Abstract
The Vibrio cholerae genome revealed the presence of multiple sets of chemotaxis genes, including three cheA gene homologs. We found that the cheA-2, but not cheA-1 or cheA-3, gene is essential for chemotaxis under standard conditions. Loss of chemotaxis had no effect on virulence factor expression in vitro.
Vibrio cholerae, the causative agent of cholera, is a gram-negative motile bacterium with a single polar flagellum. V. cholerae has a life cycle consisting of two distinct phases. Outside the host, the bacteria have a free-swimming phase, during which the major virulence factors are not expressed (for a review see reference 14). In the host, the bacteria enter a sessile virulent phase where the organisms adhere to the intestinal epithelium, replicate, and cause disease. Eventually the bacteria detach and exit the host via the profuse diarrhea that is the hallmark of cholera. Once back in the aquatic environment, the cycle can begin again.
Motility is an important virulence factor in many pathogenic species and in some cases is inversely regulated with the expression of virulence traits (12). Although the roles of motility and chemotaxis of V. cholerae in its ability to cause cholera has not been clearly established (3, 8), the production of the two major virulence factors, cholera toxin (CT) and toxin-coregulated pili (TCP), is known to be affected by the motility phenotype of the bacteria (3). Despite the potentially very important roles of chemotaxis in both the free-swimming as well as virulent phases of V. cholerae, no detailed genetic analysis of its chemotactic behavior has been performed. In the present study we generated several deletion mutants defective in putative chemotaxis genes and analyzed their motility behavior.
V. cholerae cheA genes.
The recently completed genome revealed several chemotaxis-related gene homologs in V. cholerae (7), most of which are clustered in three different regions distributed on both chromosomes (Fig. 1). Similarly, several other organisms, including Pseudomonas aeruginosa (13), Rhodobacter sphaeroides (9), Myxococcus xanthus (15), and Borrelia burgdorferi (2), have been reported to contain multiple chemotaxis operons. In V. cholerae, three putative genes with strong homology to the Escherichia coli cheA gene can be identified. The cheA-1 (VC1397) and cheA-2 (VC2063) genes are located on the larger chromosome of V. cholerae, whereas cheA-3 (VCA1095) is found on the smaller chromosome (7) (Fig. 1) and showed 38, 40, and 49% identity to the E. coli cheA gene, respectively.
FIG. 1.
Diagram of the location of the cheA genes in the two chromosomes of V. cholerae. The cheA-1 (VC1397) and cheA-2 (VC2063) genes are located on the larger chromosome of V. cholerae, whereas cheA-3 (VCA1095) is found on the smaller chromosome. MCP, methyl-accepting chemotaxis protein.
Mutants in cheA.
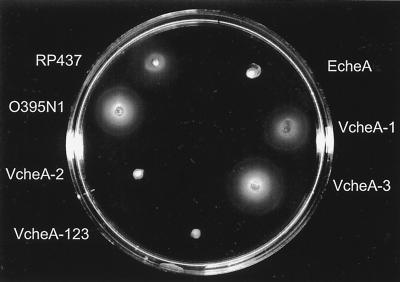
We created mutant strains carrying deletions in each of the V. cholerae cheA genes (VcheA-1, VcheA-2, VcheA-3) as well as a triple mutant strain (VcheA-123) by homologous recombination. Sequence data for V. cholerae were obtained from The Institute for Genomic Research website at http://www.tigr.org. The genes and surrounding sequences were amplified in PCRs by using specific primers and were cloned into plasmid vectors. Internal deletions were generated by using convenient restriction sites present in the genes or by cloning two PCR-derived DNA fragments containing the 5′- and 3′-flanking genomic regions adjacent to each other. The DNA fragments carrying the desired deletions were subcloned into the suicide vector pWM91 (10), and the mutated alleles were then introduced into the chromosome of the V. cholerae O395N1 strain following sucrose selection as described previously (1). The strains and plasmids used in this study are listed in Table 1. When assayed in 0.3% Luria-Bertani (LB) soft agar, the parental strain and the VcheA-1 and VcheA-3 mutant strains showed similarly large swarm circles, whereas the VcheA-2 and VcheA-123 strains showed very small swarm circles (Fig. 2). As expected, the cheA mutant derivative of an E. coli strain (EcheA) displays