Abstract
The genome of temperate phage φFC1 integrates into the chromosome of Enterococcus faecalis KBL 703 via site-specific recombination. In this study, an integration vector containing the attP site and putative integrase gene mj1 of phage φFC1 was constructed. A 2,744-bp fragment which included the attP site and mj1 was inserted into a pUC19 derivative containing the cat gene to construct pEMJ1-1. E. faecalis KBL 707, which does not contain the bacteriophage but which has a putative attB site within its genome, could be transformed by pEMJ1-1. Southern hybridization, PCR amplification, and DNA sequencing revealed that pEMJ1-1 was integrated specifically at the putative attB site within the E. faecalis KBL 707 chromosome. This observation suggested that the 2,744-bp fragment carrying mj1 and the attP site of phage φFC1 was sufficient for site-specific recombination and that pEMJ1-1 could be used as a site-specific integration vector. The transformation efficiency of pEMJ1-1 was as high as 6 × 103 transformants/μg of DNA. In addition, a vector (pATTB1) containing the 290-bp attB region was constructed. pATTB1 was transformed into Escherichia coli containing a derivative of the pET14b vector carrying attP and mj1. This resulted in the formation of chimeric plasmids by site-specific recombination between the cloned attB and attP sequences. The results indicate that the integration vector system based on the site-specific recombination mechanism of phage φFC1 can be used for genetic engineering in E. faecalis and in other hosts.
Enterococci are gram-positive anaerobic bacteria that normally occur in the intestines of most animals from cockroaches to humans. In humans, the typical concentration of enterococci in stool can be up to 108 CFU per g (9, 29). Enterococci tolerate a variety of growth conditions, including hypotonic, hypertonic, acidic, and alkaline environments, and can survive at temperatures ranging from 10 to 45°C. In addition, they are intrinsically resistant to many antibiotics and can become opportunistic pathogens in hospitalized and immunocompromised patients. Acquired resistance to antibiotics is also of great concern. Enterococci are now among the most common members of the nosocomial group of bacterial pathogens (16, 24, 31, 35).
Bacteriophage φFC1 was first isolated in our laboratory from a culture of the lysogenic strain of Enterococcus faecalis (KBL 703) following induction by UV irradiation. Bacteriophage φFC1 has a double-stranded DNA genome of approximately 40.5 kbp, an icosahedral head, and a sheathless noncontractile tail. It has been classified into Bradley's group B or Siphoviridae according to the International Committee on Taxonomy of Viruses classification system (12, 13).
Phage φFC1 integration into the host chromosome occurs by a site-specific mechanism. A gene that encodes a putative site-specific recombinase and that is upstream of the attP site has been identified. The gene, mj1, encodes a 465-amino-acid polypeptide with similarity in its N-terminal domain to site-specific recombinases (10). The MJ1 integrase displays significant overall homology (57%) with the integrases of listerial phage A118 and lactococcal bacteriophage TP901-1. Analysis of the DNA sequences around the attP region identified two predicted bacterium-phage junction regions (attL and attR). The corresponding bacterial attachment site (attB) was deduced from the sequences of these regions (11).
In this study, a vector system based on the site-specific recombination apparatus of temperate bacteriophage φFC1 of E. faecalis KBL 703 was constructed. We wanted to determine whether putative integrase gene mj1 could serve as an efficient mediator of integration and if the mj1 and attP sites were sufficient for site-specific integration into the attB site. The vector we constructed, which included the attP site and mj1, could integrate into the attB-like site on chromosomal DNA of E. faecalis strain KBL 707, which contains no bacteriophage. This strain also was easily lysogenized by phage φFC1, produced from E. faecalis KBL 703 after UV irradiation. In addition, a 290-bp fragment containing the attB site was cloned into vector pACYC184 for use in intermolecular integration assays to determine the minimum size of the attB site and the eventual necessity for other host factors required for site-specific recombination. Our results showed that the phage φFC1 integrase could function in Escherichia coli as well as in E. faecalis strains and that a 290-bp fragment containing the attB site was the only host factor required to produce a functional target site for the vector containing the attP site and the integrase gene.
MATERIALS AND METHODS
Bacterial strains, bacteriophage, and plasmids.
