Abstract
Selenophosphate synthetase (SPS), the selD gene product from Escherichia coli, catalyzes the biosynthesis of monoselenophosphate, AMP, and orthophosphate in a 1:1:1 ratio from selenide and ATP. It was recently demonstrated that selenium delivered from selenocysteine by an E. coli NifS-like protein could replace free selenide in the in vitro SPS assay for selenophosphate formation (G. M. Lacourciere, H. Mihara, T. Kurihara, N. Esaki, and T. C. Stadtman, J. Biol. Chem. 275:23769-23773, 2000). During growth of E. coli in the presence of 0.1 μM 75SeO32− and increasing amounts of l-selenocysteine, a concomitant decrease in 75Se incorporation into formate dehydrogenase H and nucleosides of bulk tRNA was observed. This is consistent with the mobilization of selenium from l-selenocysteine in vivo and its use in selenophosphate formation. The ability of E. coli to utilize selenocysteine as a selenium source for selenophosphate biosynthesis in vivo supports the participation of the NifS-like proteins in selenium metabolism.
Selenophosphate synthetases (SPS) from Escherichia coli (18) and the closely related enzyme from Haemophilus influenzae (7) have been characterized. In the presence of high levels of free selenide (1 to 5 mM) and dithiothreitol (8 to 10 mM) that are included in the in vitro assay system, the apparent Km values for ATP and selenide are 1 mM and 20 μM, respectively. A Km value of 20 μM for selenide is far above the optimal concentration (0.1 to 1 μM) needed for the growth of various bacterial species and cultured mammalian cells. In fact, levels above 10 μM are toxic for many bacterial species. Considering that intracellular concentrations of selenium should not approach toxic levels, a mechanism to provide SPS with adequate amounts of selenium may be necessary.
Genetic analysis of iron-sulfur cluster formation in the nitrogenase protein from Azotobacter vinelandii led to the identification of a pyridoxal 5′-phosphate-dependent protein. This protein, referred to as NifS, catalyzed the desulfurization of cysteine to alanine and a sulfur transfer form, referred to as S* (19). (The products of the cysteine desulfurase and selenocysteine lyase reactions have been referred to in the literature as elemental sulfur [S0] and elemental selenium [Se0]. Designation of the product as S0 or Se0 implies that the redox state of the product is known. Since the redox state has not been determined, it is now proposed by Klaus-Dieter Asmus of Notre Dame University that both products can be designated as a sulfur transfer form [S*] and as a selenium transfer form [Se*] until a redox state is determined.) In vitro experiments performed with an inactive apo-Fe nitrogenase demonstrated that the NifS protein and l-cysteine could replace high concentrations of sulfide required for the reconstitution of the iron-sulfur cluster in nitrogenase, which resulted in a recovery of 85 to 90% of the original enzymatic activity (20). NifS homologs have since been identified in both prokaryotes and eukaryotes. The cysteine desulfurase activity is essential in the delivery of sulfur for iron-sulfur cluster formation (18) and the biosynthesis of thiamine, biotin, and 4-thiouridine (s4U) in tRNA (9). In addition to cysteine, NifS-like proteins also act on selenocysteine as the substrate. Members of a similar family of proteins, the selenocysteine lyase (SCL) enzymes, resemble NifS in both structure and function (2, 3). SCL enzymes catalyze the pyridoxal 5′-phosphate-dependent decomposition of selenocysteine to alanine and a selenium transfer form referred to as Se*. SCL enzymes exhibit high specificity towards selenocysteine with little or no reactivity towards cysteine.
The chemical similarity between selenium and sulfur allows selenium to enter the bacterial metabolism via the cysteine biosynthetic pathway, where it can be incorporated into free selenocysteine and selenomethionine (14). Selenocysteine can be inserted into proteins nonspecifically, or a lyase can utilize it to generate selenium. The possibility that SCL enzymes can function as selenium delivery proteins for SPS has been considered (6, 8) The generation of selenium near SPS may be an attractive solution to the obstacle of selenide toxicity and the high Km value for selenide exhibited by SPS.