small swarm circles compared to that of its parent strain (Fig. 2). This indicates that the cheA-2 gene of V. cholerae is required for swarming in LB, consistent with previous results (8), whereas cheA-1 and cheA-3 are not. Similarly, in several other species only some of the multiple che genes appear to function in the chemotaxis behavior, including R. sphaeroides (9) and M. xanthus (15). It is possible that the multiple che genes serve as secondary chemotactic genes, and we have yet to discover the conditions under which these genes are needed, or they might function in other cellular processes such as development.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genotype or description | Source or reference |
|---|---|---|
| V. cholerae | ||
| O395N1 | Parental | J. J. Mekalanos |
| VcheA-1 | O395N1 ΔcheA-1 | This study |
| VcheA-2 | O395N1 ΔcheA-2 | This study |
| VcheA-3 | O395N1 ΔcheA-3 | This study |
| VcheA-123 | O395N1 ΔcheA-1, ΔcheA-2, ΔcheA-3 | This study |
| T:Z | O395N1 toxT::lacZ | 6 |
| T:ZcheA-1 | O395N1 toxT::lacZ ΔcheA-1 | This study |
| T:ZcheA-2 | O395N1 toxT::lacZ ΔcheA-2 | This study |
| T:ZcheA-3 | O395N1 toxT::lacZ ΔcheA-3 | This study |
| T:ZcheA-123 | O395N1 toxT::lacZ ΔcheA-1, ΔcheA-2, ΔcheA-3 | This study |
| TCP-2 | O395 tcpA::TnphoA | R. K. Taylor |
| TCP-2cheA-2 | TCP-2 ΔcheA-2 | This study |
| KP2.11 | O395 ctx::phoA | R. K. Taylor |
| KP2.11cheA-2 | KP2.11 ΔcheA-2 | This study |
| E. coli | ||
| RP437 | Parental | J. S. Parkinson |
| EcheA | (RP9535) RP437 ΔcheA | J. S. Parkinson |
| Plasmids | ||
| pBAD-TOPO | Expression vector | Invitrogen |
| pVcheA-1 | pBAD-TOPO with V. cholerae cheA-1 | This study |
| pVcheA-2 | pBAD-TOPO with V. cholerae cheA-2 | This study |
| pVcheA-3 | pBAD-TOPO with V. cholerae cheA-3 | This study |
| pEcheA | pBAD-TOPO with E. coli cheA | This study |
FIG. 2.
Analyses of mutants for motility. Swarms in LB soft agar of the V. cholerae strain O395N1 (wild type) and it's cheA-1 (VcheA-1), cheA-2 (VcheA-2), cheA-3 (VcheA-3), and triple (VcheA-123) mutant derivatives as well as the E. coli strain RP437 and its cheA mutant derivative are shown. Plates were incubated for 8 h at 37°C.
Complementation of cheA.
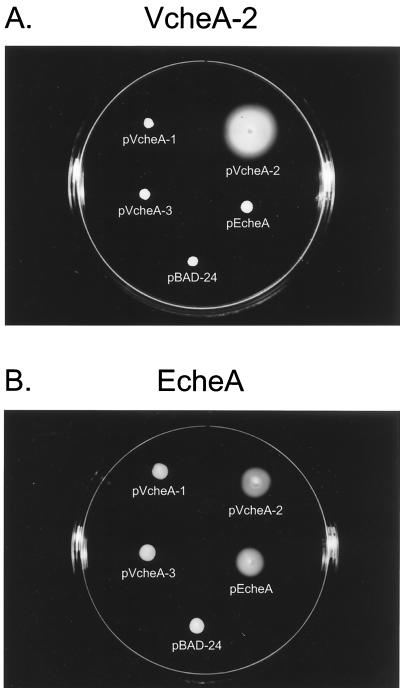
Expression plasmids were generated by cloning the PCR products of the different cheA genes into the plasmid vector pBAD-TOPO (Invitrogen) via a cloning kit. All three V. cholerae cheA genes as well as the E. coli cheA gene were cloned under the control of an arabinose-inducible promoter and were transformed into the VcheA-2, VcheA-123, and EcheA deletion strains by electroporation. As the VcheA-1 and VcheA-3 strains did not show any chemotaxis-deficient phenotype in this assay, we did not check it for complementation. We analyzed motility of the VcheA-2 (Fig. 3A) and VcheA-123 (data not shown) strains having plasmids that carry either of the three V. cholerae cheA genes or pEcheA by stabbing bacteria into LB soft agar. Both strains carrying pVcheA-2 showed marked increase in swarm circle size in the presence of 0.02% arabinose, whereas the presence of the V. cholerae cheA-1 or cheA-3 gene, even when expressed from an arabinose-inducible promoter, did not increase the swarm circle size in soft agar. However, induction of the E. coli cheA gene resulted in only very slightly larger swarm zones in both background strains compared to those of the empty plasmid control (Fig. 3A), which was more prominent after longer incubation periods (data not shown). As expected, the E. coli cheA deletion strain was complemented by the plasmid carrying the E. coli cheA gene (Fig. 3B) in the presence of the inducer. However, the E. coli cheA mutant strain was complemented well by pVcheA-2 but not by pVcheA-1 or pVcheA-3 (Fig. 3B). These data suggest that the V. cholerae cheA-2 gene encodes a functional homolog of the E. coli CheA protein. The E. coli cheA gene appears to partially compensate for the absence of the V. cholerae cheA-2 gene, whereas the V. cholerae cheA-2 gene can almost fully complement the E. coli deletion strain. Further experiments will be needed to determine the underlying mechanism of this difference.
