Abstract
We have cloned homologs of the Pseudomonas putida GPo1 alkane hydroxylase from Pseudomonas aeruginosa PAO1, Pseudomonas fluorescens CHA0, Alcanivorax borkumensis AP1, Mycobacterium tuberculosis H37Rv, and Prauserella rugosa NRRL B-2295. Sequence comparisons show that the level of protein sequence identity between the homologs is as low as 35%, and that the Pseudomonas alkane hydroxylases are as distantly related to each other as to the remaining alkane hydroxylases. Based on the observation that rubredoxin, an electron transfer component of the GPo1 alkane hydroxylase system, can be replaced by rubredoxins from other alkane hydroxylase systems, we have developed three recombinant host strains for the functional analysis of the novel alkane hydroxylase genes. Two hosts, Escherichia coli GEc137 and P. putida GPo12, were equipped with pGEc47ΔB, which encodes all proteins necessary for growth on medium-chain-length alkanes (C6 to C12), except a functional alkane hydroxylase. The third host was an alkB knockout derivative of P. fluorescens CHA0, which is no longer able to grow on C12 to C16 alkanes. All alkane hydroxylase homologs, except the Acinetobacter sp. ADP1 AlkM, allowed at least one of the three hosts to grow on n-alkanes.
Bacterial oxidation of n-alkanes is a very common phenomenon in soil and water (4) and is a major process in geochemical terms: the estimated amount of alkanes that is recycled per year amounts to several million tons from natural oil seepage and oil spills alone (37). Even more relevant are the alkanes (mainly waxes or paraffins) produced by plants, algae, and other organisms because they are available to bacteria throughout the biosphere. From a biotechnological perspective, alkane hydroxylases are versatile biocatalysts, which carry out a wide range of useful oxidation reactions (22, 61).
Biochemical and genetic studies have thus far focused on a very limited number of alkane hydroxylases, such as the Pseudomonas putida GPo1 alkane hydroxylase (33), which oxidizes C5 to C12 n-alkanes to 1-alkanols, thereby allowing the host to grow on these compounds. Only recently has it become apparent that this enzyme is the prototype of a very diverse collection of related non-heme iron integral membrane oxygenases which includes not only the alkane hydroxylases of Acinetobacter sp. ADP1 (35), Burkholderia cepacia RR10 (29), Acinetobacter calcoaceticus EB104, and P. putida P1 (43) but also xylene monooxygenases (48), fatty acid desaturases, fatty acid monooxygenases, steroid oxygenases, and decarbonylases (41).
The P. putida GPo1 alkane hydroxylase system consists of three components: alkane hydroxylase (AlkB), rubredoxin (AlkG), and rubredoxin reductase (AlkT). AlkB is a non-heme iron integral membrane protein which carries out the hydroxylation reaction (26, 31, 54). Rubredoxin (33) transfers electrons from the NADH-dependent flavoprotein rubredoxin reductase (49) to AlkB. The molecular genetics of this enzyme system has been reviewed by van Beilen et al. (53, 56).
Genes that are closely related to the alkane hydroxylase gene (alkB) of GPo1 have been detected in a large fraction of the microbial population in oil-contaminated environments (45) and in several fluorescent pseudomonads (39, 43, 55, 58). More distantly related genes were found in many gram-positive and gram-negative strains able to grow on n-alkanes ranging from C6 to C30 by using highly degenerate primers, which amplify internal fragments of alkane hydroxylase homologs (43) (J. B. van Beilen, unpublished data).
In earlier studies, the alkane hydroxylase genes of P. putida GPo1 and Acinetobacter sp. ADP1 have been cloned by using traditional cloning methods. In both cases, cosmids that restored growth of chemical mutants on alkanes were selected from a cosmid library (9, 35). However, this method was not successful for other alkane hydroxylase genes. For example, in classical mutagenesis experiments with Pseudomonas aeruginosa PG201, mutants unable to grow on alkanes usually had mutations in genes responsible for rhamnolipid biosynthesis or downstream metabolism (24). In retrospect, this can be explained by the fact that P. aeruginosa contains two alkane hydroxylases with overlapping substrate ranges. More recent data show that many other strains also contain multiple alkane hydroxlases (L. G. Whyte, T. H. M. Smits, D. Labbé, B. Witholt, C. W. Greer, and J. B. van Beilen, submitted for publication; J. B. van Beilen, unpublished data). In addition, although medium-chain-length alkane hydroxylases can be assayed in vitro, e.g., using the conversion of alkenes to epoxides or the cooxidation of NADH (51), such methods could not be developed for membrane-bound long-chain alkane hydroxylases, possibly because long-chain alkanes are hardly soluble in water (solubility around 10 μM), and activity is strongly limited by substrate mass transfer. Therefore, it was not possible to purify the alkane hydroxylases based on enzyme activity and use reverse genetics to clone the genes.
In this study, we used PCR products obtained with highly degenerate primers described earlier or information from genome sequencing projects to clone a number of alkane hydroxylases from quite diverse strains. This implies that these genes are cloned based on sequence similarity, not on function. Knockout mutants of the alkB gene homologs could in principle be used to prove that these genes indeed encode functional alkane hydroxylases. However, many strains are not easily accessible for molecular genetic studies, as tools or methods are not (yet) available. This is the case for Alcanivorax borkumensis AP1, which has recently been isolated from seawater and grows almost exclusively on n-alkanes (62). Other strains, like Mycobacterium tuberculosis and Legionella pneumophila, are important human pathogens and are difficult to cultivate. Moreover, if a strain contains multiple alkB homologs, knockout mutants may not show phenotypical changes, while in vivo substrate range studies would only give information about the sum of all (induced) alkane hydroxylase activities.
Heterologous expression of novel alkane hydroxylases is complicated by the fact that all three components of the alkane hydroxylase system are necessary for enzyme activity. Fortunately, complementation experiments with the P. putida GPo1 alkane hydroxylase system have shown that rubredoxins from various gram-positive and gram-negative alkane degraders can replace the GPo1 rubredoxin in alkane oxidation (52). This implies that the GPo1 rubredoxin and rubredoxin reductase or equivalent proteins from other sources should be able to serve as electron donors for novel alkane hydroxylases related to the GPo1 enzyme. Based on this notion, we have constructed three different recombinant hosts that allow us to test whether the novel alkane hydroxylases oxidize n-alkanes.
MATERIALS AND METHODS
Strains, plasmids, and media.
Strains used in this study are listed in Table 1. Luria-Bertani broth (38), E medium (57), and E2 medium (28) supplemented with carbon sources or antibiotics were used throughout. MT trace elements (28) were added to minimal media. All cultures were grown aerobically at 30°C. For growth on n-alkanes, petri dishes with E2 medium were incubated at 30°C with n-alkanes supplied through the vapor phase, in the case of n-octane, by placing an open Erlenmeyer flask with n-octane (C8) and n-decane (C10) in a sealed container and for n-dodecane (C12), n-tetradecane (C14), and n-hexadecane (C16), by placing a Whatman 3MM filter disk with 200 μl of n-alkane in the lid of the petri dish. Recombinants were cultured in baffled Erlenmeyer flasks with E2 medium and 1% (vol/vol) n-alkanes as carbon sources at 120 rpm. Rhamnolipids (11) were added to cultures of recombinant P. putida GPo12 strains to a concentration of 0.01% (wt/vol) to solubilize long-chain n-alkanes. Alkanes that are solid at 30°C (n-eicosane [C20], n-docosane [C22], n-tetracosane [C24] and n-octacosane [C28]) were dissolved to 10% (vol/vol) in dioctylphthalate. This solution was then added to the growth medium as a second phase at 2% (vol/vol).
TABLE 1.