E. faecalis strains were propagated at 37°C in Todd-Hewitt broth (THB; Difco Laboratories, Detroit, Mich.) without shaking. E. coli was grown with agitation at 37°C in Luria-Bertani broth. The plasmids used in this study are listed in Table 1. Temperate phage φFC1 was purified after induction by UV irradiation from E. faecalis KBL 703, and its DNA was extracted in accordance with standard procedures (32).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Bacterial strains | ||
| E. faecalis | ||
| KBL 703 | Original lysogenic strain for phage ΦFC1 | 8, 35 |
| KBL 707 | Indicator strain for phage ΦFC1 | 29 |
| E. coli JM109 | recA1 endA1 gyrA96 thi hsdR17 supE44 relA1 (lac-proAB) F traD36 proAB lacIqZM15 | 38 |
| Plasmids | ||
| pESH2.8 | Ampr Cmr; replicative ori of ColE1; pEK104 carrying a 2.8-kbp HindIII fragment of pS3.6 | 25 |
| pCONT3.7 | Ampr Cmr; replicative ori of ColE1 | This study |
| pEMJ1-1 | pCONT3.7 carrying a 2,744-bp attP fragment and int fragment of phage ΦFC1 | This study |
| pATTB1 | pACYC carrying 290-bp attB fragment | This study |
| pETMJ1 | pET14b carrying int and attP | 23 |
| pREC1 | Plasmid by recombination of pETMJ1 and pATTB1 | This study |
| pT7BlueR | Ampr; T cloning vector | Novagen |
| pATTR | pT7BlueR carrying the attR region | This study |
| pATTL | pT7BlueR carrying the attL region | This study |
UV inducibility of bacteriophage φFC1.
Bacteriophage φFC1 was induced from the E. faecalis lysogenic strain by UV irradiation (12, 13). Active strains were incubated at 37°C in THB until the optical density at 600 nm reached approximately 0.4. Cells were then harvested and resuspended in 50 ml of sterile 0.1 M MgSO4, and, after UV irradiation with a 15-W germicidal lamp emitting 16 ergs/mm2 for 15 s, they were transferred to double-strength THB and incubated at 37°C again. Changes in the turbidity of the cultures were recorded every 20 min.
Amplification of the attB, attL, attR, and attP regions.
The putative integrase gene and the mj1 and attP sites were amplified from phage φFC1 DNA with primers PHY-1 (5′-AAC TGC AGG GCG CAA GAA ACA ACT GCT T-3′) and PHY-2 (5′-GAA GAT CTT GTT CTC GAG CAT AGT CTC C-3′). The attL region was amplified from the genomic DNA of E. faecalis KBL 707 transformants with primers ON-2 (5′-CGG ATT GCC AGA TGG ATG AT-3′) and PHY-2. The attR region was amplified with primers ON-1 (5′-CGG CCA TTG AAT TAG GGT GT-3′) and PHY-1. A 290-bp fragment containing attB was amplified from E. faecalis KBL 703 genomic DNA with intermolecular integration assay primers PATB-1 (5′-CCC TCG GGC GGA TTG CCA GAT GGA TGA T-3′) and PATB-2 (5′-CCC CCG AGC GGC CAT TGA ATT AGG GTG T-3′). A junction region of pREC1 was amplified from plasmid DNA, which was extracted from E. coli cotransformed with pATTB1 and pETMJ1, with primer set PATB-1 and PHY-2. PCR was conducted as follows: 94°C for 5 min, and then 30 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 1 to 3 min. This was followed by a final extension period of 72°C for 10 min. PCR products were analyzed on 0.8 to 1.2% agarose gels in Tris-acetate-EDTA buffer.
For transformation, 20 μl of frozen cells was thawed on ice, mixed with plasmid DNA, and transferred to the electrode pin. A single electric pulse (PG 240 bacterial electrode and Progenitor II; Hoefer Co.) with the following parameters was applied: 12,000 V/cm peak voltage, 4.7 ms, and 100 μF. The cell suspension was mixed with ice-cold SGM17MC (4) and left on ice for 5 min. After incubation at 37°C for 2 h, transformed cells were spread on a streptococcal regeneration plate containing 5 μg of chloramphenicol per ml.
Southern blotting.
DNA restriction fragments separated on an agarose gel were transferred to a Hybond-N membrane (Amersham) by the capillary method (32). Appropriate probes were labeled with digoxigenin-11-dUTP, and prehybridizations and hybridizations were performed as recommended by the supplier (Boehringer-Mannheim). Digoxigenin-11-dUTP-labeled λ DNA digested with DraI was used as a molecular marker for the hybridization.