Recent in vitro experiments demonstrated that the NifS protein from A. vinelandii (6) and the three NifS-like proteins from E. coli (8) can effectively mobilize selenium from free selenocysteine to SPS as a substrate. In fact, determined SPS activity was greater than with selenide alone as the substrate. In the present study, in vivo evidence is presented to support the participation of the E. coli NifS-like proteins as components of a selenium delivery system for the biosynthesis of selenophosphate.
MATERIALS AND METHODS
Materials.
l-selenocystine was synthesized (20) and converted to l-selenocysteine as reported previously (4). [75Se]selenite was purchased from The University of Missouri Research Reactor Facility, Columbia, Mo. pFN52 was provided by August Böck, Universitat München. E. coli strain RL165 (cysK511) was obtained from the E. coli genetic stock center.
Methods. (i) Selenium-dependent expression of β-galactosidase.
E. coli RK4353 (MC4100 gyr A219 non-9) was transformed with pFM52, a plasmid containing the gene encoding the first 258 amino acids (including the in-frame TGA codon corresponding to the amino acid at position 140) of formate dehydrogenase H (FDHH) fused to the lacZ gene. Cultures were grown anaerobically at 37°C in low-sulfur minimal medium containing 100 mM K2HPO4, 100 mM NaNH4HPO4, and 150 mM citric acid, supplemented with 10 μM NiCl2, 7.5 μM FeCl3, 500 μM reduced glutathione, 10 μM Na2MoO4, 5 μM MnCl2, 0.5% glucose, 30 mM formate, 55 μg of thiamine, 420 μg of 18 amino acids (except cysteine and methionine), and the indicated concentration of selenite or l-selenocysteine. Cells were harvested by centrifugation at 3,000 × g and suspended in a solution containing 100 mM potassium phosphate buffer (pH 7.0), 10 mM KCl, 1 mM MgSO4, and 50 mM β-mercaptoethanol. β-galactosidase activity was determined according to the method of Miller (13) using O-nitrophenyl-β-d-galactopyranoside as a substrate.
(ii) Incorporation of selenium from selenocysteine into formate dehydrogenase H.
E. coli strains RK4353 or RL165 were grown anaerobically at 37°C overnight in Luria-Bertani (LB) medium containing 0.1 μM SeO32− and 20 μCi of 75SeO32−. Additional cultures were supplemented with the indicated concentrations of l-selenocysteine. After growth, cells were harvested, resuspended in 100 mM potassium phosphate buffer (pH 7.0), and lysed by freeze-thawing in liquid N2. Aliquots of supernatants containing 11 to 12 μg of protein were analyzed for 75Se-containing FDHH by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and PhosphoImager analysis.
(iii) Incorporation of selenium from selenocysteine into the nucleosides of bulk tRNA.
One milliliter of clarified extract was extracted with 1 ml of phenol. After the addition of 0.2 ml of 5 M NaCl, the mixture was centrifuged to separate phases. Two volumes of ethanol were added to the aqueous phase. After several hours at −20°C, the precipitate was collected by centrifugation. The tRNA was dissolved in 0.3 ml of 0.5 M Tris·HCl (pH 8.5) to deacylate the esterified amino acids and again precipitated with 2 volumes of ethanol.