List of strains and plasmids used in this study
| Strain or plasmid | Relevant phenotype, genotype, or characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| Acinetobacter sp. ADP1 | Grows on C12-C16 alkanes | 23 |
| A. borkumensis AP1 | Grows on C8-C16 alkanes | 62 |
| E. coli JM101 | endA hsdR, supR, thi-I, Δ(lac-proAB) F′ (traD36, proAB, laclq, lacZM15) | 63 |
| E. coli DH10B | Cloning strain | Gibco BRL |
| E. coli SF800 | W3110 polA mutant | Laboratory collection |
| E. coli GEc137 | DH-1 thi, fadR | 8 |
| P. aeruginosa PAO1 | Grows on C12-C24 alkanes | 21 |
| P. fluorescens CHA0 | Grows on C12-C32 alkanes | 47 |
| P. fluorescens KOB2 | alkB knockout mutant of CHA0, Kmr | This study |
| P. fluorescens KOB2Δ1 | alkB knockout mutant of CHA0 | This study |
| P. putida GPo12 | GPo1 cured of the OCT plasmid | 25 |
| P. rugosa NRRL B-2295 | Grows on C12 | 22 |
| Plasmids | ||
| p2295 | P. rugosa alkB fragment in pGEM7-Zf(+) | 52 |
| pAF1 | A. borkumensis AP1 alkG in pZEr0-2.1 | J. B. van Beilen |
| pAP1J1 | 5.0-kb NcoI-EcoRV fragment of A. borkumensis AP1 in pZErO2.1, alkS alkB1 | This study |
| pAP1S1 | 7.5-kb BglII fragment of A. borkumensis AP1 in pZErO2.1, alkGHJ | This study |
| pAP1T1 | 4.6-kb NheI fragment of A. borkumensis AP1 in pZErO2.1, ′alkS | This study |
| pCHA0 | P. fluorescens CHA0 alkB1 fragment in pGEM7-Zf(+) | 43 |
| pCK217 | pUC18Sfi containing res-npt-res | 27 |
| pCom5 | Expression vector with PalkB, Gmr | 44 |
| pCom7 | Expression vector with PalkB, GmroriT | 44 |
| pCom7B1 (PAO1) | P. aeruginosa PAO1 alkB1 gene in pCom7 | This study |
| pCom7B2 (PAO1) | P. aeruginosa PAO1 alkB2 gene in pCom7 | This study |
| pCom7B (GPo1) | P. putida GPo1 alkB gene in pCom7 | This study |
| pCom7alkB1 (AP1) | A. borkumensis AP1 alkB1 gene in pCom7 | This study |
| pCom7M (ADP1) | Acinetobacter sp. ADP1 alkM gene in pCom7 | This study |
| pCom8 | Broad-host-range expression vector with PalkB, GmroriT alkS | 44 |
| pCom8B1 (PAO1) | P. aeruginosa PAO1 alkB1 gene in pCom8 | This study |
| pCom8B2 (PAO1) | P. aeruginosa PAO1 alkB2 gene in pCom8 | This study |
| pCom8B (GPo1) | P. putida GPo1 alkB gene in pCom8 | This study |
| pCom8M (ADP1) | Acinetobacter sp. ADP1 alkM gene in pCom8 | This study |
| pCom8MT (H37Rv) | M. tuberculosis H37Rv alkB gene in pCom8 | This study |
| pCom8B (2295) | P. rugosa NRRL B-2295 alkB gene in pCom8 | This study |
| pEX18Tc | Gene replacement vector | 19 |
| pEXPF1rnrB2 | pPF1rnrB PvuI fragment in pEX18Tc | This study |
| pGEc47 | alkBFGHJKL alkST (GPo1) in pLAFR1 | 9 |
| pGEc47ΔB | pGEc47, deletion in alkB | 54 |
| pGEM7-Zf(+) | Cloning vector, Apr | Promega |
| pJMSB8 | Apr, PAI/04/03::parA in pJMSA8 | 27 |
| pKKPalk | E. coli expression vector with PalkB, Apr | 44 |
| pPF1 | 4.5-kb XhoI-BamHI fragment of P. fluorescens CHA0 in pGEM7-Zf(+), alkB | This study |
| pPF1rnrB | pPF1ΔSfiI-StuI, res-npt-res in alkB | This study |
| pPF21 | P. fluorescens CHA0 alkB gene in pCom5 | This study |
| pPru1 | 3.0-kb SacI-BamHI fragment of P. rugosa NRRL B-2295 in pGEM7-Zf(+), alkB | J. B. van Beilen |
| pSCYB11 | TcralkB rubA rubB (M. tuberculosis H37Rv) | 34 |
| pSK2 | A. borkumensis SK2 PCR fragment in pGEM7-Zf(+) | J. B. van Beilen |
| pUC18Sfi | Cloning vector, Apr | 16 |
| pUCP24 | E. coli and Pseudomonas shuttle vector | 60 |
| pUCPParA | Gmr, parA as EcoRI-HindIII fragment in pUCP24 | This study |
| pZErO2.1 | Cloning vector, Kmr | Invitrogen |
Escherichia coli strains and P. putida GPo12(pGEc47B) were transformed by electroporation according to the method of Dower et al. (7). Pseudomonas fluorescens CHA0 and KOB2Δ1 were transformed according to the method of Højberg et al. (20). Plasmids with an oriT (origin of transfer) were introduced in P. putida GPo12(pGEc47ΔB) by triparental matings with E. coli DH10B as the donor and E. coli CC118(RK600) as the helper strain (6). Transconjugants were selected on E medium containing the appropriate antibiotics. E. coli strains harboring plasmids were grown with appropriate antibiotics (tetracycline, 12.5 μl/ml; ampicillin, 100 μg/ml; gentamicin, 10 μg/ml). For P. fluorescens KOB2Δ1 recombinants, gentamicin was used at 100 μg/ml. For P. putida GPo12 recombinants, tetracycline (12.5 μg/ml) and gentamicin (100 μg/ml) were used.
DNA manipulations.
Plasmid DNA was isolated with the Roche High Pure Plasmid Isolation kit or according to the method of Birnboim and Doly (2) for Pseudomonas recombinants. Chromosomal DNA was isolated according to the method of Sambrook et al. (38). PCRs were carried out using the Roche Expand High Fidelity Polymerase kit on a Perkin Elmer GeneAmp PCR System 9600. The following PCR program was used: 4 min at 95°C and 25 cycles of 45 seconds at 95°C, 1 min at annealing temperature (5°C below the melting point), and 1 min at 72°C; 5 min at 72°C; and then transfer at 4°C. PCR products were purified over 1% agarose gels, cut with the respective enzymes, purified again over a 1% agarose gel, and cloned into pGEM7-Zf(+) or pKKPalk (44). All PCR fragments were checked for possible PCR artifacts by sequencing both strands of the inserts on a Li-Cor 4000L sequencer using the Amersham Thermosequenase cycle sequencing kit and IRD800-labeled −40 forward (AGGGTTTTCCCAGTCACGACGTT) and −40 reverse (GAGCGGATAACAATTTCACACAGG) primers for pGEM7-ZF(+) clones or PalkFwd (TGGCGCAAGCGTCCGATTAG), PalkFw3 (GCCAGCTCGTGTTTTTCCAGCAGACG), and pKKRev (GAGTTCGGCATGGGGTCAGGTG) for pKKPalk-derived plasmids (MWG-Biotech). Nucleotide and amino acid sequences were analyzed and compared using LASERGENE Navigator from DNASTAR. Nucleotide and amino acid sequences were compared with the EMBL, SwissProt, and GenBank databases using BLAST (1). BLAST searches were carried out at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST/).
Cloning of alkane hydroxylase gene homologs and construction of expression plasmids.
To clone complete alkane hydroxylase genes, easy-to-clone restriction fragments were identified by Southern blotting (38) with previously cloned DNA fragments as probes. Enriched gene banks were constructed by isolating restriction fragments of the desired size from a preparative gel. The DNA fragments were ligated between the appropriate sites of pGEM7-Zf(+) or pZErO2.1 and transformed into E. coli DH10B. Transformants containing the target genes were identified by colony blotting with the same probes. Both strands of the inserts were sequenced by a combination of ordered deletions, subcloning, or walking primers.