DNA sequencing.
DNA cycle sequencing was done using the ABI PRISM dye terminator cycle sequencing ready reaction kit (Perkin-Elmer Co.). Double-stranded template DNA (0.5 μg) and 3.5 pmol of primer were mixed with 8 μl of terminator ready reaction mixture, and cycle sequencing was carried out with a GeneAmp PCR system 9600 (Perkin-Elmer Co.). The amplified DNA was purified with Centri-Sep spin columns (Applied Biosystems), and the nucleotide sequence was analyzed with an ABI PRISM 310 genetic analyzer.
The left junction region, attL, which lies between the attB site and pEMJ1-1 on a 617-bp DNA fragment, was amplified from the genomic DNA of the E. faecalis KBL 707 transformant by PCR using primers ON-2 and PHY-2 and sequenced. attR, the other junction region of the 400-bp fragment, was amplified from the genomic DNA of the E. faecalis KBL 707 transformant by PCR using primers ON-1 and PHY-3 (5′-GCG TTA ACT GCC AAT ATA GC-3′) and then sequenced.
Nucleotide sequence accession numbers.
The nucleotide sequence data shown in Fig. 3 have been deposited in GenBank under accession no. AY026043 and AY026044.
FIG. 3.
Nucleotide sequences of the attB, attP, attL, and attR regions. attL and attR were sequenced after pEMJ1-1 integrated into the E. faecalis KBL 707 chromosome. Uppercase, E. faecalis KBL 707 chromosomal DNA sequences; lowercase, phage φFC1 DNA sequences; boldface, 3-bp core. In the attB sequence, the single nucleotide difference in the real attB nucleotide sequence of E. faecalis KBL 703 is underlined (T in place of A in E. faecalis KBL 703). Arrows (attP sequence), inverted repeats r1, r2, and r3.
RESULTS
Characterization of E. faecalis KBL 707.
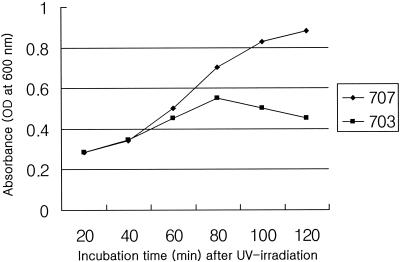
Induction of prophage is usually initiated by exposure of cells to UV radiation or DNA-damaging chemicals such as mitomycin C (22). In this study, we monitored changes in turbidity in cultures of E. faecalis KBL 703 and KBL 707 after UV irradiation (Fig. 1).
FIG. 1.
Changes in turbidity of E. faecalis KBL 707 and KBL 703 cultures after UV irradiation. OD, optical density.
E. faecalis KBL 703 strain carries three plasmids (p703/9, p703/5, and p703/4) and temperate bacteriophage φFC1, whereas E. faecalis KBL 707 strain contains neither plasmids nor bacteriophage. Previous studies have shown that among several E. faecalis strains, E. faecalis KBL 707 was the most easily lysogenized by infection with phage φFC1, produced from E. faecalis KBL 703 after UV irradiation (12, 13). In addition, when the attB site was amplified from E. faecalis KBL 707 with primers that amplify the attB site of E. faecalis KBL 703, the expected amplification product, with a size of 300 bp, was obtained (data not shown). This suggests that phage φFC1 was integrated into the attB-like site of the genome of E. faecalis KBL 707. For this reason, we tested the proficiency of putative integrase gene mj1 and the attP region of phage φFC1 for site-specific recombination in E. faecalis KBL 707.
Construction of site-specific integration plasmid pEMJ1-1.
A 3.7-kbp HindIII fragment from pESH2.8, a pUC19 derivative that contains the enterococcal replication origin from enterococcal plasmid p703/5 and the chloramphenicol acetyltransferase gene, was subjected to self-ligation and was named pCONT3.7. Since the vector was constructed as a control vector for site-specific integration vector pEMJ1-1, the possibility that pCONT3.7 could exist in E. faecalis was tested. No colonies were obtained when E. faecalis KBL 707 was transformed with pCONT3.7 and grown on a THB plate containing 5 μg of chloramphenicol per ml. This result showed that pCONT3.7, which is derived from pESH2.8, could not replicate in E. faecalis KBL 707 because it lacked an enterococcal replication origin.