FIG. 3.
Complementation of cheA mutants by plasmids carrying various cheA genes. (A) Swarming abilities in the presence of arabinose of the V. cholerae cheA-2 deletion strain (VcheA-2) complemented by plasmids carrying the V. cholerae cheA-1 (pVcheA-1), cheA-2 (pVcheA-2), or cheA-3 (pCheA-3) gene or the E. coli cheA gene. (B) Swarming abilities in the presence of arabinose of the E. coli cheA deletion strain (EcheA) complemented by plasmids carrying the V. cholerae cheA-1 (pVcheA-1), cheA-2 (pVcheA-2), or cheA-3 (pCheA-3) gene or the E. coli cheA gene. pBAD-24 is the parent vector and contains no cheA gene. Plates were incubated for 8 h at 37°C.
Detection of the CheA proteins in V. cholerae cells.
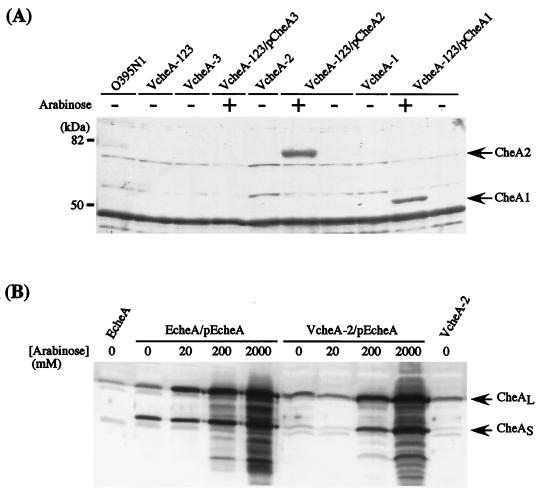
Expression of the CheA proteins was examined. Bacterial whole-cell extracts were subjected to immunoblotting with anti-E. coli CheA serum. Fresh overnight cultures were diluted 1:30 into fresh Tryptone-glycerol medium supplemented with or without arabinose. Cells were grown at 37°C for 3 h with vigorous shaking, harvested by centrifugation, and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by immunoblotting. Immunoblotting was performed, essentially as described previously (11), with rabbit anti-E. coli CheA serum as the first antibody and alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G antibody as the second antibody. Although a number of bands were detected for O395N1 cells, all of them were also detected for its derivative VcheA-123, suggesting that none of them are related to CheA (Fig. 4A). However, unique bands corresponding to CheA-1 or CheA-2 were detected for VcheA-123 cells carrying pCheA-1 or pCheA-2 in the presence of, but not in the absence of, arabinose, suggesting that these proteins were overexpressed by the induction of the araBAD promoter (Fig. 4A). In contrast, no band corresponding to CheA-3 was detected for VcheA-123 cells carrying pCheA-3, even in the presence of arabinose. Expression of the E. coli CheA protein was confirmed in both the E. coli and V. cholerae cheA deletion strains in response to the addition of arabinose (Fig. 4B).
FIG. 4.
Detection of the CheA proteins in V. cholerae and E. coli cells. (A) Cultures were grown with (+) or without (−) 20 mM arabinose. The arrows indicate the positions of the bands corresponding to the CheA-1 and CheA-2 proteins. (B) The amount of arabinose added to the cultures is indicated above the lanes. The arrows indicate the positions of the bands corresponding to the large (CheAL) and small (CheAS) forms of the E. coli CheA protein. Immunoblottings were performed with rabbit anti-E. coli CheA serum as the first antibody and alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G antibody as the second antibody. The plasmids introduced into the strains are indicated after a slash.
Virulence gene expression.