The alkB1 and alkB2 genes of P. aeruginosa PAO1 (46) were amplified by PCR from chromosomal DNA using primer combinations AlkBpaFwd (AACTGGAATTCACGATGTTTGA) and AlkBpaRv2 (CTGCCCGAAGCTTGAGCTAT) and AlkBpaBfw (GGAGAATTCTCAGACAATCT) and AlkBpaBrv (GAGGCGAATCTAGAAAAAACTG), respectively. Primers alkMfE2 (CCGGAATTCACTATGAATGCACCTGTA) and alkMrPS (AATAGGCCTGCAGTCACTTAGACTCTCTT) were used to amplify alkM of Acinetobacter sp. ADP1 (35). To amplify alkB1 of A. borkumensis AP1, primers alkB1fw (CAAGGTGATCCATATGTCAGAGAAC) and alkB1rv (GCGGATCCTCAAAGTGTGAAAGC) were used. For alkB of Prauserella rugosa NRRL B-2295, primers 2295fwEco (GGAGAATTCAGATGAGCGACGACGCAC) and 2295rvBam (CGGCGAGGATCCGGTCCAGCTC) were used. The M. tuberculosis H37Rv alkB gene was amplified from cosmid pSCYB11 (34) with primers MTalkBfw2 (CGGAATTCATATGACCACGCAAATCGGC) and MTalkBrv3 (CAGACCGGGATCCGGTAGGCGG). The P. putida GPo1 alkB gene was amplified from pGEc47 (9) with primers B5-Eco (GGAGAATTCCAAATGCTTGAG) and B3-Hind (TTTGTGAAAGCTTTCAACGCC). All PCR fragments were digested with the respective restriction enzymes (sites underlined in the primer sequences) and inserted in pCom7 or pCom8 (44). The alkB2 (PAO1) gene was first cloned between the EcoRI and XbaI sites of pUC18Sfi and then recloned in pCom8 with EcoRI and HindIII. The alkB gene of P. fluorescens CHA0 was cloned directly as a 1.6-kb MunI-SalI fragment, including 133 bp upstream of the alkB gene, and inserted in the EcoRI-SalI restriction sites of pCom5 (44).
Construction of a P. fluorescens CHA0 alkB knockout mutant.
Plasmid pPF1 was digested with SfiI and StuI to remove a 0.5-kb internal segment of alkB and blunt ended with T4 DNA polymerase. Subsequently, the fragment was ligated to a blunt-ended (Klenow) 2.1-kb res-npt-res cassette cut from pCK217 (27) with HindIII and Ecl136II. After digestion of the resulting plasmid (pPF1rnrB2) with PvuI and treatment with Klenow, the large fragment was ligated in SmaI-digested pEX18Tc (19). The final plasmid, pEXPF1rnrB2, was introduced into P. fluorescens CHA0 by electroporation using kanamycin selection (50 μg/ml). A Kmr Tcs colony was obtained, which was shown by Southern blot hybridization and PCR to have a chromosomal insertion of the res-npt-res cassette in the alkB gene (data not shown). This mutant was named P. fluorescens KOB2. To remove the kanamycin resistance gene with its transcription terminator, the parA gene was supplied on pUCPParA. The latter plasmid was constructed by digesting pJMSB8 (27) with EcoRI and HindIII. The fragment containing the parA gene was isolated over a gel and ligated in pUCP24 digested with the same enzymes. Gentamicin-resistant colonies were tested for the absence of the kanamycin cassette by PCR and sensitivity to kanamycin. One positive colony, subsequently cured of pUCPParA, was designated P. fluorescens KOB2Δ1.
Sequences.
Nucleotide sequences used in this study are available at EMBL under the following accession numbers: Acinetobacter sp. ADP1 alkRM, AJ002316; M. tuberculosis H37Rv alkB (Rv3252c), Z46863; P. aeruginosa PAO1 alkB1 (PA2574), AE004685; alkB2 (PA1525), AE004581; P. putida GPo1 alkBFGHJKL alkN alkST, AJ245436; and P. putida P1 alkBFGHJKL alkST, AJ233397.
Nucleotide sequence accession numbers. Novel sequences were submitted to GenBank and are available under the following accession numbers: A. borkumensis Ap1, AJ295164; P. rugosa NRRL B-2295, AJ009587; P. fluorescens CHA0, AJ009579.
RESULTS
Selection of strains.
For this study, we selected a number of alkane-oxidizing strains from different environments (soil, seawater, and cow rumen) and different research fields (biodegradation, biocatalysis, and pathogenesis). The alkane hydroxylase genes of Acinetobacter sp. ADP1 and P. putida strains GPo1 and P1, which are isolates of common soil and water organisms, have been cloned and sequenced before (35, 53). A. borkumensis AP1 is a marine γ-Proteobacterium (62) which grows almost exclusively on n-alkanes. The same species was isolated from seawater at many geographic locations and forms a major part of the biomass in oil-polluted marine habitats (14). P. fluorescens CHA0 was originally not studied for the ability to grow on n-alkanes but is of interest as a biocontrol strain which excretes secondary metabolites toxic to soil-borne plant pathogens (12). P. aeruginosa PAO1 represents a species which is of clinical importance as the primary opportunistic pathogen among the pseudomonads (3) but is also common in soil and water and on plants. In contrast, M. tuberculosis is not found in the environment but is a typical and notorious example of the slow-growing pathogenic Mycobacteria. It is not able to grow on the paraffin-coated slides used in the so-called paraffin baiting method, unlike P. aeruginosa PAO1 and Mycobacterium avium (30, 32). P. rugosa NRRL B-2295 was isolated from cow rumen and was selected because it is a useful biocatalyst that converts cumene to 2-phenyl-1-propionic acid (22) if it is grown on n-alkanes.
Cloning of novel alkane hydroxylase genes and analysis of flanking regions.
The P. aeruginosa PAO1 genome sequence (46) encodes two alkane hydroxylase homologs (AlkB1 and AlkB2) while the genome sequence of M. tuberculosis H37Rv (5) encodes one alkane hydroxylase homolog. These putative alkane hydroxylase genes were cloned as described in Materials and Methods. Several unfinished genome sequences, such as those of L. pneumophila strain Philadelphia-1, Burkholderia pseudomallei K96243 and several mycobacteria, also contain an alkane hydroxylase homolog each. These sequences were included in the phylogenetic analysis but not in the functional studies. In a separate paper, the cloning and functional analysis of the B. cepacia RR10 alkB gene have been described (29). This sequence was also included in the phylogenetic analysis. Of the above alkB genes or gene homologs, only the H37Rv alkB gene is located close to other genes that are likely to be involved in alkane oxidation: it is followed by two rubredoxin genes and a regulatory protein with a TetR signature that is similar to proteins encoded downstream of other alkane hydroxylase genes (Whyte et al., submitted).
Additional alkane hydroxylase genes were cloned by using PCR fragments obtained previously with degenerate primers, which amplify internal segments of genes that are homologous to the P. putida GPo1 alkane hydroxylase and rubredoxin 2 (43, 52). A 7.8-kb fragment of P. fluorescens CHA0 DNA containing the alkane hydroxylase was cloned by using a 550-bp CHA0 alkB fragment (43) as a probe in Southern and colony blots. The DNA segment downstream of the alkane hydroxylase encodes two proteins (PraA and PraB) with homology to the so-called protein activator for alkane oxidation of P. aeruginosa PAO1 (15) and an outer membrane protein homologous to the OmpP1 proteins of Haemophilus influenzae.