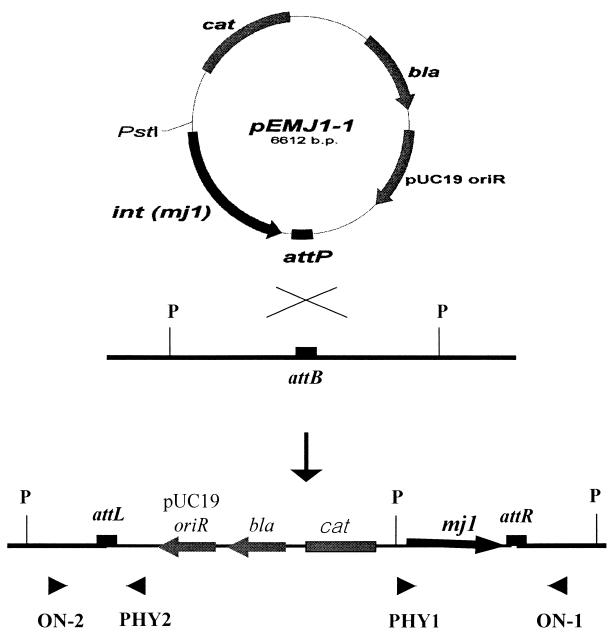
A 2,744-bp fragment containing putative integrase gene mj1 and the attP site from phage φFC1 DNA was ligated to pCONT3.7 to create pEMJ1-1, which was then transformed into E. faecalis KBL 707. The electroporation efficiency was 6 × 103 colonies/μg of DNA. Although colonies were obtained, no plasmid DNA could be isolated from the E. faecalis KBL 707 transformant because pEMJ1-1 does not have an enterococcal replication origin. The result suggested that putative integrase MJ1 had elicited recombination between the attP site on pEMJ1-1 and the putative attB site in E. faecalis KBL 707 chromosomal DNA (Fig. 2).
FIG. 2.
Schematic representation of the site-specific integration of pEMJ1-1 into the attB locus of the E. faecalis KBL 707 chromosome. P, PstI site; arrowheads, primers.
Site-specific integration of pEMJ1-1 into the E. faecalis chromosome.
Five colonies of E. faecalis KBL 707 transformants were randomly selected and subjected to Southern blotting with a fragment containing mj1 and the attP site as the probe. Intact E. faecalis KBL 707 DNA was used as a control. If strand exchange between the attB-like site on the chromosomal DNA of E. faecalis KBL 707 and the attP site on pEMJ1-1 occurred, the probe containing the attP site and mj1 should hybridize to both ends of the vector that had been integrated into the host genome. Two signals, approximately 7 and 5.5 kbp, were detected by Southern blotting, which confirmed that pEMJ1-1 had integrated into the host genome as a single copy (data not shown). If pEMJ1-1 used the attP site to integrate into the putative attB site on the chromosomal DNA of E. faecalis KBL 707, transformants would have junction regions attL and attR. We were able to amplify the attL and attR regions as 617- and 2,417-bp fragments, respectively, from the genomic DNA of E. faecalis KBL 707 transformants. These fragments had the predicted sizes (data not shown). The results verified our findings that two junction regions were generated by integration of pEMJ1-1 into the putative attB locus of E. faecalis KBL 707.
To determine whether the attB site on the chromosomal DNA of E. faecalis KBL 703 and the attB-like site of E. faecalis KBL 707 had the same sequence or not and to confirm that pEMJ1-1 was integrated into the chromosome of E. faecalis KBL 707 via site-specific recombination, the junction regions were sequenced. The 617- and 400-bp fragments were used to analyze the left and right junction regions, respectively, and the sequences were compared to those of the natural junction regions between E. faecalis KBL 703 and phage φFC1 (Fig. 3). The sequence of the attB-like site of E. faecalis KBL 707 was identical to that of the original attB site of E. faecalis KBL 703 except for 1 nucleotide. The results also showed that pEMJ1-1 was integrated into the attB-like site in a manner similar to that for phage φFC1, through strand exchange between the attP and the attB sites mediated by the integrase. In addition, the core sequences (ACT) on the attP and attB sites, in which direct recombination occurred, were conserved in the attL and attR regions after strand exchange between the attP and attB sites. These results confirmed that pEMJ1-1 was integrated into the attB-like site through the action of integrase MJ1 and that the 2,744-bp fragment containing the attP site and mj1 was sufficient to allow site-specific recombination of pEMJ1-1, as well as phage φFC1.