RESULTS AND DISCUSSION
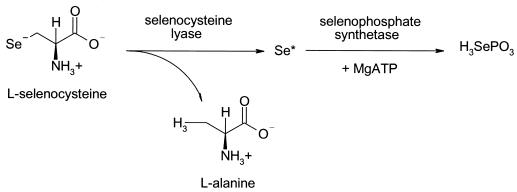
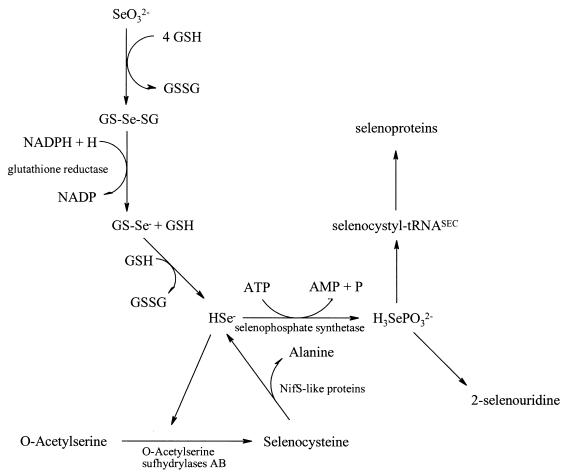
The mobilization of sulfur from free cysteine is a major step in the pathways for iron-sulfur cluster formation, biotin, s4U in tRNAs, and thiamine biosynthesis (9, 15). The participation of cysteine desulfurase enzymes allows the direct delivery of sulfur from cysteine to occur and prevents intracellular levels of sulfide from increasing to toxic levels. Since selenium is much more toxic than sulfur, E. coli must be able to tightly control levels of free selenium. A similar mechanism which utilizes SCL enzymes as selenium delivery proteins for selenophosphate biosynthesis has been considered (Fig. 1).
FIG. 1.
Proposed mechanism for selenocysteine lyase enzymes participating as selenium delivery proteins for selenophosphate synthetase.
The apparent participation of SCL enzymes in selenophosphate biosynthesis was previously observed in vivo. In early attempts to understand the mechanism of incorporation of selenocysteine into proteins, 3H-, 14C-, and 75Se-labeled selenocysteine samples were prepared and added to cultures of Clostridium sticklandii. 75Se derived from [75Se]selenocysteine was found to be incorporated into selenoprotein A of the glycine reductase complex more efficiently than 75SeO32−. In contrast, there was no detectable incorporation of 14C or 3H (16). In view of the fact that the specific insertion of selenocysteine into selenoproteins requires selenophosphate as a selenium donor, the involvement of an SCL as a provider of the essential selenium substrate for SPS seems likely.
It was also demonstrated that each of the three E. coli NifS-like proteins as well as the A. vinelandii NifS protein can participate as a component of a selenium delivery system for SPS in vitro (6, 8). In fact, synthesis of selenophosphate was even greater when selenium was supplied from l-selenocysteine by a NifS-like protein than when free selenide was provided as a substrate. To establish a relevant role for the E. coli NifS-like proteins in selenium metabolism, in vivo experiments designed to mobilize selenium from free selenocysteine and to detect its incorporation into FDHH and 2-selenouridine nucleosides of tRNA were carried out.
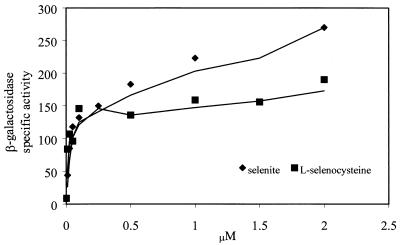
The fdhF-lacZ fusion plasmid pFM52 was originally constructed to demonstrate that selenocysteine enters a polypeptide chain as directed by UGA in the mRNA (22). pFM52 contains the nucleotide sequence encoding the first 268 amino acids of the selenocysteine-containing FDHH fused to the full-length β-galactosidase gene. The insertion of selenocysteine at amino acid 140 of FDHH is dependent on the selC gene product tRNASec. Initially the tRNASec is charged with serine. The conversion of seryl-tRNASec to selenocystyl-tRNASec requires the selA gene product, selenocysteine synthetase, and selenophosphate. In this study, cells transformed with pFM52 were tested for β-galactosidase activity after growth in selenium-deficient minimal medium as well as in medium supplemented with SeO32− or l-selenocysteine. In the absence of added selenium, a low level of β-galactosidase activity could be detected (8.8 nmol/min/mg). A selenium-dependent expression of the reporter β-galactosidase was observed when cultures were supplemented with selenium in the form of SeO32− or l-selenocysteine (Fig. 2).
