Abstract
Flow cytometric analysis showed that the hns205 and hns206 mutants, lacking the abundant nucleoid-associated protein H-NS, have decreased origin concentration, as well as a low number of origins per cell (ploidy). The most striking observation was that the low ploidy was due to a very short replication time, e.g., at 30°C it was halved compared to that of the hns+ strain. The decreased origin concentration was not caused by a decreased dnaA gene expression, and the hns206 mutant had normal DnaA protein concentrations. The replication phenotypes of the hns206 mutant were independent of RpoS. Cells overproducing H-NS from a LacI-controlled plasmid had a normal origin concentration, indicating that H-NS is not controlling initiation. A wild-type H-NS concentration is, however, required to obtain a wild-type origin concentration, since cells with an intermediate H-NS concentration had an intermediate origin concentration. Two lines of evidence point to an indirect effect of H-NS on initiation. First, H-NS did not show high-affinity binding to any part of oriC, and H-NS had no effect on transcription entering oriC from the mioC promoter. Second, in a shift experiment with the hns206 mutant, when H-NS protein was induced to wild-type levels within 10 min, it took more than one generation before the origin concentration started to increase.
Escherichia coli contains a number of small abundant DNA-binding proteins (8), which are believed to play an important role in structuring the bacterial nucleoid. These proteins include the site-specific DNA-binding proteins IHF and FIS and the general DNA-binding proteins HU and H-NS, the latter of which has a preference for binding to intrinsically curved DNA (43). All of these proteins have been shown to play a role in the initiation of replication from the chromosomal origin, oriC. The HU protein has been shown to be required as an oriC specificity factor for initiation of replication in vitro (22). The minimal oriC sequence contains binding sites, DnaA boxes R1 to R4, for the essential initiator protein DnaA, and binding sites for IHF and FIS are located between R1 and R2 and between R2 and R3, respectively (34). Minichromosome replication is compromised in mutants lacking IHF or FIS and by mutation in their binding sites in oriC (34). Furthermore, fis and ihf mutants have been reported to show the asynchrony phenotype (11, 42), suggesting that the initiation cascade might not be working properly.
The oriC DNA exhibits a strong intrinsic curvature with a major bend localized at the right border of the minimal oriC (29), and thus H-NS might also bind to oriC. Only a few studies of replication-related effects of H-NS have been reported. An hns mutant produces anucleate cells, indicating that it is affected in chromosome localization (27). The same study also showed that a lack of H-NS, in contrast to the lack of FIS or IHF, did not lead to asynchrony but results in reduced ploidy, indicating an effect on the length of the cell cycle. There are confusing reports on the effects of hns mutations on initiation: it seems to confer reduced DnaA activity leading to suppression of the dnaA(cos) mutant and increased temperature sensitivity of a dnaA(Ts) mutant (28), but it allows replication of compromised minichromosomes carrying DnaA box mutations or an FIS-binding site mutation (31).
hns mutants exhibit a very pleiotropic phenotype, and expression of more than 50 proteins is affected by inactivation of the hns gene. In many cases the effect of H-NS on gene expression is direct, being mediated by binding of H-NS to intrinsically curved DNA present in the promoter region (4). In many other cases the effect is, at least partially, mediated by the increased concentration of RpoS found in hns mutants (10). The function of H-NS seems primarily to be modulation of the regulation by environmental stimuli, most notably the temperature regulation of genes involved in adhesion and virulence (4). Furthermore, H-NS is a cold shock protein (32) and hns mutants are cold sensitive, in the sense that the decrease in growth rate observed at normal temperature is much more pronounced at low temperature (19).
Here we present a thorough study of the replication phenotype of hns mutants at different growth temperatures and of strains with different amounts of wild-type H-NS protein. We investigated the effects on ploidy and origin concentration by flow cytometry and quantitative Southern blots and on dnaA gene expression by using a DnaA-β-galactosidase fusion and by immunoblot.
MATERIALS AND METHODS
Bacterial strains, growth media and conditions, and enzyme measurements.