Intermolecular integration assay with E. coli.
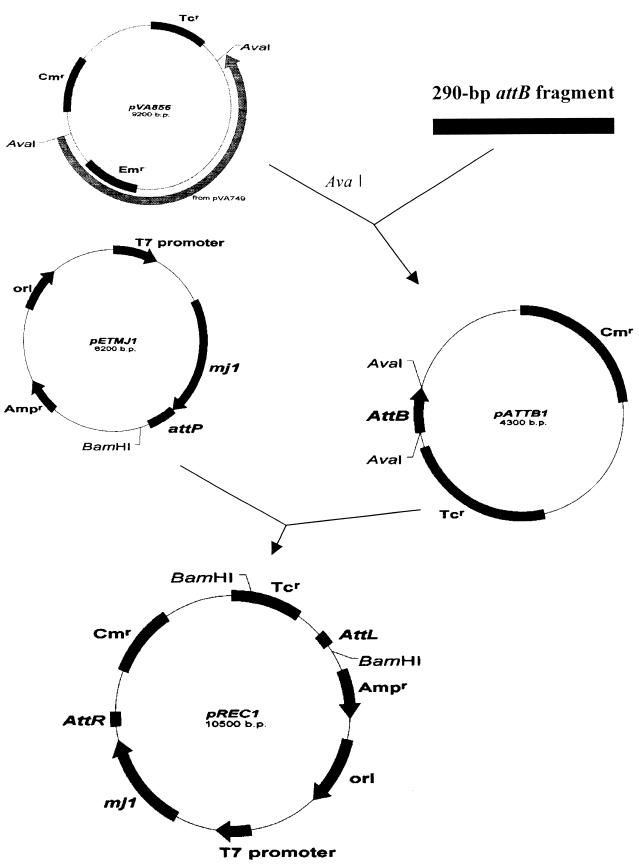
A 290-bp fragment containing bacterial attachment site attB, amplified from E. faecalis KBL 703 genomic DNA, was ligated into the AvaI site of pACYC184 to create pATTB1 (Fig. 4). The mj1 expression plasmid pETMJ1, which is a pET14b derivative carrying integrase gene mj1 and the attP site (11, 23), and pATTB1 were cotransformed into E. coli JM 109. Restriction enzyme digestion of plasmid DNA isolated from the transformed cells indicated that, in addition to the original plasmids, a chimeric plasmid (pREC1) had formed through an intermolecular integration reaction between the attB site on pATTB1 and the attP site on pETMJ1 (Fig. 4 and 5). To determine more accurately if site-specific recombination between pATTB1 and pETMJ1 had occurred, plasmid DNA from E. coli JM 109 cotransformed with pATTB1 plus pETMJ1 was analyzed by junction amplification and sequencing. The junction was amplified as expected, and the attL and attR sequences were identical to those determined for E. faecalis (Fig. 3 and 5). The results demonstrated that site-specific recombination resulting in formation of the chimeric plasmid had occurred, that plasmids pATTB1 and pETMJ1 carried functional attB and attP sites, respectively, and that the phage φFC1 integrase could function in E. coli as well as in E. faecalis without the need for any additional cofactors.
FIG. 4.
Schematic representation of the construction of attB-containing plasmid pATTB1 and chimeric plasmid pREC1. pVA856 is a shuttle vector constructed by joining pACYC184 and pVA749 at the AvaI site. The pACYC184 fragment from pVA856 was used for construction of pATTB1. A 290-bp attB fragment was amplified from the E. faecalis KBL 703 chromosome by PCR. pATTB1 and pETMJ1 were cotransformed into E. coli JM 109 for the intermolecular integration assay.
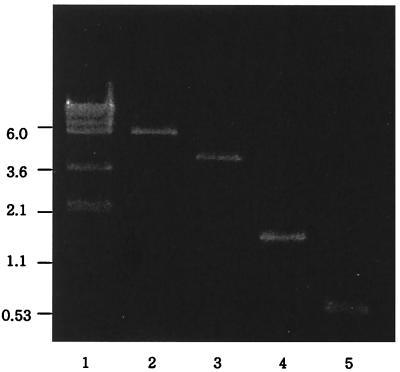
FIG. 5.