FIG. 2.
Effect of selenite and l-selenocysteine on the expression level of β-galactosidase in the pFM52 fdhF-lacZ fusion plasmid.
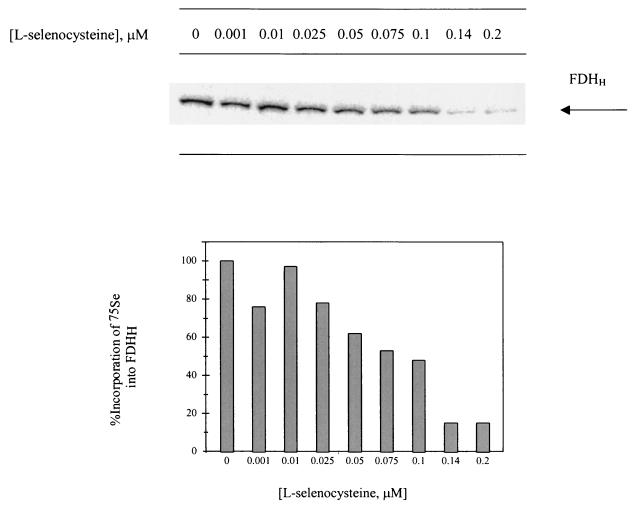
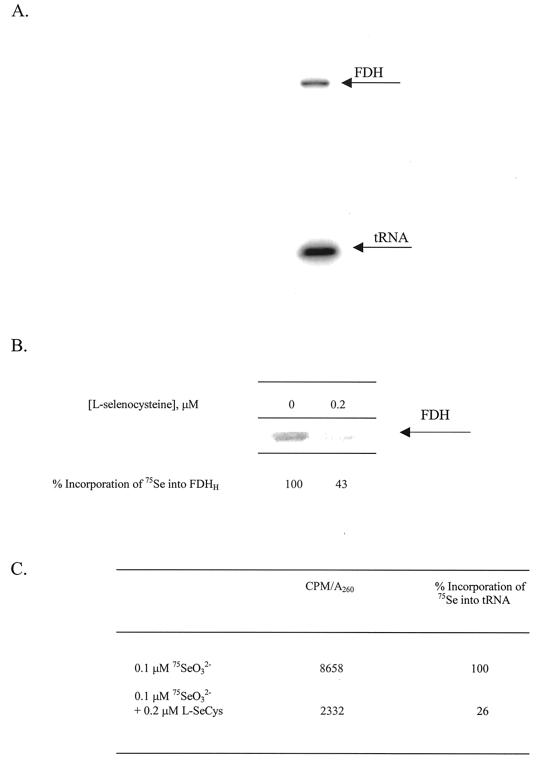
Cultures of E. coli strain RK4353 were grown anaerobically in LB containing 0.1 μM 75SeO32− at 37°C overnight. Quantitation of 75Se incorporation in FDHH from a culture grown with no added l-selenocysteine revealed 603 cpm in the FDHH protein band. A decrease in the [75Se]selenocysteine content of FDHH was observed when the same E. coli strain was grown on 75SeO32− in the presence of increasing concentrations of unlabeled l-selenocysteine. (Selenite could react with free selenocysteine as it does readily with any sulfyhryl compound in the medium. These reactions have not been studied in detail. However, RS-Se-SR derivatives are commonly made with selenite and 4 RSH. Eventually, with excess RSH, the selenium is reduced to RS-Se− and eventually HSe−. For growth of anaerobic bacteria, 0.5 to 1 μM selenite and 30 μM sodium sulfide are routinely added to cultures. Under these conditions up to 60% or more of the selenium from selenite is incorporated into proteins and tRNAs. Thus, based on wide experience with these selenite-reducing system conditions, the possibility of loss of selenium from added selenite is unlikely.) Comparison of the amounts of radioactivity incorporated into FDHH showed that as the concentration of l-selenocysteine is increased in cultures, there is a progressive dilution of radioactivity into FDHH (Fig. 3). Supplementation of l-selenocysteine up to 0.2 μM in cultures containing 0.1 μM 75SeO32− decreased incorporation of 75Se into FDHH by 85%. Conversely, attempts were made to place [75Se] selenocysteine in competition with selenite by monitoring incorporation of 75Se into FDHH. Although the chemical synthesis of unlabeled selenocysteine yields was acceptable, the level of incorporation of 75Se into the product was too low for cell labeling. Examination of cell extract after growth of E. coli in the presence of [75Se]selenocysteine detected no 75Se incorporation specifically or nonspecifically into proteins; further attempts were abandoned.