The strains used in this study are listed in Table 1. Strains derived from BBC119 (17) carry λRB1 containing a dnaA"lacZ fusion (13), which allows determination of dnaA gene expression by measuring DnaA-β-galactosidase activity as described previously (2, 35). Strains derived from TC3983, which carry a pappY′-lacZ fusion, were used for the studies in which H-NS synthesis was induced by IPTG (isopropyl-β-d-thiogalactopyranoside), since this fusion, unlike λRB1, does not include a lacY+ gene and therefore allows graduated induction by varying the IPTG concentration in the medium. AB minimal medium (18) supplemented with 1% Casamino Acids (Difco), 0.2% glucose, and 1 μg of thiamine/ml was used for all experiments. Cultures were kept in exponential growth at the different temperatures for more than 15 mass doublings before the start of the experiment.
TABLE 1.
E. coli K-12 strains
| Strain | Genotypea | Source, reference, or construction |
|---|---|---|
| LJ24 | thi-1 leu-6 lacY1 lacI-ZΔ(Mlu) glnV44 tonA21 rpsL rfbD1 rpoS396(Am) | 6, 36 |
| BBC119 | λRB1b | 17 |
| MC4100 | araD139 Δ(argF-lac)U169 deoC1 flb5301 relA1 rpsL150 ptsF25 rbsR | 38 |
| GM230 | hns-205::Tn10 φ(proU-lacZ)hyb2(λ placMu15)c | 26 |
| PD23 | hns-206::Aprc | 19 |
| RH90 | rpoS359::Tn10c | 30 |
| BL21/λDE3 | gal met r− m−hsdS placIq/λ placUV5-T7-gene 1 | 41 |
| TC4061 | λRB1 hns-205::Tn10b | GM230(P1) × BBC119 |
| TC4136 | λRB1 hns206::Aprb | PD32(P1) × BBC119 |
| TC4409 | λRB1 rpoS359::Tn10b | RH90(P1) × BBC119 |
| TC4410 | λRB1 rpoS359::Tn10 hns206::Aprb | RH90(P1) × TC4136 |
| TC3983 | attB::p appY′-lacZb | 15 |
| TC4033 | hns205::Tn10 attB::pappY′-lacZb | 7 |
| TC4135 | hns206::AprattB::pappY′-lacZb | 7 |
| TC4300 | attB::pmioC4140-lacZb | This work |
| TC4301 | attB::pmioC4141-lacZb | This work |
| TC4302 | attB::pmioC4142-lacZb | This work |
| TC4623 | hns206::AprattB::pmioC4140-lacZb | PD32(P1) × TC4300 |
| TC4624 | hns206::AprattB::pmioC4141-lacZb | PD32(P1) × TC4301 |
| TC4625 | hns206::AprattB::pmioC4142-lacZb | PD32(P1) × TC4302 |
| TC4617 | hns206::AprattB::pappY′-lacZb/pTAC4617 | This work |
Genetic symbols are as described elsewhere (9).
Genotype otherwise like LJ24.
Genotype otherwise like MC4100.
Immunoblot procedure.
Sample preparation and immunoblot analysis were carried out as described previously (3, 23).
Flow cytometric procedures.
Samples were prepared and flow cytometry was performed exactly as described by Christensen et al. (17), based on procedures described elsewhere (33, 39). The average number of origins per cell and the average light scatter per cell were determined in samples treated for 4 h at 37°C with 300 μg of rifampin and 36 μg of cephalexin/ml. The DNA concentration was determined by analysis of the DNA content and the light scatter of samples taken directly from the exponentially growing cultures.
Determination of C time (i.e., the replication time) by Southern blot marker frequency analysis.
Chromosomal DNA was prepared, and the origin per terminus ratio was determined by Southern blot analysis exactly as described previously (3).
Plasmid constructions.