Intermolecular integration assay with E. coli. The presence of a BamHI fragment of approximately 1.3 kbp and a 600-bp PCR product is indicative of site-specific recombination between pATTB1 and pETMJ1. Lane 1, λ DNA digested with DraI as a size marker; lane 2, pETMJ1 digested with BamHI; lane 3, pATTB1 digested with BamHI; lane 4, the 1.3-kbp BamHI fragment from E. coli cotransformed with pATTB1 and pETMJ1; lane 5, the 600-bp PCR product from the junction on chimeric plasmid pREC1.
DISCUSSION
Characteristic features of the sequences of numerous attP sites include (i) a central core sequence, where synapsis and strand exchange occur, which is also present in the three other attachment sites (attB, attL, and attR), and (ii) two unique elements termed the P and P′ arm sequences, which surround the core region. Both arm and core DNA sequences contain inverted and direct repeats that act as the binding site for the specific integrase (13, 18, 20, 29). The attP site of phage φFC1 contains a core sequence (5′-ACT-3′), which is relatively short compared to those of other bacteriophages, and two inverted repeats surrounding it. The site of specific recombination is within the core sequence (5′-ACT-3′), as determined by comparison of the attL, attR, attB, and attP regions. This recombination event thus occurs without DNA synthesis or sequence duplication, a typical feature of other temperate phages (26).
Identification of the locations of attB sites shows that integration into tRNA genes, especially at their 3′ ends, is a common occurrence among gram-negative phages such as P22, P4, 186, HP1, 16-3, and φCTX and in actinomycete integrative plasmids such as SLP1, pSAM2, pMEA100, and pSE211 (1, 27). One explanation for this target site preference is that the integrase uses the symmetrical sequence in the tRNA gene as a recognition site (1). Studies of phages that have inserted their genomes into a tRNA gene have shown that the length of the homologous region between attB and attP is usually large, ranging from 17 bp for mv4 (5) to 182 bp for HP1 (average of 40 to 60 bp) (36). Insertion of phage into the tRNA gene does not cause disruption because of the presence of a truncated tRNA gene. This insertion is therefore not lethal. In contrast, phages of gram-positive bacteria integrate either into a gene coding for a protein (L54a [17], φ13 [3], φLC3 [21], or TP901-1 [2]) or into an intergenic region (φ11 [18]), except for mycobacteriophage L5 and Lactobacillus delbrueckii subsp. bulgaricus bacteriophage mv4, which integrate into tRNA genes (15, 18). From analyses of DNA sequence data, it appears that the attB site for phage φFC1 is a part of an open reading frame which encodes a protein with 43% identity to the C-terminal end of E. coli RadC. RadC is a protein involved in DNA repair following damage by UV or ionizing radiation or alkylating agents (6).
The integration vectors based on the site-specific recombination of temperate phages that we have developed offer a number of advantages. They could be used to introduce a single copy of heterologous DNA into the attB site on the bacterial chromosome. It is possible that large fragments of DNA could be integrated, because this recombination system should be able to accommodate a fragment at least as large as the phage genome. For a system using sequences from phage φFC1, this could be up to 40.5 kbp. In a system that does not necessitate packaging of DNA in the phage head, such as the one described here, there is no theoretical upper size limit for the passenger DNA. Because a single copy is integrated, expression studies may be performed under conditions that mimic those for chromosomal genes or operons present in only one copy. The specific integration of such vectors should not affect the viability of the transformants, since integration occurs in nonessential sites on the genome. Moreover, easy modification of these new vectors will allow us to extend the use of this methodology to other biotechnologically important bacteria and even to mammalian cells (7, 14).
This study demonstrated that plasmid pEMJ1-1, containing integrase gene mj1 and the attP region of the enterococcal bacteriophage φFC1, could integrate into the chromosomal DNA of E. faecalis KBL 707 via a site-specific recombination process that obeys Campbell's classic model of integration for phage lambda. Integrative recombination took place precisely between the attP and attB sites. Sequence data from the junctions confirmed that the attB region of E. faecalis KBL 707 was similar to that of E. faecalis KBL 703. It also showed that specific interaction between the attP and the attB sequences occurred during integration of pEMJ1-1 and that the core sequence was preserved during this process. All of the genes required for integration of phage φFC1 could be localized within a 2,744-bp DNA fragment which contained 1.4-kbp integrase gene mj1 and the attP site. In addition, a 290-bp fragment containing the attB sequence could serve as the attB locus for the attP site in recombination mediated by the integrase. Use of the enterococcal attB site as an integration target enables plasmids containing the attP site, such as pEMJ1-1 and pETMJ1, to transform not only E. faecalis strains containing the attB site within their genomes but also other cells that have been engineered to carry the attB sequence. Further studies will enable development of vectors derived from phage φFC1, which are expected to provide powerful tools for the stable, single-copy gene insertion of DNA into industrial and laboratory strains of bacteria, and even mammalian cells.
