A789 raiI7::Tn5 | A802 raiR8::Tn5 | A700 | |
| None | + | − | − | − |
| pIJ9228 (raiI raiR) | +++ | +++ | +++ | +++ |
| pIJ9231 (raiI7::Tn5 raiR) | + | − | + | − |
| pIJ9234 (raiI raiR8::Tn5) | + | +/− | − | +/− |
AHL production was tested on a plate assay using C. violaceum CV026 as the indicator strain.
−, no complementation; +/−, weak complementation (less than 10% of AHL levels produced by 8401); +, complementation; +++, strong complementation (the AHL production level was greater than that of the control 8401).
There was essentially no (or very low levels of) production of AHLs by cloned raiI in the absence of raiR, as seen with A802/pIJ9234 (Table 3). The observation (Table 3) that A789 (raiI7::Tn5) was very poorly complemented (AHL levels were less than 10% of that seen in the 8401 control) by pIJ9234 (carrying raiI and raiR::Tn5) indicates that the raiI mutation in A789 may have a polar effect on raiR. When the raiI7::Tn5 mutation was on the plasmid (pIJ9231) in the raiR mutant A802, the polarity was less evident. This may have been due to the multicopy plasmid allowing expression of raiR.
We analyzed the complementation characteristics of the original mutant A700. There was weak complementation with pIJ9234 (carrying raiI and a mutation in raiR) but no complementation with pIJ9231, carrying a mutation in raiI. Therefore, A700 probably carries a mutation in raiI. Genomic DNA was hybridized with a raiIR probe, and this revealed that there was an insert of about 1.7 kb in the raiI region (data not shown). Given that the insertion is around 1.7 kb, possibly an IS50 element may have transposed separately from the Tn5-gus transposon used for the mutagenesis. Similar events have been observed previously in R. leguminosarum (19).
raiI is positively autoregulated by RaiR.
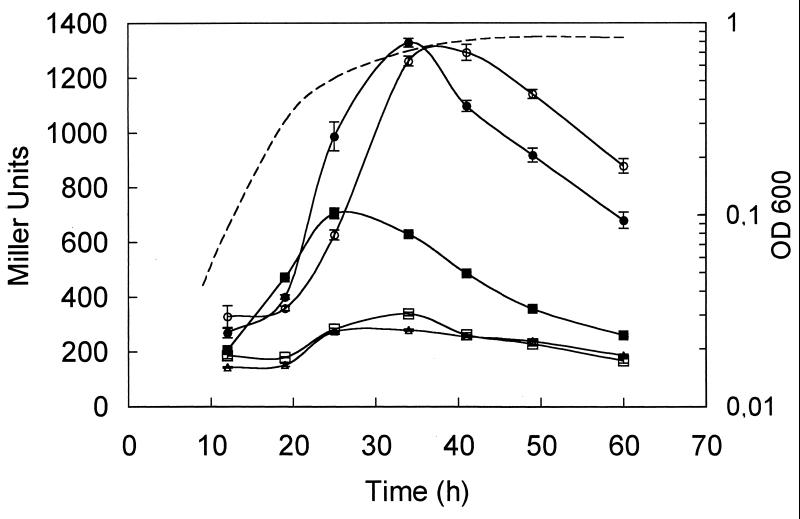
Analysis of β-galactosidase activity with 8401/pIJ9280 (raiI-lacZ) revealed that raiI is upregulated as the cells enter the late-exponential phase of growth. Mutation of raiI or raiR in strain 8401 blocked induction of raiI-lacZ (Fig. 5). When pRL1JI was present (A34), raiI-lacZ expression was similar to that seen with 8401, except that the induction was slightly earlier (Fig. 5). This effect was consistent in different tests, and we did not observe any differences between the growth of A31 and that of A34. In the presence of pRL1JI, mutation of raiI (A793) did not block raiI-lacZ expression, although the level of induction was lower than that seen in the control, A34 (Fig. 5). These observations are consistent with the hypothesis that raiI is positively autoregulated (by AHLs made by RaiI) but that pRL1JI may determine the production of AHLs which can also induce raiI-lacZ. As shown above (Table 2), the rhiI-made AHLs (C6-HSL, C7-HSL, and C8-HSL) are unlikely to be responsible for this activation. In other work (V. Danino, A. Wilkinson, and J. A. Downie, unpublished data) we have established that the other AHL synthase (traI) on pRL1JI produces AHLs that activate raiR-dependent raiI-lacZ expression.
FIG. 5.