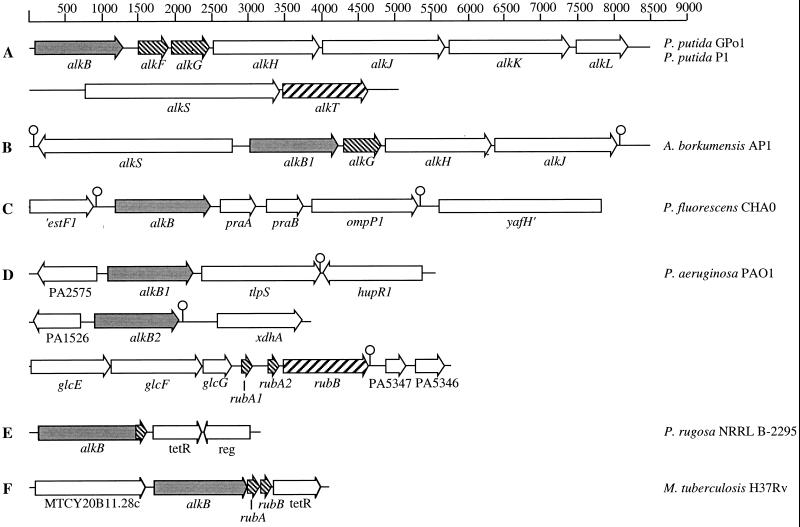
In the case of A. borkumensis AP1, we cloned a 3.5-kb EcoRI-BamHI fragment encoding a rubredoxin similar to the P. putida GPo1 AlkG (52). By cloning and sequencing adjacent DNA fragments, a contiguous sequence of 11.2 kb was assembled. Sequence analysis of this region showed that the alk gene organization in this strain is quite similar to that of P. putida strains GPo1 and P1 (Fig. 1) (53). The P. rugosa NRRL B-2295 alkB gene was cloned as a 3.0-kb SacI-BamHI chromosomal DNA fragment by using the 559-bp EcoRI-SacI alkB fragment of p2295 (43) as a probe. Sequence analysis of the 3.0-kb fragment showed that it encodes an alkane hydroxylase with a C-terminal extension that is highly homologous to rubredoxins involved in alkane hydroxylation (52). The region immediately downstream of the alkB gene encodes a possible regulatory protein with a TetR signature at its N terminus (pfam00440) quite homologous to the TetR protein encoded downstream of the M. tuberculosis H37Rv alkB-rubAB genes (26% full-length sequence identity) (Fig. 1). In the related Nocardioides sp. strain CF8, genes encoding very similar proteins were found: a rubredoxin domain fused to an alkane hydroxylase and a TetR protein encoded upstream of the alkB gene (13).
FIG. 1.
Organization of alk genes in different organisms. Alkane hydroxylases are indicated in grey; rubredoxin and rubredoxin reductase genes are indicated by fine and coarse hatches, respectively. (A) P. putida GPo1 and P. putida P1. alkB, alkane hydroxylase; alkF, rubredoxin 1; alkG, rubredoxin 2; alkH, aldehyde dehydrogenase; alkJ, alcohol dehydrogenase; alkK, acyl coenzyme A (acyl-CoA) synthetase; alkL, outer membrane protein; alkS, regulatory protein; alkT, rubredoxin reductase. (B) A. borkumensis AP1. alkS, putative regulatory protein; alkB1, alkane hydroxylase; alkG, rubredoxin; alkH, putative aldehyde dehydrogenase; alkJ, putative alcohol dehydrogenase. (C) P. fluorescens CHA0. ′estF1, esterase; alkB, alkane hydroxylase; praA and praB, protein activators of alkane oxidation; ompP1, outer membrane protein; yafH′, acyl-CoA dehydrogenase. (D) P. aeruginosa PAO1. PA2575, hypothetical protein; alkB1, alkane hydroxylase 1; tlpS, methyl-accepting chemotaxis protein; hupR1, regulatory protein of hydrogen uptake; PA1526, regulatory protein of GntR family; alkB2, alkane hydroxylase 2; xdhA, xanthine dehydrogenase homolog; glcEFG, genes homologous to the glcEFG genes of E. coli; rubA1, rubredoxin 1; rubA2, rubredoxin 2; rubB, rubredoxin reductase; PA5347 and PA5346, hypothetical proteins. (E) P. rugosa NRRL B-2295. alkB, alkane hydroxylase; tetR, putative regulatory protein of the TetR family; reg, putative regulatory protein. (F) M. tuberculosis H37Rv. MTCY20B11.28c, cationic transporter; alkB, alkane hydroxylase; rubA, rubredoxin 1; rubB, rubredoxin 2; tetR, putative regulatory protein of the TetR family.
Comparison of alkane hydroxylases.
In a peptide alignment of the full-length alkane hydroxylases (not shown), the six membrane-spanning segments identified in the P. putida GPo1 alkane hydroxylase (54), three histidine motifs conserved among the hydrocarbon oxygenases and desaturases (42), and a fourth motif (NYXEHYG[L/M]) identified in the alkane hydroxylases (43) are well conserved in all sequences. Most insertions and deletions in each sequence relative to the other AlkB sequences are located in predicted cytoplasmic or periplasmic domains before the third transmembrane helix. For example, a 40-amino-acid insertion in the P. fluorescens CHA0 AlkB is located in the periplasmic loop between the third and fourth putative transmembrane helices while other sequences have smaller insertions in the same loop. Insertions between the conserved histidine motifs are present in the M. tuberculosis, P. rugosa, and Nocardioides sp. alkane hydroxylases. Especially the hydrophobic segments and the N- and C-terminal ends of the alkane hydroxylases vary strongly in sequence.
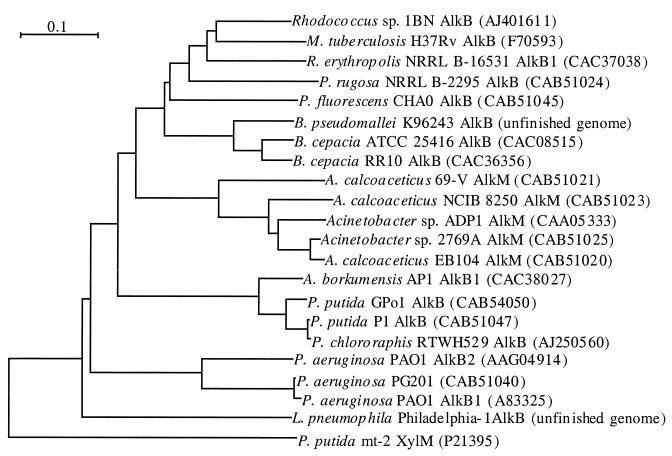
For a phylogenetic analysis of known and new alkane hydroxylases (Fig. 2) with Clustal (17), a peptide sequence alignment of the AlkB sequences corresponding to the 550-bp internal gene fragments (the sequence between the first and last histidine clusters) was generated and manually optimized. The peptide sequence identity among the alkane hydroxylases ranged from 37 to 99%. The corresponding fragment of the closest relative of the alkane hydroxylases, which does not oxidize n-alkanes, xylene monooxygenase (XylM), was also included in the analysis. It has 20 to 26% peptide sequence identity to the different alkane hydroxylases and is most closely related to the L. pneumophila Philadelphia-1 AlkB and the P. aeruginosa PAO1 AlkB1 and AlkB2.
FIG. 2.
Unrooted phylogenetic tree of partial sequences of published and hypothetical alkane hydroxylases. The distance tree was generated by Clustal from a manual alignment of the (putative) alkane hydroxylases. Only the segments corresponding to the 550-bp fragments obtained in PCR experiments with highly degenerate primers (based on histidine box 1 and histidine box 4) were used for the alignment. The XylM sequence was included as the out-group sequence.
Alkane hydroxylases from strains that oxidize medium-chain alkanes (P. putida GPo1, P. putida P1, A. borkumensis AP1, and P. chloroaphis RWTH529 [58]) are clustered in the phylogenetic tree (Fig. 2), whereas the putative alkane hydroxylases from long-chain alkane-degrading strains are highly divergent. Interestingly, alkane hydroxylases cloned from fluorescent pseudomonads are as divergent as the entire collection; while the P. fluorescens and Burkholderia sequences are quite closely related to gram-positive sequences, the P. aeruginosa sequences are quite distantly related to both the P. putida and P. fluorescens sequences.
Construction of P. fluorescens CHA0 alkB knockout mutants.
An alkB knockout mutant of P. fluorescens CHA0 was constructed by inserting a kanamycin resistance gene flanked by two res sites (27) in the alkB gene. This mutant, named KOB2, did not grow on C12 to C16 n-alkanes, unlike the parent strain. To reduce polar effects of the kanamycin resistance gene and its terminator on the expression of the PraA and PraB proteins, a homolog of which enhances the oxidation of alkanes in P. aeruginosa PAO1 (15, 18), these sequences were removed using resolvase (ParA) (27) expressed from the lac promoter on plasmid pUCPParA. The resulting strain was named KOB2Δ1.