REFERENCES
- 1.Campbell, A. 1992. Chromosomal insertion sites for phages and plasmids. J. Bacteriol. 174:7495-7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christiansen, B., L. Brondsted, F. K. Vogensen, and K. Hammer. 1996. A resolvase-like protein is required for the site-specific integration of the temperate lactococcal bacteriophage TP901-1. J. Bacteriol. 178:5164-5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman, C., J. Knights, R. Russel, D. Shanley, T. H. Birkbeck, G. Dougan, and I. Charles. 1991. Insertional inactivation of the Staphylococcus aureus β-toxin by bacteriophage phi-13 occurs by site- and orientation-specific integration of the phi-13 genome. Mol. Microbiol. 5:933-939. [DOI] [PubMed] [Google Scholar]
- 4.Cruz-Rodz, A. L., and M. S. Gilmore. 1990. High efficiency introduction of plasmid DNA into glycine treated Enterococcus faecalis by electroporation. Mol. Gen. Genet. 224:152-154. [DOI] [PubMed] [Google Scholar]
- 5.Dupont, L., B. Boizet-Bonhoure, M. Coddeville, F. Auvray, and P. Ritzenthaler. 1995. Characterization of genetic elements required for site-specific integration of Lactobacillus delbrueckii subsp. bulgaricus bacteriophage mv4 and construction of an integration-proficient vector for Lactobacillus plantarum. J. Bacteriol. 177:586-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felzenszwalb, I., S. Boiteux, and J. Laval. 1992. Identification of the radC102 mutant. Order of the genes in the 81.5-82.0 min region of the Escherichia coli chromosome. Nucleic Acids Res. 20:366.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groth, A. C., E. C. Olivares, B. Thyagarajan, and M. P. Calos. 2000. A phage integrase directs efficient site-specific integration in human cells. Proc. Natl. Acad. Sci. USA 97:5995-6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeon, Y. W., S. Y. Jeong, Y. W. Kim, and H. I. Chang. 1994. Cloning of replication origin from enterococcal plasmid p703/5. Kor. J. Appl. Microbiol. Biotechnol. 22:18-22. [Google Scholar]
- 9.Jett, B. D., and M. S. Gilmore. 1994. Virulence of enterococci. Clin. Microbiol. Rev. 7:462-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim, M.-J. 1995. Sequencing and expression of a putative site-specific recombinase gene (mj1) of an enterococcal bacteriophage φFC1. M.S. thesis. Korea University, Seoul, Korea.
- 11.Kim, M.-J., J. Y. Lee, Y. W. Kim, and H. I. Chang. 1996. Molecular characterization of the region encoding integrative functions from enterococcal temperate phage φFC1. J. Biochem. Mol. Biol. 29:448-454. [Google Scholar]
- 12.Kim, Y. W. 1999. Genetic studies of bacteriophage φFC1. from Enterococcus faecalis. Ph.D. thesis. Korea University, Seoul, Korea.