FIG. 3.
PhosphoImager analysis of a SDS-12% PAGE gel containing cell extracts from cultures of E. coli RK4353 grown anaerobically in LB containing 0.1 μM 75SeO32− and the indicated concentration of l-selenocysteine. Quantitation of 75Se content in FDHH is reported in the graph below the gel.
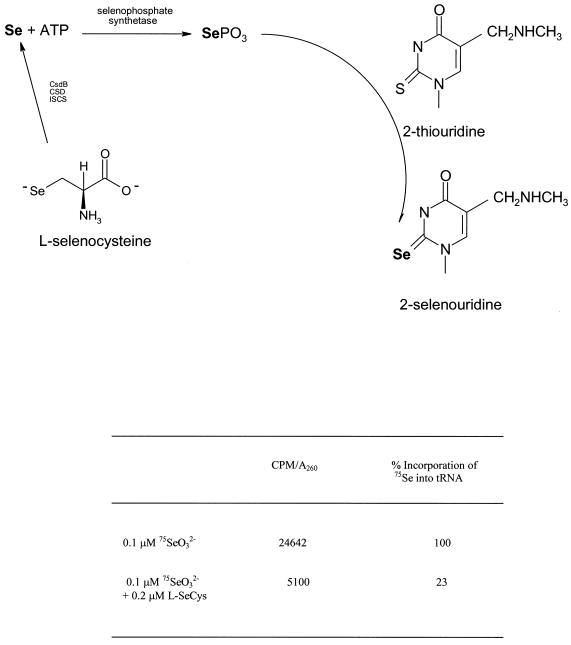
Since the substitution reaction in which the 2-thiouridine residue in tRNA is converted to 2-selenouridine requires selenophosphate as a selenium donor, a decrease in incorporation of 75Se into tRNA also is expected. The observed incorporation of 75Se into tRNA was decreased by 77% when E. coli RK4353 cultures were grown in the presence of 0.1 μM 75SeO32− supplemented with 0.2 μM l-selenocysteine (Fig. 4). The decrease in incorporation of 75Se into tRNA, with added selenocysteine, is similar to the decrease in 75Se content observed in FDHH and supports a common pathway for selenium from SeO32− and for the mobilized selenium from selenocysteine.
FIG. 4.
Conversion of 2-thiouridine to 2-selenourindine in nucleosides of tRNA. Effect of incorporation of 75Se into 2-selenouridine in RK4353 when grown in the presence of 0.1 μM 75SeO32− and 0.2 μM l-selenocysteine.