Expression of raiI-lacZ. The expression of raiI-lacZ (pIJ9280) was analyzed by measuring β-galactosidase levels throughout the growth of strains 8401 (open circles), 8401/pRL1JI (solid circles), A789 (8401 raiI7:Tn5) (open squares), A793 (8401/pRL1JI raiI7:Tn5) (solid squares), and A802 (8401 raiR8:Tn5) (open triangles). The growth curve (OD600) shown as a broken line corresponds to that obtained with 8401/pIJ9280; the growth of the other strains was very similar.
Two other lacZ fusion plasmids (Fig. 3), pIJ9271 (carrying raiI raiR-lacZ) and pIJ9272 (carrying raiR-lacZ but lacking raiI and the predicted raiI promoter region), were constructed. The raiR-lacZ fusion (pIJ9272) was expressed in strain 8401 at a low level (330 ± 20 Miller units), indicating that either raiR does not have a separate promoter or, if it does, it has a relatively weak one. The level of raiR-lacZ expression in the raiR mutant (A802) was similar (295 ± 17 Miller units), indicating that there is not a RaiR-regulated promoter between raiI and raiR. The raiIR-lacZ fusion (pIJ9271) was expressed at a slightly higher level (395 ± 20 Miller units) in 8401, and this was decreased in the raiR mutant (320 ± 20 Miller units). These results indicate that raiR may be expressed at a low level that is independent of the raiI promoter, but as raiI expression is induced, so is that of raiR, such that the raiIR genes may be positively autoregulated (by RaiR). In strain 8401, there was significantly less expression of raiIR-lacZ (395 ± 20 Miller units) than of raiI-lacZ (1,261 ± 28 Miller units), suggesting that there may be some kind of transcriptional attenuation between raiI and raiR. The 90-bp region between raiI and raiR was examined for possible secondary structures. A region of dyad symmetry was found flanking either side of the predicted translation start of RaiR. This may influence the level of raiR expression.
Adding raiR (on pIJ9276) to the control strain (8401) greatly increased the level of raiI-lacZ expression (Table 4). This indicates that the availability of RaiR is a limiting factor in raiI expression. The cloned raiR gene also increased the expression of the raiIR-lacZ fusion in 8401 (from 395 ± 20 to 1,200 ± 37 Miller units) but not that of the raiR-lacZ fusion, which remained constant (330 ± 30 Miller units). This result confirms that expression of raiR is influenced by the raiI promoter; however, the low-level constitutive expression of raiR was unaffected by cloned raiR.
TABLE 4.
Effect of raiR on raiI-lacZ expressiona
| Strain |
raiI-lacZ (pIJ9280) expression
|
||
|---|---|---|---|
| Alone | + 3OH,C8-HSL | + raiR (pIJ9276) | |
| 8401 | 1,261 ± 18 | 4,640 ± 29 | 21,517 ± 340 |
| A789 raiI7::Tn5 | 462 ± 16 | 3,854 ± 31 | 1,695 ± 31 |
| A802 raiR8::Tn5 | 344 ± 13 | 299 ± 11 | 18,606 ± 278 |
| A643 cinI3::Spc | 346 ± 18 | 1,330 ± 19 | 16,222 ± 271 |
| A552 cinR1::Spc | 364 ± 17 | 1,738 ± 53 | 14,194 ± 205 |
| A797 cinI3::Spc raiI7::Tn5 | 326 ± 13 | 1,338 ± 19 | 364 ± 15 |
β-Galactosidase activity was assayed after 24 h of growth and is expressed in Miller units (21) ± standard errors.
raiI expression is controlled by the cinRI locus.
We previously observed that mutation of cinI in 8401 greatly reduces the production of AHLs detected by C. violaceum CV026 (17). This implies that the cinRI locus influences raiI expression. This was tested by transferring raiI-lacZ (pIJ9280) into cinI and cinR mutant derivatives of 8401. As shown (Table 4), both of these genes are required for normal expression of raiI. Although mutation of raiI strongly reduced raiI-lacZ expression, this expression was further reduced (from 462 to 326 Miller units) in the rai cinI double mutant A797 (Table 4). This could be consistent with the hypothesis that the raiI promoter may respond to the CinI-made 3OH,C14:1-HSL in addition to RaiI-made AHLs. However, added 3OH,C8-HSL only partially suppressed the effects of mutations in cinI or cinR on raiI-lacZ expression (Table 4). This may imply that cinI and cinR have an indirect effect on the expression of raiI.