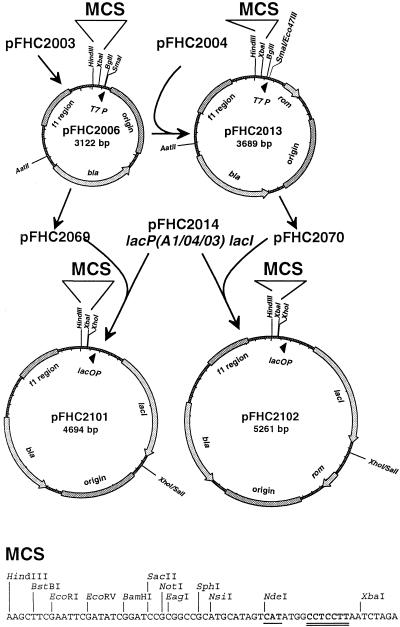
The T7 promoter-controlled expression vectors pFHC2006[rom−] and pFHC2013[rom+] and the corresponding LacI-controlled vectors pFHC2101[rom−] and pFHC2102[rom+] carrying the lacP(A1/04/03) promoter and the lacI gene were constructed as described in Fig. 1. Both the T7 promoter and the lacP(A1/04/03) promoter plasmid derivatives are well suited for cloning of PCR-amplified genes in which the start codon of the gene in question lies in an NdeI restriction site, and the identical multiple cloning sites make it easy to move genes between these expression vectors. Only the rom+ plasmid pFHC2102, which does not amplify excessively going to stationary phase on plates or in liquid medium, was used in this study. To construct plasmid pFHC2242, from which H-NS production can be induced, the hns gene was amplified from chromosomal DNA with the primers 9.4 (CTAGGAATTCCATATGAGCGAAGCACTTAAAATTCTGAAC) and 9.5 (CTGAAAGCTTGCAAGTGCAATCTACAAAAGATTATTGCTTG), and the PCR fragment was digested with NdeI and HindIII and ligated to NdeI-HindIII-digested pFHC2102. The sequence of the inserted hns gene in pFHC2242 was verified by sequencing with the Thermosequenase kit from Amersham, by using the internal [35S]dATP primer labeling protocol and the primers pFH2102-2 (GTGTTGACTTGTGAGCGGAT) and pFH2102-3 (AAGCTGGGATTTAGGTGACAC), which are located upstream and downstream of the multiple cloning site, respectively. pTAC4617 is a chloramphenicol-resistant derivative of pFH2242 obtained by insertion of the 1,347-bp Eco47III fragment of pACYC184 (16) into SspI-digested pFH2242. Plasmid pTAC1257 carries the mioC gene and oriC (bp 365 to 1,617 in GenBank under accession no. K00826) inserted into the ClaI site of a HindIII− derivative of pBR322. Different restriction fragments were excised from pTAC1257 and inserted in front of the lacZ gene in the promoter cloning vector pTAC3953 (14) to obtain plasmids pTAC4140, pTAC4141, pTAC4142, and pTAC4144 (see Fig. 7). The pmioC-lacZ fusions from the first three plasmids were subsequently transferred into the λ attachment site as described previously (5) to obtain strains TC4300, TC4301, and TC4302.
FIG. 1.
Construction of expression vectors. The starting point for the T7 promoter expression vectors was plasmid pFHC2003, a pGEMEX-1 derivative lacking the XbaI fragment (positions 30 to 942 in GenBank under accession no. X65317) and with the NdeI site filled in with Klenow polymerase. Two oligonucleotides were synthesized to form a HindIII-XbaI multiple cloning site (MCS) adapter with an NdeI site encompassing a translation start signal (underlined) positioned favorably to a good Shine-Dalgarno sequence (double underline). This adapter was used to replace the HindIII-XbaI sequences of pFHC2003 to construct pFHC2006[rom−]. A rom+ derivative, pFHC2013, was constructed by combining the Eco47III-AatII fragment of plasmid pFHC2004 (pBR322 with the NdeI site filled in with Klenow polymerase) and the AatII-SmaI fragment of pFHC2006. Thus, pFHC2013 carries the origin and the bla gene from pBR322, whereas pFHC2006 carries the corresponding pUC variants. The T7 promoter was removed from plasmids pFHC2006 and pFHC2013 by replacing an XbaI-BglII fragment with an adapter formed by oligonucleotides 5.6 (CTAGAGCTCGAGTACTGTCGACA) and 5.7 (GATCTGTCGACAGTACTCGAGCT) to produce plasmids pFHC2069 and pFHC2070. Finally, an XhoI fragment carrying lacP(A1/04/03) and lacI from pFHC2014 (a derivative of pBEX5BA [21], wherein an SphI site was treated with T4 DNA polymerase to remove this site and an overlapping NsiI site) was inserted into the plasmids pFHC2069 and pFHC2070 cut with XhoI and SalI to construct the two new lac promoter cloning vectors pFHC2101[rom−] (GenBank accession no. AY070364) and pFHC2102[rom+] (GenBank accession no. AY070365).