To determine if SeO32− could be used in selenophosphate biosynthesis without first being converted to free selenocysteine, an experiment was performed using E. coli strain RL165. This strain contains a cysK mutation (O-acetylserine sulfurhydrylases AB) which results in its inability to catalyze the biosynthesis of free cysteine. Since it has been proposed that O-acetylserine sulfurhydrylases AB can nondiscriminately utilize both sulfur and selenium (14), free selenocysteine cannot be generated through this pathway. Growth of RL165 in the presence of 75SeO32− results in the incorporation of selenium into specific selenocysteine-containing proteins and tRNAs with no nonspecific incorporation observed (Fig. 5A). However, when RL165 was grown in the presence of 0.1 μM 75SeO32− and 0.2 μM l-selenocysteine, there was a 67% decrease of 75Se incorporation into the specific selenocysteine-containing protein FDHH (Fig. 5B) and a 74% decrease in the tRNA nucleosides (Fig. 5C). Although RL165 cannot generate free selenocysteine, through the cysteine biosynthetic pathway, it can effectively utilize it as a selenium source for selenophosphate biosynthesis if it is supplemented in the medium during growth. It is also possible that an independent pathway for the synthesis of free selenocysteine may exist. This pathway may not be dependent on the O-acetylserine sulfurhydrylases AB and is not inhibited by the cysK mutation found in RL165. This pathway could prevent selenocysteine from becoming incorporated nonspecifically into proteins in place of cysteine as well as providing a source of selenocysteine for the NifS-like proteins. Furthermore, when RL165 was grown in the presence of 1 μM 75SeO32− and high concentrations of cystine (50 to 200 μg/ml), the specific incorporation of 75Se into FDHH and tRNA nucleosides was observed (data not shown). Growth in the presence of high concentrations of cystine should temporarily shut down the uptake of sulfate from the medium. The ability of E. coli to transport and metabolize selenium under conditions where sulfur uptake is turned off would further support a specific selenium transport and metabolism pathway.
FIG. 5.
(A) PhosphoImager analysis of a SDS-12% PAGE gel containing cell extract from E. coli strain RL165 grown with 0.1 μM 75SeO32−. (B) FDHH visualized by PhosphoImager analysis of an SDS-12% PAGE gel containing cell extract from E. coli strain RL165 grown with 0.1 μM 75SeO32− and 0.2 μM l-selenocysteine. (C) Effect of incorporation of 75Se into 2-selenouridine in RL165 when grown in the presence of 0.1 μM 75SeO32− and 0.2 μM l-selenocysteine.
The results obtained from the fdhF-lacZ fusion experiments show that when strain RK4353 was grown in minimal media with selenocysteine as a source of selenium, a read-through of the UGA codon from the fdhF gene occurred. This read-through gives evidence that selenophosphate can be generated from selenium derived from free selenocysteine. The dilution of 75Se into FDHH and tRNA nucleosides from 75SeO32−, when unlabeled free selenocysteine was added to the cultures of E. coli strains RK4353 and RL165, simply indicates that as more free selenocysteine is available, more selenophosphate is generated from selenium derived from free selenocysteine rather than a decrease in the expression level of FDHH. The generation of selenophosphate from selenium derived from selenocysteine supports the participation of a selenocysteine lyase enzyme in selenium metabolism. The three E. coli NifS-like proteins, cysteine sulfinate desulfinase (CSD), CsdB, and IscS, are ideal candidates for an in vivo selenocysteine lyase. All three proteins exhibit both cysteine desulfurase and selenocysteine lyase activities, with considerable differences in the degree of discrimination between the two substrates (10, 11). A unique selenocysteine lyase mechanism for these enzymes has been established based on mutagenesis studies. Cysteine desulfurase activity was found to be dependent on a conserved nucleophilic cysteine residue corresponding to the catalytically essential Cys325 of the A. vinelandii NifS (21). Although the substitution of Ala for Cys358 in CSD, Cys364 in CsdB, and Cys328 in IscS resulted in the complete loss of enzyme activity toward l-cysteine, there was little effect on activity toward l-selenocysteine. This difference in lyase mechanisms for the two substrates may support a dual function for each enzyme in vivo (12). Although it has been demonstrated in vitro that each NifS-like protein can function as a selenium delivery protein, it is not known if one or all three of these enzymes mobilize selenium in vivo. Lauhon and Kambampati recently reported that an iscS mutant strain of E. coli lacked 4-thiouridine and required thiamine and nicotinic acid for growth in minimal media, implying that IscS is directly involved in the mobilization of sulfur in these pathways (9). Additional deletions of the CSD and CsdB genes did not produce a similar phenotype, nor were both genes able to complement the IscS deletion. This may support a different function for both CSD and CsdB in vivo. Considering that the CsdB protein was 290 times more active on l-selenocysteine in vitro than on l-cysteine, it may function effectively as a selenocysteine lyase in vivo (10).