KOB2Δ1 and KOB2 were transformed with pPF21, which encodes the P. fluorescens CHA0 alkane hydroxylase gene under the control of its own promoter, and compared with CHA0 with respect to growth on n-hexadecane. The growth rates of the three strains were similar, but the lag time of KOB2(pPF21) (around 200 h) was much longer than that of KOB2Δ1(pPF21) (around 100 h). We expect that KOB2 needs more time to produce sufficient amounts of PraAB for growth on alkanes, due to the polar effect of the kanamycin cassette. As the lag time of KOB2Δ1 is still somewhat longer than that of CHA0 (100 versus 80 h), the polar effect of the insertion in the alkB gene may not be eliminated completely. A 16-kDa band corresponding to PraB was detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in the culture supernatant of CHA0 and KOB2Δ1(pPF21) at time points t = 120 h and t = 168 h after inoculation but not in KOB2(pPF21). A very weak PraB band [estimated to be 10-fold-less strong than for CHA0 and KOB2Δ1(pPF21)] was observed in KOB2(pPF21) only at t = 216 h and later time points (data not shown).
Characterization of P. fluorescens KOB2Δ1: a second alkane hydroxylase system?
P. fluorescens KOB2Δ1 did not grow on alkanes up to 16 carbon atoms in length, unlike the parent strain CHA0. However, when KOB2Δ1 was grown on n-octadecane, growth was observed, with a growth rate 2 to 3 times lower than that of CHA0 (Table 2). Longer alkanes (C20 to C28), which are solid at 30°C, supported growth of both KOB2Δ1 and CHA0 when supplied as a 10% (vol/vol) solution in dioctylphthalate (an inert high-boiling solvent that is not a growth substrate for the strains used in this study). The growth rates of CHA0 and KOB2Δ1 on C20 to C28 n-alkanes dissolved in dioctylphthalate were comparable for both strains (data not shown). From these data we conclude that P. fluorescens CHA0 possesses a second alkane oxidation system, which oxidizes n-alkanes from C18 up to at least C28.
TABLE 2.
Growth rates of P. fluorescens CHA0 and KOB2Δ1 on n-alkanes ranging from C12 to C20
| Strain | Growth rate (h−1) ona:
|
||||
|---|---|---|---|---|---|
| C12 | C14 | C16 | C18 | C20 in DOPh | |
| CHA0 | 0.019 | 0.019 | 0.053 | 0.049 | 0.031 |
| KOB2Δ1 | —b | — | — | 0.015 | 0.028 |
C12, dodecane; C14, tetradecane; C16, hexadecane; C18, octadecane; C20 in DOPh, eicosane added as a 10% solution in dioctylphthalate (DOPh).
—, no growth.
Attempts to clone the second alkane hydroxylase gene by using PCR with the degenerate alkane hydroxylase primers described before (43) were not successful. In addition, Southern blots using probes based on several alkane hydroxylase genes, including the cloned CHA0 alkB gene, did not yield specific signals other than that corresponding to the cloned CHA0 alkB gene (43) (data not shown). Therefore, the second alkane hydroxylase gene must have clearly less than 70% DNA sequence identity to any of the cloned alkane hydroxylase genes, or it is a completely unrelated enzyme.
In vivo functional analysis of alkane hydroxylases.
Three different host systems for the functional expression of alkane hydroxylases were developed based on the observation that the second component of the P. putida GPo1 alkane hydroxylase system, the electron transfer protein rubredoxin (AlkG), can be replaced by rubredoxins from other alkane hydroxylase systems (52). The broad-host-range cosmid pGEc47ΔB, a deletion derivative of pGEc47 (9) that lacks part of the alkane hydroxylase gene alkB (54), expresses the P. putida GPo1 rubredoxin, rubredoxin reductase, and other proteins involved in the degradation of n-alkanes, in E. coli as well as in Pseudomonas species. Two hosts were used for this plasmid. E. coli GEc137 is a fadR mutant of E. coli DH-1, which grows on medium-chain-length fatty acids. The strain also grows on n-alkanes of the same length if it contains pGEc47 (9). P. putida GPo12 is a derivative of GPo1, cured of the OCT plasmid (25). Both host strains containing pGEc47ΔB did not show any detectable alkane hydroxylase activity (<0.01 U g of cells−1, or less than 0.06% of full activity) (54). Plasmids carrying the alkane hydroxylase gene of P. putida GPo1 fully restored the alkane hydroxylase activity of both recombinant strains, based on growth rates on n-octane and activity assays (data not shown).
All alkB gene homologs were expressed from PalkB, the promoter of the P. putida GPo1 alkane hydroxylase, by using the vectors pKKPalk, pCom7, and pCom8 (44), and a gratuitous inducer of PalkB, dicyclopropylketone (10). The alkane hydroxylases of P. putida P1 and A. borkumensis AP1, which are quite closely related to the P. putida GPo1 AlkB, complement the alkB deletion in E. coli GEc137(pGEc47ΔB) and P. putida GPo12(pGEc47ΔB) for growth on n-octane (Table 3). However, similar E. coli recombinants containing alkane hydroxylase genes cloned from strains able to grow on C12 to C16 n-alkanes did not grow on these n-alkanes. Some P. putida GPo12 recombinants containing the same genes grew on n-dodecane and also (but only poorly) on n-tetradecane if rhamnolipids were added (Table 4). Therefore, the third host, P. fluorescens KOB2Δ1, an alkB deletion mutant of P. fluorescens CHA0, was constructed as described above. KOB2Δ1 is not able to grow on C12 to C16 n-alkanes, unlike the parent strain, and could be complemented for growth on these n-alkanes by the native (CHA0) alkane hydroxylase gene cloned in pCom5 (Table 4). For the long-chain alkane hydroxylases, P. fluorescens KOB2Δ1 proved to be a much better host than E. coli or P. putida. The P. aeruginosa PAO1, P. fluorescens CHA0, M. tuberculosis H37Rv, and P. rugosa NRRL B-2295 alkane hydroxylases all restored growth of P. fluorescens KOB2Δ1 recombinants on at least one of the n-alkanes tested, albeit with growth rates that were sometimes clearly below that of the wild-type strain P. fluorescens CHA0 (Tables 3 and 4). Only the Acinetobacter sp. ADP1 alkM gene did not allow any of the three hosts to grow on any of the alkanes tested. The KOB2Δ1 recombinant containing the P. putida GPo1 alkB gene did not grow on C8 and C10 (known substrates of the GPo1 alkane hydroxylase), showed weak growth on n-dodecane, and did not grow on C14 and C16.
TABLE 3.
Functional analysis of alkane hydroxylases based on growth on alkane vapor on minimal medium plates
| Alkane hydroxylase | Resulta of functional analysis on host strain:
|
||
|---|---|---|---|
| E. coli GEc137(pGEc47ΔB) | P. putida GPo12(pGEc47ΔB) | P. fluorescens KOB2Δ1 | |
| P. putida GPo1 alkB | ++ (C6-C12) | ++ (C6-C12) | +(C12) |
| P. putida P1 alkB | ++ (only C8 was tested) | NT | NT |
| A. borkumensis AP1 alkB1 | ++ (C8-C12) | ++ (C8-C12) | NT |
| Acinetobacter sp. ADP1 alkM | − | − | − |
| P. aeruginosa PAO1 alkB1 | − | NT | + (C12-C16) |
| P. aeruginosa PAO1 alkB2 | − | + (C12-C14) | ++ (C12-C16) |
| P. fluorescens CHA0 alkB | − | + (C12-C14) | ++ (C12-C16) |
| M. tuberculosis H37Rv alkB | − | ++ (C10-C12) | ++ (C12-C16) |
| P. rugosa NRRL B-2295 alkB | NT | ++ (C10-C12) | ++ (C12-C16) |
−, no growth; +, poor growth; ++, good growth; NT, not tested. The substrates indicated in parentheses were tested and positive.
TABLE 4.