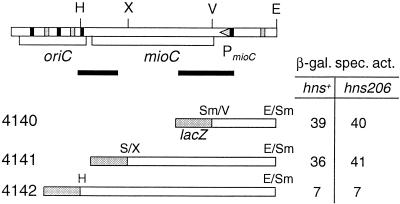
FIG. 7.
Effect of the hns206 mutation on mioC transcription. At the top is a map of the oriC region contained in pTAC1257. Abbreviations: E, EcoRI; H, HindIII; S, SmaI; V, EcoRV; X, XhoI. Triangle, promoter; rectangles, DnaA boxes (black = consensus boxes; gray = consensus with 1 misfit). Black lines indicate the location of regions of bend DNA according to Kimura et al. (29). The extent of mioC DNA carried by the different fusions is given below, together with the specific β-galactosidase activity in cultures grown at 30°C in glucose-Casamino Acids-supplemented medium.
Purification of H-NS protein and gel retardation assay.
H-NS protein was overproduced and purified, and the protein concentration was determined exactly as described previously (20). Gel retardation assays were carried out as described earlier (20) with 10-μl reactions containing increasing concentrations of purified H-NS protein (0.03 to 0.3 μg). The DNA used for the reaction was either 0.25 μg of plasmid pTAC4144 digested with HinfI plus BstEII or a mixture of purified oriC PCR fragments (5 to 10 ng) mixed with the control pbla (10-ng) and tet (20-ng) PCR fragments.
RESULTS
Initiation synchrony in hns mutants.
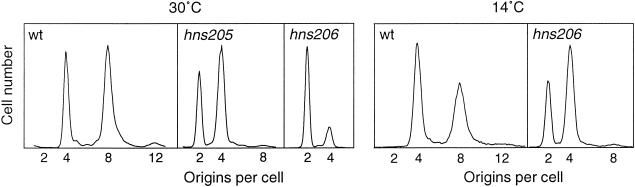
Normally, all origins present in a single cell of E. coli initiate nearly simultaneously. Thus, if initiation is inhibited and ongoing rounds of replication are allowed to terminate, the cells will contain predominantly 2n genome equivalents, i.e., four or eight for fast-growing wild-type cells. To test the requirement of H-NS for synchronous initiation, isogenic hns+, hns205, and hns206 strains were grown in rich medium and samples were treated with rifampin and cephalexin and analyzed by flow cytometry. Figure 2 shows that initiations in the hns mutants are synchronous, indicating that the initiation cascade is working perfectly in the absence of H-NS protein.
FIG. 2.
Initiation synchrony. Strains BBC119, TC4061 (hns205), and TC4136 (hns206) were grown in AB glucose-Casamino Acids medium at the indicated temperatures. Samples were treated with rifampin and cephalexin and fixed, stained, and analyzed by flow cytometry.
Effect of hns mutations on cell cycle parameters.
It is striking that the hns mutants have fewer origins per cell than the wild type (Fig. 2). From the logistics of the bacterial cell cycle (24), the number of origins per cell (or the ploidy) is a function of three parameters: tD, the generation time; C, the replication time; and D, the time from termination to division (cell separation). The hns206 mutant has an increased generation time (Table 2) which contributes significantly to the reduced number of origins per cell. There must, however, also be a significant reduction in the C+D time in the mutant, as estimated from the origins per cell.
TABLE 2.
Comparison of cell cycle and initiation parameters in the wild type and the hns206 mutanta
| Growth temp (°C) | Genotype | Origins/cell | DNA (g.e.)b/cell | tD (min) | C + Dc (min) | Ce (min) | Df (min) | Cell size (LS/cell)g | Origins/LSh | DnaA-β-galactosidase contenti |
|---|---|---|---|---|---|---|---|---|---|---|
| 37 | hns+ | 7.2 | 5.0 | 27 | 75 | 41 | 40 | 1.00 | 1.00 | 1.00 |
| hns206 | 4.1 | 3.4 | 31 | 63 | 34 | 36 | 0.69 | 0.84 | 1.28 | |
| 30 | hns+ | 7.1 | 5.0 | 44 | 124 | 62 | 69 | 0.97 | 1.01 | 1.00 |
| hns206 | 3.1 | 2.5 | 58 | 95 | 29 | 62 | 0.64 | 0.67 | 1.42 | |
| 14 | hns+ | 6.2 | 3.1 | 450 | NCd | 630 | 400 | 0.86 | 1.00 | NDj |
| hns206 | 3.5 | 2.4 | 960 | NC | 750 | 800 | 0.91 | 0.54 | ND |
Strains BBC119 and TC4136 were grown in glucose-Casamino Acids-supplemented medium at the indicated temperatures. Samples were obtained for flow cytometric analysis of DNA, origins, and light scatter (LS); for Southern blot analysis of origins per termini (O/T); and for determination of DnaA-β-galactosidase specific activity.
g.e., genome equivalents.