Cloned raiR almost completely suppressed the effects of mutating cinI or cinR (Table 4). In a raiI mutant background, no such strong suppression was seen, demonstrating that RaiI-made AHLs are required for this effect. There is expression of raiI-lacZ in the raiI mutant carrying cloned raiR (1,695 Miller units), and this is probably due to CinI-made 3OH,C14:1-HSL, because the level of expression is reduced (to 364 Miller units) in the cinI raiI double mutant A797 (Table 4). One explanation for these observations is that the raiIR genes are initially induced in response to CinI-made 3OH,C14:1-HSL, and then as RaiI-made AHLs accumulate, expression of raiI is autoinduced. In support of this model, we have consistently observed a delay (of about 2 to 3 h) in the induction of raiI-lacZ expression in strain 8401 compared with assays of cinI-lacZ described previously (17). The timing of raiI induction is somewhat earlier in growth if pRL1JI is present (Fig. 5), indicating that AHLs determined by pRL1JI (traI) could also stimulate raiI expression.
Conclusions.
It is evident that the raiIR genes constitute part of a quorum-sensing network in R. leguminosarum and probably also in R. etli (25). The next step is to identify the genes regulated by RaiR in response to RaiI-made AHLs. The types of genes regulated are likely to play some sort of role in environmental adaptation that is not readily seen in laboratory tests of growth or nodulation. The observation that the raiIR genes may be absent from some strains of R. leguminosarum points toward a potentially subtle role for these genes. Recent comparisons of the plasmid and genome sequences of Sinorhizobium strains have revealed that many genes present in one strain are absent from another (11). Identifying the roles of such genes may give insights into aspects of subtle but specific interactions between rhizobia and their host plants and environment.
ADDENDUM IN PROOF
After submission of this work, R. S. Blosser-Middleton and K. M. Gray (J. Bacteriol. 183:6771-6777, 2001) described an analysis of [α-14C]methionine-labeled AHLs made by strains of R. leguminosarum used here also. In their work, only comigrating standards were used to identify the AHLs made by strains carrying or lacking pRL1JI. They assumed that 3O,C8-HSL was being made by both strains and concluded that the kinetics of production of this AHL was different depending on whether pRL1JI was present or absent. A lower rate of formation was thought to occur when pRL1JI was absent. However, the chemical characterization of 3OH,C8-HSL in the work described here means that the data of Blosser-Middleton and Gray should be reinterpreted. It is evident that 3O,C8-HSL and 3OH,C8-HSL comigrate, so some of their conclusions are probably incorrect. We propose that the different kinetics of appearance of the AHL that comigrates with 3O,C8-HSL is due to the formation of two products, one being 3OH,C8-HSL made by RaiI and one being 3O,C8-HSL made by TraI. In the strain carrying pRL1JI, we detected both 3OH,C8-HSL and 3O,C8-HSL, but when pRL1JI (and hence traI) was absent, no 3O,C8-HSL was detected. The slower induction of raiI-lacZ (and hence the appearance of 3OH,C8-HSL) compared to that of cinI-lacZ (Fig. 5) is consistent with delayed production of 3OH,C8-HSL during growth of the strain lacking pRL1JI. There are several other aspects of the analysis of AHL formation as described by Blosser-Middleton and Gray that need to be reassessed in light of the work described here.
Acknowledgments
We thank John Firmin for collecting data on mass spectra, A. Wilkinson and V. Danino for making unpublished data available, Chris Harty for help with the synthesis of AHLs, and P. Williams for helpful discussions. We also thank V. Danino and K. Wilson for help with preparative TLC and A. Davies for assistance with maintenance of bacterial strains and plasmids.
This work was supported by a grant in aid and a grant (208/PRS12210) from the Biotechnology and Biological Sciences Research Council, and in part by contracts (B104-CT96-0181 and QLK3-CT-2000-31759) from the European Union.