Growth rates of wild-type and alkB recombinant strains on n-alkanes
| Strain | Additional genec | Growth rate (h−1) ona:
|
||
|---|---|---|---|---|
| C12 | C14 | C16 | ||
| P. aeruginosa PAO1 | 0.036 | 0.069 | 0.1 | |
| P. fluorescens CHA0 | 0.019 | 0.019 | 0.053 | |
| P. putida GPo1b | 0.063 | − | − | |
| GPo12(pGEc47ΔB)b | alkB2 (PAO1) | 0.021 | 0.011 | − |
| alkB (CHA0) | 0.025 | 0.009 | − | |
| alkB (2295) | 0.033 | 0.010 | − | |
| alkB (H37Rv) | 0.077 | 0.011 | − | |
| KOB2Δ1 | alkB (GPo1) | 0.007 | − | − |
| alkB1 (PAO1) | 0.004 | 0.019 | 0.016 | |
| alkB2 (PAO1) | 0.033 | 0.032 | 0.046 | |
| alkB (CHA0) | 0.017 | 0.029 | 0.053 | |
| alkB (2295) | 0.014 | 0.050 | 0.049 | |
| alkB (H37Rv) | 0.043 | 0.041 | 0.038 | |
C12, n-dodecane; C14, n-tetradecane; C16, n-hexadecane; −, no growth.
Rhamnolipids were added to the GPo1 and GPo12(pGEc47ΔB) cultures.
See Fig. 1 for full name of original host strain.
DISCUSSION
Functional analysis of alkane hydroxylases.
To increase our understanding of enzyme systems involved in aerobic alkane degradation, we have started to clone alkane hydroxylase genes from a collection of about 200 bacteria able to grow on long- and short-chain alkanes. In this study, we used PCR products obtained with highly degenerate primers described earlier and information from genome sequencing projects to clone a number of alkane hydroxylase homologs from quite diverse strains. To be able to show that these genes encode functional alkane hydroxylases, we have developed several hosts for the expression of novel alkane hydroxylases. The best activity assay (in the absence of an in vitro assay) is growth on n-alkanes. Here, we have observed that the minimum level of activity needed for growth of a recombinant on n-octane is around 10 to 20% of the full GPo1 alkane hydroxylase activity (50). Therefore, any growth of the recombinants on n-alkanes indicates a significant level of alkane hydroxylase activity.
E. coli GEc137(pGEc47ΔB) and P. putida GPo12(pGEc47ΔB) were found to be suitable hosts to test medium-chain alkane hydroxylase genes (oxidizing alkanes ranging from C6 to C12) (Table 3). Some recombinants derived from GPo12 are also able to grow on n-tetradecane if rhamnolipids are added but do so quite slowly. It is likely that uptake of longer n-alkanes requires factors (for example, porins and alkane-solubilizing compounds) that are not produced or expressed by GPo12. For this reason, we constructed an alkB knockout mutant of P. fluorescens CHA0 in which the polar effects of the alkB gene knockout on the alkane solubilization proteins and (putative) uptake system, encoded further downstream, are reduced. This host, KOB2Δ1, does indeed allow complementation for growth on longer alkanes by alkane hydroxylases cloned from quite diverse organisms, including two gram-positive strains (Tables 3 and 4). The Acinetobacter sp. ADP1 alkM gene could not be expressed successfully in the E. coli or Pseudomonas hosts, possibly because of the markedly different distribution of positive charges near the transmembrane stretches (data not shown): about seven positive charges are located in the three periplasmatic loops of AlkM and other Acinetobacter sp. AlkB homologs versus, on average, only one in AlkB homologs from other bacteria. The distribution of positive charges is important for correct folding in the cytoplasmic membrane (59), and it is conceivable that AlkM does not fold properly in an E. coli or Pseudomonas membrane. In contrast, the Acinetobacter sp. ADP1 rubredoxin could be expressed successfully in E. coli and shows exactly the same charge distribution as other rubredoxins involved in alkane hydroxylation (52).
The GPo1 alkB gene did not allow KOB2Δ1 to grow on alkanes shorter than C12, possibly because the CHA0 rubredoxin and rubredoxin reductase, which we have not cloned yet, are not induced by alkanes shorter than C12. The related P. fluorescens strain Pf0-1, the genome of which is being sequenced, possesses a rubredoxin and a rubredoxin reductase that are closely related to proteins that were shown to function in alkane hydroxylation (52), but the Pf0-1 genome does not encode an alkane hydroxylase homolog.
Structure of membrane-bound alkane hydroxylases.
The above expression and complementation systems allowed us to demonstrate that homologs of the GPo1 alkB gene indeed encode functional alkane hydroxylases. This allows the comparison of sequences to gain insight in the structure-function relationships of this class of enzymes. The P. putida GPo1 alkane hydroxylase is well studied, mainly because of its potential applications in biocatalysis (61). However, our present knowledge on the structure-function relationship of this class of integral membrane oxygenases is limited to the folding topology (54) and the likely involvement of conserved histidines in the binding of the two active-site iron atoms (40). The alkane hydroxylases that were analyzed in this study show significant sequence divergence, but the membrane folding of these alkane hydroxylases appears to follow the same pattern: the six hydrophobic stretches that are likely to span the cytoplasmic membrane (54) are conserved in all sequences. The same is true for the conserved histidines (42, 43) that are essential for enzymes related to the alkane hydroxylases and are likely to contribute to the nitrogen-rich ligand sphere of the catalytic diiron center (40). It is not yet possible to identify residues involved in other aspects of alkane hydroxylase function. Residues that are involved in the binding of rubredoxin are likely to be conserved between all alkane hydroxylases, as rubredoxins can be exchanged between alkane hydroxylase systems from all strains tested (52). However, these residues cannot be distinguished from other conserved residues. Similarly, the four medium-chain-length alkane hydroxylases are too closely related to each other to distinguish residues conserved by chance from residues conserved because of functional constraints.
Organization of genes involved in alkane degradation.
The organization of genes involved in alkane oxidation varies strongly among the different alkane-degrading bacteria (Fig. 1). In most strains, genes involved in alkane degradation seem to be distributed over the genome. None of the rubredoxin reductase genes is located close to an alkane hydroxylase (except in the case of Rhodococcus erythropolis [Whyte et al., submitted]), perhaps because they are also involved in other pathways and require a different type of regulation. In contrast, most rubredoxin genes are located immediately downstream of the alkane hydroxylase genes. Those that are located elsewhere are encoded directly upstream of rubredoxin reductase genes. Interestingly, the alk gene cluster around alkB1 of A. borkumensis is very similar to the alk gene clusters of P. putida strains GPo1 and P1, with respect to gene organization as well as sequence. The P. putida alk genes have a significantly lower G+C content than the rest of the genome, and are encoded on a putative catabolic transposon (53). These comparisons suggest that the A. borkumensis alk genes may have ended up in this strain by horizontal gene transfer as well. However, in this strain, the alkane hydroxylase can be considered an almost essential enzyme, as this bacterium grows on very few other carbon sources, such as pyruvate, and is found mainly in oil-contaminated seawater. Moreover, the G+C content of these genes closely resembles that determined for the total genome of A. borkumensis (62).
The presence of alkane hydroxylases in the genome sequences of L. pneumophila Philadelphia-1, B. pseudomallei K96243, and P. aeruginosa PAO1 may reflect the double nature of these organisms as pathogens and as common soil or water organisms. Alkanes are omnipresent in the environment, and microorganisms are likely to utilize these highly reduced compounds as carbon and energy sources. This explains why it is easy to isolate alkane-degrading strains from pristine soil, aquifers recently polluted with oil (36), and oil-polluted seawater. The alkane hydroxylase of M. tuberculosis H37Rv may be a relic of an earlier lifestyle. Alternatively, M. tuberculosis still has an unrecognized reservoir in the environment where alkanes are available as C sources.
Conclusions.