The results presented here provide evidence that E. coli has evolved a mechanism to utilize either SeO32− or l-selenocysteine as a source of selenium for selenophosphate biosynthesis. Little is known about the transport and metabolism of SeO32− in vivo. It has been proposed that SeO32− reacts with free thiols such as glutathione to form selenodiglutathione (5) (Fig. 6). Subsequent reactions with glutathione reductase, with reduced thioredoxin, or with excess free glutathione will lead to further reduction to selenide (1, 4). Selenide may be used as a substrate for SPS or it can be converted to free selenocysteine through either the cysteine biosynthetic pathway or a specific selenocysteine biosynthetic pathway. Although it is not clear if all SeO32− is converted first to free selenocysteine prior to selenophosphate formation, it can be seen that when free selenocysteine competes with SeO32− the selenium derived from selenocysteine is incorporated readily into FDHH and tRNA nucleosides. Recent evidence has been presented in the literature to support the participation of the E. coli NifS-like proteins in several pathways requiring sulfur (9, 15). The work presented in this paper provides evidence for an additional function for one or more of the E. coli NifS-like proteins in selenium metabolism.
FIG. 6.
Proposed pathway of selenium for selenophosphate biosynthesis. Selenophosphate synthetase may utilize either HSe− as a substrate, after reduction from SeO32−, or Hse−, which is mobilized from free selenocysteine by an E. coli NifS-like protein.
Acknowledgments
I acknowledge the support and encouragement offered by Thressa C. Stadtman during the course of this work.
REFERENCES
- 1.Björnstedt, M., S. Kumar, and A. Holmgren. 1992. Selenodiglutathione is a highly effective oxidant of reduced thioredoxin and a substrate for mammalian thioredoxin reductase. J. Biol. Chem. 267:8030-8034. [PubMed] [Google Scholar]
- 2.Chocat, P., N. Esaki, K. Tanizawa, K. Nakamura, H. Tanaka, and K. Soda. 1985. Purification and characterization of selenocysteine β-lyase from Citrobacter freundii. J. Bacteriol. 163:669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esaki, N., T. Nakamura, H. Tanaka, and K. Soda. 1982. Selenocysteine lyase, a novel enzyme that specifically acts on selenocysteine. Mammalian distribution and purification and properties of pig liver enzyme. J. Biol. Chem. 257:4386-4391. [PubMed] [Google Scholar]
- 4.Ganther, H. E. 1971. Reduction of the selenotrisulfide derivative of glutathione to persulfide analog by glutathione reductase. Biochemistry 10:4089-4098. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh, H. S., and H. E. Ganther. 1975. Acid-volatile selenium formation catalyzed by glutathione reductase. Biochemistry 14:1632-1636. [DOI] [PubMed] [Google Scholar]
- 6.Lacourciere, G. M., and T. C. Stadtman. 1998. The NifS protein can function as a selenide delivery protein in the biosynthesis of selenophosphate. J. Biol. Chem. 273:3091-3096. [DOI] [PubMed] [Google Scholar]