- 13.Kim, Y. W., and H. I. Chang. 1994. Isolation and molecular characterization of φFC1, a new temperate phage from Enterococcus faecalis. Mol. Cell 4:155-158. [Google Scholar]
- 14.Kolot, M., N. Silberstein, and E. Yagil. 1999. Site-specific recombination in mammalian cells expressing the Int recombinase of bacteriophage HK022. Mol. Biol. Rep. 26:207-213. [DOI] [PubMed] [Google Scholar]
- 15.Landy, A. 1989. Dynamic, structural, and regulatory aspects of site-specific recombination. Annu. Rev. Biochem. 58:913-949. [DOI] [PubMed] [Google Scholar]
- 16.Leclercq, R., E. Derlot, J. Duval, and P. Courvalin. 1988. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N. Engl. J. Med. 319:157-161. [DOI] [PubMed] [Google Scholar]
- 17.Lee, C. Y., and J. J. Iandolo. 1986. Integration of staphylococcal phage L54a occurs by site-specific recombination: structural analysis of the attachment sites. Proc. Natl. Acad. Sci. USA 83:5474-5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, C. Y., and J. J. Iandolo. 1988. Structural analysis of staphylococcal bacteriophage φ11 attachment sites. J. Bacteriol. 170:2409-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, M. H., L. Pascopella, W. R. Jacobs, and G. H. Hatfull. 1991. Site-specific integration of mycobacteriophage L5. Proc. Natl. Acad. Sci. USA 88:3111-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leong, J. M., S. Nunes-Düby, C. F. Lesser, P. Youderian, M. M. Susskind, and A. Landy. 1985. The phi-80 and P22 attachment sites. J. Biol. Chem. 260:4468-4477. [PubMed] [Google Scholar]
- 21.Lillehaug, D., and N. K. Birkeland. 1993. Characterization of genetic elements required for site-specific integration of the temperate lactococcal bacteriophage φLC3 and construction of integration-negative φLC3 mutants. J. Bacteriol. 175:1745-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsushiro, A., K. Sato, H. Miyamoto, T. Yamamura, and T. Honda. 1999. Induction of prophages of enterohemorrhagic Escherichia coli O157:H7 with norfloxacin. J. Bacteriol. 181:2257-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moitoso de Vargas, L., C. A. Pargellis, N. M. Hansan, E. W. Bushman, and A. Landy. 1988. Autonomous DNA binding domains of lambda integrase recognize two different sequence families. Cell 54:923-929. [DOI] [PubMed] [Google Scholar]
- 24.Murray, B. E., K. V. Singh, S. M. Markowitz, H. A. Lopardo, J. E. Patterson, and M. J. Zervos. 1991. Evidence for clonal spread of a single strain of β-lactamase-producing Enterococcus (Streptococcus) faecalis to six hospitals in five states. J. Infect. Dis. 163:780-785. [DOI] [PubMed] [Google Scholar]
- 25.Park, J.-H. 1997. Cloning of replication origin of enterococcal plasmid p703/5 and construction of a promoter probe vector for Enterococcus faecalis. M.S. thesis. Korea University, Seoul, Korea.
- 26.Raya, R. R., C. Fremaux, G. L. de Antoni, and T. R. Klaenhammer. 1992. Site-specific integration of the temperate bacteriophage φadh into the Lactobacillus gasseri chromosome and molecular characterization of the phage (attP) and bacterial (attB) attachment sites. J. Bacteriol. 174:5584-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiter, W., P. Palm, and S. Yeats. 1989. Transfer RNA genes frequently serve as integration sites for prokaryotic genetic elements. Nucleic Acids Res. 17:1907-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rew, J. 1991. Catalogue of strains, 4th ed., p. 23. Korean Culture Center of Microorganisms, Yonsei University, Seoul, Korea.
- 29.Rice, E. W., J. W. Messer, C. H. Johnson, and D. J. Reasoner. 1995. Occurrence of high-level aminoglycoside resistance in environmental isolates of enterococci. Appl. Environ. Microbiol. 61:374-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romeo, D. A., and T. R. Klaenhammer. 1992. IS 946-mediated integration of heterologous DNA into the genome of Lactococcus lactis subsp. lactis. Appl. Environ. Microbiol. 58:699-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahm, D. F., J. Kissinger, M. S. Gilmore, P. R. Murray, R. Mulder, and J. Solliday. 1989. In vitro susceptibility studies of vancomycin-resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 33:1588-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 33.Segall, A. M., and H. A. Nash. 1993. Synaptic intermediates in bacteriophage lambda site-specific recombination: integrase can align pairs of attachment sites. EMBO J. 12:4567-4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song, J. S., J. H. Park, C. W. Kim, Y. W. Kim, W. J. Lim, I. Y. Kim, and H. I. Chang. 1999. Characterization of the replication region of the Enterococcus faecalis plasmid p701/5. J. Microbiol. Biotechnol. 9:91-97. [Google Scholar]
- 35.Uttley, A. H. C., C. H. Collins, J. Naidoo, and R. C. George. 1988. Vancomycin-resistant enterococci. Lancet i:57-58. [DOI] [PubMed]
- 36.Waldman, A. S., S. D. Goodman, and J. J. Scocca. 1987. Nucleotide sequences and properties of the sites involved in lysogenic insertion of the bacteriophage HPc1 genome into the Haemophilus influenzae chromosome. J. Bacteriol. 169:238-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]