While the previous PCR studies (43) and other data (J. B. van Beilen, T. H. M. Smits, L. G. Whyte, S. Schorcht, M. Röthlisberger, T. Plaggemeier, K.-H. Engesser, and B. Witholt, submitted for publication) show that many alkane-degrading strains possess homologs of the P. putida GPo1 alkane hydroxylase, this study shows that a random selection of such homologs indeed encode functional alkane hydroxylases. The three host systems constructed in this study allowed us to show that nearly all alkane hydroxylase homologs are functional and allowed us to demonstrate that they oxidize medium-chain (C5 to C11) or long-chain (C12 to C16) n-alkanes. Because CHA0 contains a second alkane hydroxylase responsible for the oxidation of C18 to C28 n-alkanes, the KOB2Δ1 recombinants did not yield information on the ability of cloned alkane hydroxylases to oxidize n-alkanes longer than C16. The complementation experiments show that long-chain alkane hydroxylases show little activity with medium-chain-length alkanes and vice versa, thereby providing us with a powerful selection method to change the substrate range of the cloned alkane hydroxylases and to identify residues involved in substrate binding.
Acknowledgments
We thank M. Röthlisberger for DNA sequencing; P. Golyshin for the gift of A. borkumensis isolates AP1, SK2, and SK7; A. G. Franchini for assistance with cloning of the A. borkumensis AP1 alkB gene; H. P. Schweizer for supplying us with pUCP24 and pEX18Tc; J. M. Sanchez-Romero for pCK217 and pJMSB8; and S. T. Cole for pSCYB11. Special thanks for helpful discussions go to L. Whyte, NRC, Montreal, Canada.
This research was supported by the Swiss Priority Program in Biotechnology of the Swiss National Science Foundation, project no. 5002-037023.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Birnboim, H. C., and J. Doly. 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Botzenhart, K., and G. Döring. 1993. Etiology and epidemiology of Pseudomonas aeruginosa, p. 1-18. In M. Campa, M. Bendinelli, and H. Friedman (ed.), Pseudomonas aeruginosa as an opportunistic pathogen. Plenum Press, New York, N.Y.
- 4.Bühler, M., and J. Schindler. 1984. Aliphatic hydrocarbons, p. 329-385. In K. Kieslich (ed.), Biotransformations, vol. 6a. Verlag Chemie Weinheim, Weinheim, Germany. [Google Scholar]
- 5.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLeah, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Soeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrett. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 6.Ditta, G., S. Stanfield, D. Corbin, and D. R. Helinski. 1980. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 77:7347-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dower, W. J., J. F. Miller, and C. W. Ragsdale. 1988. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 16:6127-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eggink, G., H. Engel, W. Meijer, J. Otten, J. Kingma, and B. Witholt. 1988. Alkane utilization in Pseudomonas oleovorans. Structure and function of the regulatory locus alkR. J. Biol. Chem. 263:13400-13405. [PubMed] [Google Scholar]
- 9.Eggink, G., R. G. Lageveen, B. Altenburg, and B. Witholt. 1987. Controlled and functional expression of Pseudomonas oleovorans alkane utilizing system in Pseudomonas putida and Escherichia coli. J. Biol. Chem. 262:17712-17718. [PubMed] [Google Scholar]
- 10.Grund, A., J. Shapiro, M. Fennewald, P. Bacha, J. Leahy, K. Markbreiter, M. Nieder, and M. Toepfer. 1975. Regulation of alkane oxidation in Pseudomonas putida. J. Bacteriol. 123:546-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerra-Santos, L. H., O. Käppeli, and A. Fiechter. 1986. Dependence of Pseudomonas aeruginosa continuous culture biosurfactant production on nutritional and environmental factors. Appl. Microbiol. Biotechnol. 24:443-448. [Google Scholar]
- 12.Haas, D., C. Blumer, and C. Keel. 2000. Biocontrol ability of fluorescent pseudomonads genetically dissected: importance of positive feedback regulation. Curr. Opin. Biotechnol. 11:290-297. [DOI] [PubMed] [Google Scholar]
- 13.Hamamura, N., C. M. Yeager, and D. J. Arp. 2001. Two distinct monooxygenases for alkane oxidation in Nocardioides sp. strain CF8. Appl. Environ. Microbiol. 67:4992-4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harayama, S., H. Kishira, Y. Kasai, and K. Shutsubo. 1999. Petroleum biodegradation in marine environments. J. Mol. Microbiol. Biotechnol. 1:63-70. [PubMed] [Google Scholar]
- 15.Hardegger, M., A. K. Koch, U. A. Ochsner, A. Fiechter, and J. Reiser. 1994. Cloning and heterologous expression of a gene encoding an alkane-induced extracellular protein involved in alkane assimilation from Pseudomonas aeruginosa. Appl. Environ. Microbiol. 60:3679-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 172:6557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins, D. G., and P. M. Sharp. 1988. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene 73:237-244. [DOI] [PubMed] [Google Scholar]
- 18.Hisatsuka, K., T. Nakahara, and K. Yamada. 1972. Protein-like activator for n-alkane oxidation by Pseudomonas aeruginosa S7B1. Agric. Biol. Chem. 36:1361-1369. [Google Scholar]
- 19.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 20.Højberg, O., U. Schnider, H. V. Winteler, J. Sørensen, and D. Haas. 1999. Oxygen-sensing reporter strain of Pseudomonas fluorescens for monitoring the distribution of low-oxygen habitats in soil. Appl. Environ. Microbiol. 65:4085-4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holloway, B. W. 1969. Genetics of Pseudomonas. Bacteriol. Rev. 33:419-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou, C. T., M. A. Jackson, M. O. Bagby, and L. A. Becker. 1994. Microbial oxidation of cumene by octane-grown cells. Appl. Microbiol. Biotechnol. 41:178-182. [Google Scholar]
- 23.Juni, E., and A. Janik. 1969. Transformation of Acinetobacter calco-aceticus (Bacterium anitratum). J. Bacteriol. 98:281-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koch, A. K., O. Käppeli, A. Fiechter, and J. Reiser. 1991. Hydrocarbon assimilation and biosurfactant production in Pseudomonas aeruginosa mutants. J. Bacteriol. 173:4212-4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kok, M. 1988. Alkane utilization by Pseudomonas oleovorans. Ph.D. thesis. University of Groningen, Groningen, The Netherlands.
- 26.Kok, M., R. Oldenhuis, M. P. G. van der Linden, P. Raatjes, J. Kingma, P. H. van Lelyveld, and B. Witholt. 1989. The Pseudomonas oleovorans alkane hydroxylase gene. Sequence and expression. J. Biol. Chem. 264:5435-5441. [PubMed] [Google Scholar]
- 27.Kristensen, C. S., L. Eberl, J. M. Sanchez-Romero, M. Givskov, S. Molin, and V. de Lorenzo. 1995. Site-specific deletions of chromosomally located DNA segments with the multimer resolution system of broad-host-range plasmid RP4. J. Bacteriol. 177:52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lageveen, R. G., G. W. Huisman, H. Preusting, P. E. F. Ketelaar, G. Eggink, and B. Witholt. 1988. Formation of polyester by Pseudomonas oleovorans: the effect of substrate on the formation and composition of poly-(R)-3-hydroxyalkanoates and poly-(R)-3-hydroxyalkenoates. Appl. Environ. Microbiol. 54:2924-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marin, M. M., T. H. M. Smits, J. B. van Beilen, and F. Rojo. 2001. The alkane hydroxylase gene of Burkholderia cepacia RR10 is under catabolite repression control. J. Bacteriol. 183:4202-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massengale, A. R. D., R. A. Ollar, S. J. Giordano, M. S. Felder, and S. C. Aronoff. 1999. Use of the paraffin wax baiting system for identification of Pseudomonas aeruginosa clinical isolates. Diagn. Microbiol. Infect. Dis. 35:177-183. [DOI] [PubMed] [Google Scholar]
- 31.McKenna, E. J., and M. J. Coon. 1970. Enzymatic ω-oxidation. IV. Purification and properties of the ω-hydroxylase of Pseudomonas oleovorans. J. Biol. Chem. 245:3882-3889. [PubMed] [Google Scholar]
- 32.Ollar, R. A., J. W. Dale, M. S. Felder, and A. Favate. 1990. The use of paraffin wax metabolism in the speciation of Mycobacterium avium-intracellulare. Tubercle 71:23-28. [DOI] [PubMed] [Google Scholar]
- 33.Peterson, J. A., D. Basu, and M. J. Coon. 1966. Enzymatic ω-oxidation. I. Electron carriers in fatty acid and hydrocarbon hydroxylation. J. Biol. Chem. 241:5162-5164. [PubMed] [Google Scholar]
- 34.Philipp, W. J., S. Poulet, K. Eiglmeier, L. Pascopella, V. Balasubramanian, B. Heym, S. Bergh, B. R. Bloom, W. R. J. Jacobs, and S. T. Cole. 1996. An integrated map of the genome of the tubercle bacillus, Mycobacterium tuberculosis H37Rv, and comparison with Mycobacterium leprae. Proc. Natl. Acad. Sci. USA 93:3132-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ratajczak, A., W. Geißdörfer, and W. Hillen. 1998. Alkane hydroxylase from Acinetobacter sp. strain ADP-1 is encoded by alkM and belongs to a new family of bacterial integral-membrane hydrocarbon hydroxylases. Appl. Environ. Microbiol. 64:1175-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ridgway, H. F., J. Safarik, D. Phipps, P. Carl, and D. Clark. 1990. Identification and catabolic activity of well-derived gasoline-degrading bacteria from a contaminated aquifer. Appl. Environ. Microbiol. 56:3565-3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenberg, E., and E. Z. Ron. 1996. Bioremediation of petroleum contamination, p. 100-124. In R. L. Crawford and D. L. Crawford (ed.), Bioremediation: principles and applications, vol. 6. Cambridge University Press, Cambridge, England. [Google Scholar]
- 38.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, New York, N.Y.