REFERENCES
- 1.Allaway, D., E. M. Lodwig, L. A. Crompton, M. Wood, R. Parsons, T. R. Wheeler, and P. S. Poole. 2000. Identification of alanine dehydrogenase and its role in mixed secretion of ammonium and alanine by pea bacteroids. Mol. Microbiol. 36:508-515. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Meyers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Bassler, B. L., M. Wright, R. E. Showalter, and M. R. Silverman. 1994. Multiple signalling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol. Microbiol. 13:273-286. [DOI] [PubMed] [Google Scholar]
- 4.Beringer, J. E. 1974. R factor transfer in Rhizobium leguminosarum. J. Gen. Microbiol. 84:188-198. [DOI] [PubMed] [Google Scholar]
- 5.Beynon, J. L., J. E. Beringer, and A. W. B. Johnston. 1980. Plasmids and host-range in Rhizobium leguminosarum and Rhizobium phaseoli. J. Gen. Microbiol. 120:421-429. [Google Scholar]
- 6.Buchanan-Wollaston, A. V. 1979. Generalised transduction in Rhizobium leguminosarum. J. Gen. Microbiol. 112:135-142. [Google Scholar]
- 7.Cha, C., P. Gao, Y.-C. Chen, P. D. Shaw, and S. K. Farrand. 1998. Production of acyl-homoserine lactone quorum-sensing signals by Gram-negative plant-associated bacteria. Mol. Plant-Microbe Interact. 11:1119-1129. [DOI] [PubMed] [Google Scholar]
- 8.Chhabra, S. R., P. Stead, N. J. Bainton, G. P. C. Salmond, G. S. A. B. Stewart, and B. J. Bycroft. 1993. Autoregulation of carbapenem antibiotic synthesis in Erwinia carotovora ATCC 39048 by analogues of N-(3-oxohexanoyl)-l-homoserine lactone. J. Antibiot. 46:441-454. [DOI] [PubMed] [Google Scholar]
- 9.Cubo, M. T., A. Economou, G. Murphy, A. W. B. Johnston, and J. A. Downie. 1992. Molecular characterisation and regulation of the rhizosphere-expressed genes rhiABCR that can influence nodulation by Rhizobium leguminosarum biovar viciae. J. Bacteriol. 174:4026-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Downie, J. A., C. D. Knight, A. W. B. Johnston, and L. Rossen. 1985. Identification of genes and gene products involved in the nodulation of peas by Rhizobium leguminosarum. Mol. Gen. Genet. 198:255-262. [Google Scholar]
- 11.Galibert, F., T. Finan, S. R. Long, A. Puhler, P. Abola, F. Ampe, et al. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293:668-672. [DOI] [PubMed] [Google Scholar]
- 12.Gray, K. M., J. P. Pearson, J. A. Downie, B. E. A. Boboye, and E. P. Greenberg. 1996. Cell-to-cell signalling in the symbiotic nitrogen-fixing bacterium Rhizobium leguminosarum: autoinduction of a stationary phase and rhizosphere-expressed genes. J. Bacteriol. 178:372-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch, P. R. 1979. Plasmid-determined bacteriocin production by Rhizobium leguminosarum. J. Gen. Microbiol. 113:219-228. [Google Scholar]
- 14.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 15.Lamb, J. W., G. Hombrecher, and A. W. B. Johnston. 1982. Plasmid-determined nodulation and nitrogen-fixation abilities in Rhizobium phaseoli.Mol. Gen. Genet. 186:449-452. [Google Scholar]
- 16.Laue, B. E., Y. Jiang, S. R. Chhabra, S. Jacob, G. S. A. B. Stewart, A. Hardman, J. A. Downie, F. O'Gara, and P. Williams. 2000. The biocontrol strain Pseudomonas fluorescens F113 produces the Rhizobium small bacteriocin, N-(3-hydroxy-7-cis-tetradecenoyl) homoserine lactone, via HdtS, a putative novel N-acylhomoserine lactone synthase. Microbiology 146:2469-2480. [DOI] [PubMed] [Google Scholar]
- 17.Lithgow, J. K., A. Wilkinson, A. Hardman, B. Rodelas, F. Wisniewski-Dyé, P. Williams, and J. A. Downie. 2000. The regulatory locus cinRI in Rhizobium leguminosarum controls a network of quorum-sensing loci. Mol. Microbiol. 37:81-97. [DOI] [PubMed] [Google Scholar]
- 18.Lithgow, J. K., V. E. Danino, J. Jones, and J. A. Downie. 2001. Analysis of N-acyl homoserine-lactone quorum-sensing molecules made by different strains and biovars of Rhizobium leguminosarum containing different symbiotic plasmids. Plant Soil 232:3-12. [Google Scholar]
- 19.Mavridou, A., M.-A. Barny, P. Poole, K. Plaskitt, A. E. Davies, A. W. B. Johnston, and J. A. Downie. 1995. Rhizobium leguminosarum nodulation gene (nod) expression is lowered by an allele-specific mutation in the dicarboxylate transport gene dctB. Microbiology 141:103-111. [DOI] [PubMed] [Google Scholar]