- 7.Lacourciere, G. M., and T. C. Stadtman. 1999. Catalytic properties of selenophosphate synthetases: comparison of the selenocysteine-containing enzyme from Haemophilus influenzae with the corresponding cysteine-containing enzyme from Escherichia coli. Proc. Natl. Acad. Sci. USA 96:44-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacourciere, G. M., H. Mihara, T. Kurihara, N. Esaki, and T. C. Stadtman. 2000. Escherichia coli NifS-like proteins provide selenium in the pathway for the biosynthesis of selenophosphate. J. Biol. Chem. 275:23769-23773. [DOI] [PubMed] [Google Scholar]
- 9.Lauhon, C. T., and R. Kambampati. 2000. The iscS −gene in Escherichia coli is required for the biosynthesis of 4-thiouridie, thiamin, and NAD. J. Biol. Chem. 275:20096-20103. [DOI] [PubMed] [Google Scholar]
- 10.Mihara, H., T. Kurihara, T. Yoshimura, K. Soda, and N. Esaki. 1997. Cysteine sulfinate desulfinase, a NifS-like protein of Escherichia coli with selenocysteine lyase and cysteine desulfurase activities. J. Biol. Chem. 272:22417-22424. [DOI] [PubMed] [Google Scholar]
- 11.Mihara, H., M. Maeda, T. Fujii, T. Kurihara, Y. Hata, and N. Esaki. 1999. A nifS-like gene, csdB, encodes an Escherichia coli counterpart of mammalian selenocysteine lyase. J. Biol. Chem. 274:14768-14772. [DOI] [PubMed] [Google Scholar]
- 12.Mihara, H., T. Kurihara, T. Yoshimura, and N. Esaki. 2000. Kinetic and mutational studies of the three NifS homologs from Escherichia coli: mechanistic difference between L-cysteine desulfurase and L-selenocysteine lyase reactions. J. Biochem. 127:559-567. [DOI] [PubMed] [Google Scholar]
- 13.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 14.Muller, S., J. Heider, and A. Böck. 1997. The path of unspecific incorporation of selenium in Escherichia coli. Arch. Microbiol. 168:421-427. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz, C. J., O. Djaman, J. A. Imlay, and P. J. Kiley. 2000. The cysteine desulfurase, IscS, has a major role in in vivo Fe-S cluster formation in Escherichia coli. Proc. Natl. Acad. Sci. USA 97:9009-9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stadtman, T. C., G. L. Dilworth, and C. S. Chen. 1979. Selenium dependent bacterial enzymes, p. 115-130. In D. Cagniant and G. Kirsch (ed.), Proceedings of the Third International Symposium on Organic Selenium and Tellurium Compounds, Mety, France.
- 17.Tanaka, H., and K. Soda. 1987. Selenocysteine. Methods Enzymol. 143:240-243. [DOI] [PubMed] [Google Scholar]
- 18.Veres, Z., I. Y. Kim, T. D. Scholz, and T. C. Stadtman. 1994. Selenophosphate synthetase enzyme properties and catalytic function. J. Biol. Chem. 269:10597-10603. [PubMed] [Google Scholar]
- 19.Zheng, L., R. H. White, V. L. Cash, R. F. Jack, and D. R. Dean. 1993. Cysteine desulfurase activity indicates a role for NIFS in metalocluster biosynthesis. Proc. Natl. Acad. Sci. USA 90:2754-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng, L., and D. R. Dean. 1994. Catalytic formation of a nitrogenase iron-sulfur cluster. J. Biol. Chem. 269:18723-18726. [PubMed] [Google Scholar]
- 21.Zheng, L., R. H. White, V. L. Cash, and D. R. Dean. 1994. Mechanism for the desulfurization of L-cysteine catalyzed by the nifS gene product. Biochemistry 33:4714-4720. [DOI] [PubMed] [Google Scholar]
- 22.Zinoni, F., A. Birkmann, W. Leinfelder, and A. Böck. 1987. Cotranslational insertion of selenocysteine into formate dehydrogenase from Escherichia coli directed by a UGA codon. Proc. Natl. Acad. Sci. USA 84:3156-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]