Determined as: origins per cell = 2(C+D)/tD.
NC, not calculated due to rifampin-resistant initiations (3).
Determined as: O/T = 2C/tD.
Determined as: g.e./cell = [(O/T − 1)/ln(O/T)]·2D/tD (24).
Normalized to the value for BBC119 grown at 37°C.
That is, (origins/cell)/(LS/cell) normalized to the value for BBC119 grown at 37°C.
Measured as the specific activity normalized to that of BBC119 grown at the same temperature.
ND, not determined.
In order to distinguish between an effect on C and an effect on D, we determined the C time in the wild type and in the hns206 mutant by Southern blot marker frequency analysis of the origin-to-terminus ratio. We found that the hns mutant had a much shorter C time than the wild type, especially at 30°C, where it was only half of that in the hns+ strain. When the C time is known, the D time can be calculated from the amount of DNA per cell, or D can be estimated from origins per cell, which generally gives somewhat lower values, probably due to slightly inefficient inhibition of cell division by cephalexin (Table 2). By either method we found that the D time in the hns206 mutant grown at 37 or 30°C is a little shorter than that of the wild type.
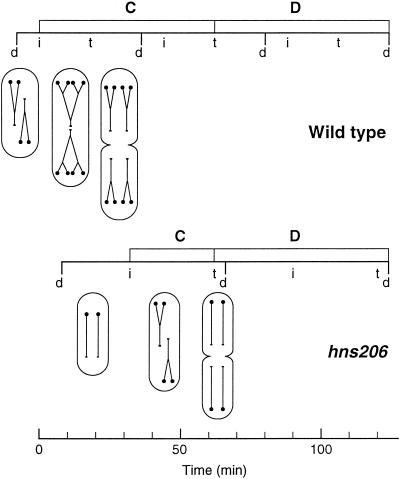
The cell cycles of the wild type and the hns206 mutant grown at 30°C were summarized in schematic form (Fig. 3). Both the wild type and the mutant grow as diploid cells since the D times are longer than the generation times. The wild-type cell is born as a cell with four origins and two more-than-half-replicated chromosomes. After ca. one-fifth of a cell cycle, a second set of replication forks is initiated, yielding a cell with eight origins and six pairs of replication forks. After three-fifths of the cell cycle, the old replication forks terminate, but this termination only gives rise to division at the end of the next cell cycle. The replication pattern of the hns mutant is simpler. It is born as a cell with two fully replicated chromosomes and initiates replication around the middle of the cell cycle, and termination takes place just before the division elicited by the round of replication in the previous cycle.
FIG. 3.
Schematic representation of the cell cycles in wild type and hns206 mutant at 30°C as a function of time. Nonreplicating chromosomes are drawn as lines with the small circle symbolizing the origin. Replicating chromosomes are shown as forked or multiforked lines. Cell mass is reflected in the relative size of the cells assuming that the difference between wild type and mutant leads to proportional changes in width and length. Division (d), initiation (i), and termination (t) are indicated along the line representing the successive cycles.
Effect of hns mutations on initiation control.
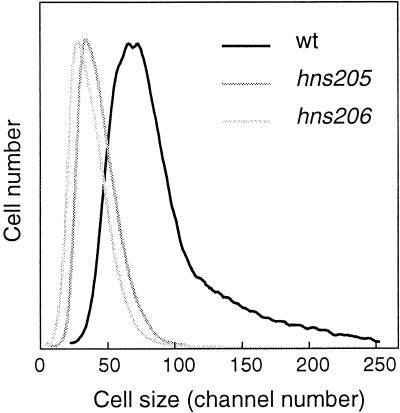
Although H-NS is not required for initiation synchrony within the single cell, it might affect the timing of replication, the interinitiation time, or the initiation mass (i.e., the mass per origin at initiation). We therefore determined the cell size distributions of hns mutants relative to that of the wild type (Fig. 4). It is clear that the size distributions of the mutants are as homogeneous as that of the hns+ strain, indicating that the coefficients of variation on interinitiation and the C and D times are very similar. The mean cell size is a function of the cell cycle parameters and the initiation mass; thus, the longer tD and shorter C and D times lead to smaller cells in the mutants.