- 20.McLean, K. H., M. K. Winson, L. Fish, A. Taylor, S. Chhabra, M. Camara, M. Daykin, J. H. Lamb, S. Swift, B. W. Bycroft, G. S. A. B. Stewart, and P. Williams. 1997. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 143:3703-3711. [DOI] [PubMed] [Google Scholar]
- 21.Miller, J. A. 1976. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 22.Milton, B. L., V. J. Chalker, D. Kirke, A. Hardman, M. Camara, and P. Williams. 2001. The LuxM homologue VanM from Vibrio anguillarum directs the synthesis of N-(3-hydroxyhexanoyl)homoserine lactone and N-hexanoylhomoserine lactone. J. Bacteriol. 183:3537-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in Gram negative bacteria. Gene 127:15-21. [DOI] [PubMed] [Google Scholar]
- 24.Rodelas, B., J. K. Lithgow, F. Wisniewski-Dyé, A. Hardman, A. Wilkinson, A. Economou, P. Williams, and J. A. Downie. 1999. Analysis of quorum-sensing-dependent control of rhizosphere-expressed (rhi) genes in Rhizobium leguminosarum bv. viciae. J. Bacteriol. 181:3816-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosemeyer, V., J. Michiels, C. Verreth, and J. Vanderleyden. 1998. luxI- and luxR-homologous genes of Rhizobium etli CNPAF512 contribute to synthesis of autoinducer molecules and nodulation in Phaseolus vulgaris. J. Bacteriol. 180:815-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Sharma, S. B., and E. Signer. 1990. Temporal and spatial regulation of the symbiotic genes of Rhizobium meliloti in planta revealed by transposon Tn5-gusA. Genes Dev. 4:344-356. [DOI] [PubMed] [Google Scholar]
- 28.Shaw, P. D., G. Ping, S. L. Daly, J. E. Cronan, Jr., K. L. Rinehart, and S. K. Farrand. 1997. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. USA 94:6036-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon, R., J. Quandt, and W. Klipp. 1989. New derivatives of transposon Tn5 suitable for mobilisation of replicons, generation of operon fusions and induction of genes in Gram-negative bacteria. Gene 80:161-169. [DOI] [PubMed] [Google Scholar]
- 30.Sourjik, V., P. Muschler, B. Scharf, and R. Schmitt. 2000. VisN and VisR are global regulators of chemotaxis, flagellar, and motility genes in Sinorhizobium (Rhizobium) meliloti. J. Bacteriol. 182:782-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spaink, H. P., J. H. Okker, C. A. Wijffelman, E. Pees, and B. J. J. Lugtenberg. 1987. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1JI. Plant Mol. Biol. 9:27-39. [DOI] [PubMed] [Google Scholar]
- 32.Swift, S., J. A. Downie, N. A. Whitehead, A. M. L. Barnard, G. P. C. Salmond, and P. Williams. 2001. Quorum sensing as a population-density-dependent determinant of bacterial physiology. Adv. Microb. Physiol. 45:99-270. [DOI] [PubMed] [Google Scholar]
- 33.Thorne, S. H., and H. D. Williams. 1999. Cell density-dependent starvation survival of Rhizobium leguminosarum bv. phaseoli: identification of the role of an N-acyl homoserine lactone in adaptation to stationary-phase survival. J. Bacteriol. 181:981-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tibertius, A., N. G. de Luca, H. Hussain, and A. W. B. Johnston. 1996. Expression of the exoY gene, required for exopolysaccharide synthesis in Agrobacterium, is activated by the regulatory ros gene. Microbiology 142:2621-2629. [DOI] [PubMed] [Google Scholar]
- 35.Vaudequin-Dransart, V., A. Petit, C. Poncet, C. Ponsonnet, X. Nesme, J. B. Jones, H. Bouzar, W. S. Chilton, and Y. Dessaux. 1995. Novel Ti plasmids in Agrobacterium strains isolated from fig tree and chrysanthemum tumors and their opinelike molecules. Phytopathology 8:311-321. [DOI] [PubMed] [Google Scholar]
- 36.Wheatcroft, R., D. G. McRae, and R. W. Miller. 1990. Changes in the Rhizobium meliloti genome and the ability to detect supercoiled plasmids during bacteroid development. Mol. Plant-Microbe Interact. 3:9-17. [Google Scholar]
- 37.Wilkinson, A. 1998. Acyl-homoserine lactone signalling in Rhizobium leguminosarum. Ph.D. thesis. University of East Anglia, Norwich, United Kingdom.
- 38.Williams, P., T. Baldwin, and J. A. Downie. 1999. Bacterial crosstalk--communication between bacteria, plant and animal cells. Soc. Gen. Microbiol. Symp. 57:1-35. [Google Scholar]
- 39.Winson, M. K., M. Camara, A. Latifi, M. Foglino, S. R. Chhabra, M. Daykin, M. Bally, V. Chapon, G. P. C. Salmond, B. W. Bycroft, A. Ladzunski, G. S. A. B. Stewart, and P. Williams. 1995. Multiple N-acyl-l-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:9427-9431. [DOI] [PMC free article] [PubMed] [Google Scholar]