- 39.Schorcht, S. 1998. Mikrobiologische und molekularbiologische Charakterisierung alkanabbauender Bakteriengemeinschaften. Ph.D. thesis. Universität Bremen, Bremen, Germany.
- 40.Shanklin, J., C. Achim, H. Schmidt, B. G. Fox, and E. Münck. 1997. Mössbauer studies of alkane ω-hydroxylase: evidence for a diiron cluster in an integral-membrane protein. Proc. Natl. Acad. Sci. USA 94:2981-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shanklin, J., and E. B. Cahoon. 1998. Desaturation and related modifications of fatty acids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49:611-641. [DOI] [PubMed] [Google Scholar]
- 42.Shanklin, J., E. Whittle, and B. G. Fox. 1994. Eight histidine residues are catalytically essential in a membrane-associated iron enzyme, stearoyl-CoA desaturase, and are conserved in alkane hydroxylase and xylene monooxygenase. Biochemistry 33:12787-12794. [DOI] [PubMed] [Google Scholar]
- 43.Smits, T. H. M., M. Röthlisberger, B. Witholt, and J. B. van Beilen. 1999. Molecular screening for alkane hydroxylase genes in Gram-negative and Gram-positive strains. Environ. Microbiol. 1:307-318. [DOI] [PubMed] [Google Scholar]
- 44.Smits, T. H. M., M. A. Seeger, B. Witholt, and J. B. van Beilen. 2001. New alkane-responsive expression vectors for E. coli and Pseudomonas. Plasmid 46:16-24. [DOI] [PubMed] [Google Scholar]
- 45.Sotsky, J. B., C. W. Greer, and R. M. Atlas. 1994. Frequency of genes in aromatic and aliphatic hydrocarbon biodegradation pathways within bacterial populations from Alaskan sediments. Can. J. Microbiol. 40:981-985. [DOI] [PubMed] [Google Scholar]
- 46.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. L. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K.-S. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. W. Hancock, S. Lory, and M. V. Olsen. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 47.Stutz, E. W., G. Défago, and H. Kern. 1986. Naturally occurring fluorescent pseudomonads involved in suppression of black root rot of tobacco. Phytopathology 76:181-185. [Google Scholar]
- 48.Suzuki, M., T. Hayakawa, J. P. Shaw, M. Rekik, and S. Harayama. 1991. Primary structure of xylene monooxygenase: similarities to and differences from the alkane hydroxylation system. J. Bacteriol. 173:1690-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ueda, T., E. T. Lode, and M. J. Coon. 1972. Enzymatic ω-oxidation. VI. Isolation of homogeneous reduced diphosphopyridine nucleotide-rubredoxin reductase. J. Biol. Chem. 247:2109-2116. [PubMed] [Google Scholar]
- 50.van Beilen, J. B. 1994. Alkane oxidation by Pseudomonas oleovorans: genes and proteins. Ph.D. thesis. University of Groningen, Groningen, The Netherlands.
- 51.van Beilen, J. B., J. Kingma, and B. Witholt. 1994. Substrate specificity of the alkane hydroxylase of Pseudomonas oleovorans GPo1. Enzyme Microb. Technol. 16:904-911. [Google Scholar]
- 52.van Beilen, J. B., M. Neuenschwander, T. H. M. Smits, C. Roth, S. B. Balada, and B. Witholt. 2002. Rubredoxins involved in alkane oxidation. J. Bacteriol. 184:1722-1732. [DOI] [PMC free article] [PubMed]
- 53.van Beilen, J. B., S. Panke, S. Lucchini, A. G. Franchini, M. Röthlisberger, and B. Witholt. 2001. Analysis of Pseudomonas putida alkane degradation gene clusters and flanking insertion sequences: evolution and regulation of the alk-genes. Microbiology 147:1621-1630. [DOI] [PubMed] [Google Scholar]
- 54.van Beilen, J. B., D. Penninga, and B. Witholt. 1992. Topology of the membrane-bound alkane hydroxylase of Pseudomonas oleovorans. J. Biol. Chem. 267:9194-9201. [PubMed] [Google Scholar]
- 55.van Beilen, J. B., L. Veenhoff, and B. Witholt. 1998. Alkane hydroxylase systems in Pseudomonas aeruginosa strains able to grow on n-octane, p. 211-215. In K. Kieslich, C. P. V. D. Beek, J. A. M. D. Bont, and W. J. J. V. D. Tweel (ed.), New frontiers in screening for microbial biocatalysts, vol. 53. Elsevier Science B.V., Amsterdam, The Netherlands. [Google Scholar]
- 56.van Beilen, J. B., M. G. Wubbolts, and B. Witholt. 1994. Genetics of alkane oxidation by Pseudomonas oleovorans. Biodegradation 5:161-174. [DOI] [PubMed] [Google Scholar]
- 57.Vogel, H. J., and D. M. Bonner. 1956. Acetylornithinase of Escherichia coli: partial purification and some properties. J. Biol. Chem. 218:97-106. [PubMed] [Google Scholar]
- 58.Vomberg, A., and U. Klinner. 2000. Distribution of alkB genes within n-alkane-degrading bacteria. J. Appl. Microbiol. 89:339-348. [DOI] [PubMed] [Google Scholar]
- 59.Von Heijne, G., and Y. Gavel. 1988. Topogenic signals in integral membrane proteins. Eur. J. Biochem. 174:671-678. [DOI] [PubMed] [Google Scholar]
- 60.West, S. E. H., H. P. Schweizer, C. Dall, A. K. Sample, and L. J. Runyenjanecky. 1994. Construction of improved Escherichia-Pseudomonas shuttle vectors derived from pUC18/19 and sequence of the region required for their replication in Pseudomonas aeruginosa. Gene 148:81-86. [DOI] [PubMed] [Google Scholar]
- 61.Witholt, B., M. J. de Smet, J. Kingma, J. B. van Beilen, M. Kok, R. G. Lageveen, and G. Eggink. 1990. Bioconversions of aliphatic compounds by Pseudomonas oleovorans in multiphase bioreactors: background and economic potential. Trends Biotechnol. 8:46-52. [DOI] [PubMed] [Google Scholar]
- 62.Yakimov, M. M., P. N. Golyshin, S. Lang, E. R. B. Moore, W.-R. Abraham, H. Lünsdorf, and K. N. Timmis. 1998. Alcanivorax borkumensis gen. nov., sp. nov., a new, hydrocarbon-degrading and surfactant-producing marine bacterium. Int. J. Syst. Bacteriol. 48:339-348. [DOI] [PubMed] [Google Scholar]
- 63.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]