FIG. 4.
Flow cytometric cell size distributions of strains BBC119, TC4061 (hns205), and TC4136 (hns206) grown in AB glucose-Casamino Acids medium at 30°C.
The relative origin concentration was determined from the number of origins per cell, and the mean cell size in samples incubated in rifampin and cephalexin. The hns206 mutant (Table 2) and the hns205 mutant (data not shown) both had significantly lower origin concentrations than the wild type. The defect in initiation was more pronounced the lower the growth temperature (Table 2). The lower origin concentration was partially compensated by the shorter C time resulting in more normal DNA concentrations. Thus, although the mutants have smaller cells than the wild type, the initiation mass (i.e., the mass per origin) is actually larger, e.g., at 30°C the hns206 mutant has a 1.5-fold larger initiation mass.
Effect of hns mutations on dnaA gene expression.
The DnaA protein is the most important factor determining the initiation mass and, normally, origin and DnaA concentrations are proportional (25). The effect of the hns206 mutation on the origin per mass could therefore be due to a decreased dnaA gene expression. The strains used in this study are lysogens carrying a dnaA"lacZ fusion, allowing us to monitor dnaA gene expression. The results (Table 2) show that dnaA gene expression is not decreased in the absence of H-NS protein; on the contrary, the mutant strain has slightly increased DnaA-β-galactosidase activity, suggesting that H-NS in the wild-type strain might have a small inhibitory effect on dnaA promoter activity.
Since the effect of H-NS might be on translation of the dnaA"lacZ fusion or on stability of the fusion protein, we determined DnaA protein by immunoblot and found that the hns206 mutant had a 20% increase in DnaA protein concentration at both 37 and 30°C (data not shown). The larger effect on DnaA-β-galactosidase activity compared to DnaA concentration is due to the change in relative concentration of the dnaA gene (located 1 min from oriC) and the dnaA"lacZ fusion located in attB (33 min from oriC) caused by the shorter C time in the hns206 mutant. In conclusion, the hns mutants seem to require more DnaA protein per origin for initiation.
Replication phenotypes of hns mutants are independent of rpoS.
Some of the replication phenotypes of the hns mutants might be mediated by the increased σS concentration found in such strains (10). Our hns+ strain BBC119 is derived from C600 and carries the rpoS396 amber mutation but is phenotypically RpoS+ (6) since it is suppressed by the glnV44 mutation, which is also present in the strain. Isogenic rpoS359::Tn10 derivatives (TC4409 and TC4410) of the hns+ and hns206 strains, however, behaved exactly like the parental strains, having the same number of origins per cell, the same origin concentration, and the same specific activity of DnaA-β-galactosidase (data not shown). We also analyzed the effect of the hns205 and hns206 mutations in the MC4100 background, which is genetically rpoS+, and found the same effects on origins per cell and origins per mass. We therefore conclude that σS plays no role in the cell cycle of exponentially growing cells.
Replication phenotypes at low temperature.
H-NS is a cold shock protein (32), and hns mutants are cold sensitive for growth (19). We therefore studied the replication phenotype of the hns206 mutant strain grown at 14°C to determine whether the cold sensitivity might be due to accentuated replication problems.
As expected, the growth defect of the hns206 strain becomes more pronounced the lower the temperature (Table 2). Despite the very slow growth at 14°C, both origins per cell and DNA per cell were similar to those at 30°C and the origin concentration (Table 2) was only slightly more affected at 14°C than at 30°C, indicating that the accentuated growth defect is not primarily related to replication.
Overproduction of H-NS.