Random nonmotile mutants of V. cholerae showed increased expression of the essential virulence factors, CT and TCP (3), and we recently reported elevated levels of toxT::lacZ expression in defined nonmotile strains (5). Furthermore, at least two ToxR-regulated genes encode proteins with homology to methyl-accepting chemotaxis proteins, and one of them, tcpI, has been suggested to be involved in virulence gene regulation (4). This prompted us to analyze the effects of the different V. cholerae cheA mutations on the expression of toxT. Single mutations in the three cheA genes as well as triple mutations were introduced into the V. cholerae O395N1 toxT::lacZ reporter strain previously described (6). Similar β-galactosidase levels of the mutant strains compared to those of the parental strain were observed (data not shown). Further, we introduced deletions in the cheA-2 genes into V. cholerae strains carrying ctx::phoA or tcpA::phoA reporter constructs and observed no differences in alkaline phosphotase expression levels between the parental and chemotaxis-deficient strains (data not shown), indicating no direct link between lack of chemotaxis and virulence gene regulation in V. cholerae in vitro. Interestingly, Lee et al. recently reported that several V. cholerae chemotaxis genes, including cheA-2, regulate virulence gene expression in an in vivo model (8).
Acknowledgments
This work was supported in part by the Cancer Center Support Grant (CA 21765) and ALSAC (American Lebanese Syrian Associated Charities).
REFERENCES
- 1.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fraser, C. M., S. Cajens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. C. Venter, et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:553-555. [DOI] [PubMed] [Google Scholar]
- 3.Gardel, C., and J. J. Mekalanos. 1996. Alterations in Vibrio cholerae motility phenotypes correlate with changes in virulence factor expression. Infect. Immun. 64:2246-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harkey, C. W., K. D. Everiss, and K. M. Peterson. 1994. The Vibrio cholerae toxin-coregulated-pilus gene tcpI encodes a homolog of methyl-accepting chemotaxis proteins. Infect. Immun. 62:2669-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Häse, C. C., and J. J. Mekalanos. 1999. Effects of changes in membrane sodium flux on virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 96:3183-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Häse, C. C., and J. J. Mekalanos. 1998. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 95:730-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heidelberg, J., J. Eisen, W. Nelson, R. Clayton, M. Gwinn, R. Dodson, D. Haft, E. Hickey, J. Peterson, L. Umayam, S. Gill, K. Nelson, T. Read, H. Tettelin, D. Richardson, M. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. Fleishmann, W. Nierman, and O. White. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee, S. H., S. M. Butler, and A. Camilli. 2001. Selection for in vivo regulators of bacterial virulence. Proc. Natl. Acad. Sci. USA 98:6889-6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin, A. C., G. H. Wadhams, and J. P. Armitage. 2001. The roles of the multiple CheW and CheA homologues in chemotaxis and in chemoreceptor localization in Rhodobacter sphaeroides. Mol. Microbiol. 40:1261-1272. [DOI] [PubMed] [Google Scholar]
- 10.Metcalf, W. W., W. Jiang, L. L. Daniels, S. K. Kim, A. Haldimann, and B. L. Wanner. 1996. Conditionally replicative and conjugative plasmids carrying lacZ alpha for cloning, mutagenesis, and allele replacement in bacteria. Plasmid 35:1-13. [DOI] [PubMed] [Google Scholar]
- 11.Okumura, H., S.-I. Nishiyama, A. Sasaki, M. Homma, and I. Kawagishi. 1998. Chemotactic adaptation is altered by changes in the carboxyl-terminal sequence conserved among the major methyl-accepting chemoreceptors. J. Bacteriol. 180:1862-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ottemann, K. M., and J. F. Miller. 1997. Roles for motility in bacterial-host interactions. Mol. Microbiol. 24:1109-1117. [DOI] [PubMed] [Google Scholar]
- 13.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, and I. T. Paulse. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:947-948. [DOI] [PubMed] [Google Scholar]
- 14.Waldor, M. K., and J. J. Mekalanos. 1996. Vibrio cholerae: molecular pathogenesis, immune response, and vaccine development, p. 37-56. In L. J. Paradise et al. (ed.), Enteric infections and immunity. Plenum Press, New York, N.Y.
- 15.Yang, Z., Y. Geng, D. Xu, H. B. Kaplan, and W. Shi. 1998. A new set of chemotaxis homologues is essential for Myxococcus xanthus social motility. Mol. Microbiol. 30:1123-1130. [DOI] [PubMed] [Google Scholar]