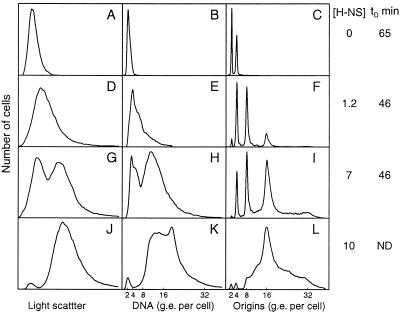
To study the effects of various levels of H-NS on initiation of replication and the cell cycle parameters, we constructed a plasmid (pFH2242 and the Camr derivative pTAC4617) in which the hns gene expression is under the control of the Lac repressor. Plasmid pTAC4617 was introduced into the hns206 mutant, and the cells were grown at 30°C and shifted to different concentrations of IPTG, giving different concentrations of H-NS protein. Selected flow cytometric distributions of samples taken after four to five generations of growth are shown in Fig. 5, and the results are summarized in Fig. 6. In the absence of IPTG, the cells contained no detectable H-NS protein (data not shown), the cell size and DNA and origin distributions were very similar to those of the strain without plasmid (compare Fig. 5A with Fig. 4 and Fig. 5C with Fig. 2), and the generation time was as long. At 0.125 mM IPTG, the H-NS concentration was close to that of the wild type (1.2-fold increased), and the growth rate, average cell size, and DNA and origin contents were the same as in the wild type. At higher H-NS concentrations the generation time was still the same, but both cell size and DNA distributions showed two peaks (Fig. 5G and H), indicating that the cultures consisted of a mixture of two populations of cells: one with near-wild-type cell size and DNA content and one with twice the size and DNA content. In the rifampin-treated sample the subpopulation of large cells contained predominantly 16 origins per cell. At IPTG concentrations greater than 0.18 mM (10 times higher than normal H-NS levels), growth gradually slowed down after the shift and the cultures consisted nearly entirely of large cells with more than eight genome equivalents of DNA per cell (Fig. 5J and K). At these high H-NS levels the cells were not able to complete replication in rifampin (Fig. 5L).
FIG. 5.
Flow cytometry analysis of cells containing increasing amounts of H-NS protein. Strain TC4617 (hns206 pTAC4617) was grown at 30°C in glucose-Casamino Acids-supplemented medium. The culture was divided into parts that received different amounts of IPTG. After four generations, samples were taken for analysis of the amount of H-NS protein by immunoblot and for analysis of cell size, DNA content, and origin distributions by flow cytometry. The concentration of H-NS relative to the hns+ strain TC3983 is given to the right of each row of panels, together with the generation time (tD) in min. ND, the tD could not be determined for this culture since growth slowed down progressively after induction. Panels: A to C, no IPTG; D to F, 0.125 mM IPTG; G to I, 0.15 mM IPTG; J to L, 0.18 mM IPTG. g.e., genome equivalents.
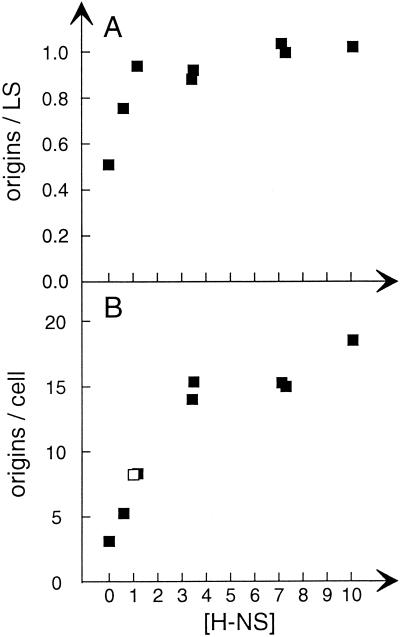
FIG. 6.
Origin concentration and ploidy as a function of H-NS concentration. Strains TC4617 (hns206 pTAC4617) and TC3983 (hns+) were grown at 30°C in glucose-Casamino Acids-supplemented medium. The TC4617 culture was divided into parts that received different amounts of IPTG. After four generations samples were taken for analysis of the amount of H-NS protein by immunoblot and for analysis of cell size and origins per cell by flow cytometry. The concentration of H-NS and origins/light scatter (LS) values are given relative to the values of strain TC3983. Symbols: ▪, TC4617; □, TC3983.
Origins per mass increased with increasing H-NS concentrations until the normal concentration was reached, and then it leveled off (Fig. 6A). dnaA gene expression was not affected by higher-than-normal H-NS levels in the range that gave a wild-type growth rate (data not shown). Thus, higher-than-normal H-NS levels do not stimulate initiation. Origins per cell, however, increased with increasing H-NS levels by up to threefold greater than that of the wild type. Since the level of DNA per cell increased in parallel with origins per cell (see Fig. 5 for examples), the C time must be fairly normal at the moderately high H-NS levels, whereas the D time becomes much longer. At the higher toxic levels of H-NS the C time is probably also becoming longer. Very similar results with both subnormal and above normal H-NS concentrations were obtained in experiments with the hns205 mutant carrying pFHC2242 (data not shown).
Analysis of H-NS binding to the oriC region by competitive gel retardation assays.
The decreased origin concentration in hns mutants might be due to a direct effect by binding of H-NS to oriC, e.g., to the major bend located just to the right of the minimal oriC (Fig. 7) or elsewhere. We therefore carried out competitive gel retardation assays with different PCR fragments from the oriC region to see whether it contained a high-affinity H-NS binding site. We did find preferential binding of H-NS both to a fragment containing the minimal oriC and the gidA promoter and to a fragment containing the major bend, but these fragments both showed rather poor affinity—lower than the medium affinity bla promoter fragment (data not shown). We found the same relative affinities for oriC and the pbla fragments when a restriction digest of plasmid DNA was used for the assay (data not shown), indicating that, unlike the major bend (29), the binding affinity of H-NS to oriC was not influenced by methylation of the numerous GATC sites present in the region.
Effect of H-NS on mioC transcription.
Although H-NS showed virtually no preferential binding to the mioC promoter in the gel retardation assays, it might have an effect on transcription from the promoter entering oriC. We therefore introduced the hns206 mutation into strains carrying pmioC-lacZ fusions with three different fusion points downstream of the mioC promoter (Fig. 7). The hns206 mutation, however, had no effect on the mioC transcription, thus ruling out the possibility that the decreased origin concentration was due to increased transcription from mioC into oriC.
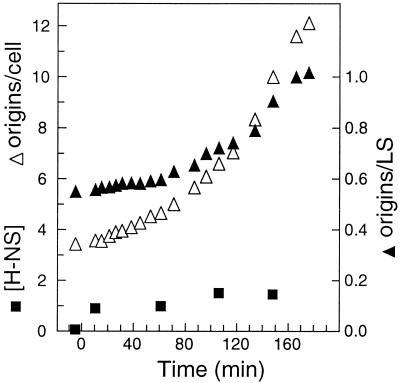
Kinetics of initiation of replication upon induction of H-NS synthesis.
To distinguish between a direct effect of H-NS on initiation at oriC or an indirect effect, we carried out a “shift” experiment from zero H-NS to near-normal levels of H-NS. The hns206 strain carrying pTAC4617 was grown in the absence of IPTG and then induced with high IPTG for a short time, just enough to accumulate wild-type levels of H-NS, and then the culture was diluted to a lower IPTG concentration giving a steady-state approximately wild-type H-NS concentration (Fig. 8). The stimulatory effect of H-NS induction on growth rate was almost immediate (data not shown). Initiation was followed by flow cytometry of samples treated with rifampin and cephalexin. If H-NS affects initiation directly by binding to oriC and facilitating a subsequent step, we would expect the cells to start initiating very shortly after induction, leading to an increased origin-per-mass ratio. An analysis of origins per light scatter shows that it takes more than one generation time (43 min) before the effect of normal levels of H-NS on initiation starts to take effect (Fig. 8). In contrast, the origins per cell started to increase soon after normal H-NS levels were reached, as predicted from the immediate decrease in generation time from 65 to 43 min, since origins/cell = 2(C+D)/tD.
FIG. 8.
Kinetic analysis of initiation after H-NS induction. Strain TC4617 (hns206 pTAC4617) was grown at 30°C in glucose-Casamino Acids-supplemented medium. At time zero, IPTG was added to 0.3 M for 10 min, and then the culture was diluted to obtain an IPTG concentration of 0.13 mM. Samples were taken before and after the shifts for analysis of the amount of H-NS protein by immunoblot and for analysis of cell size and origins per cell by flow cytometry. The concentration of H-NS and origins/light scatter (LS) are given relative to the values of the hns+ strain TC3983.
DISCUSSION
We confirmed here the previous observation (27) that hns mutants show normal initiation synchrony and have a reduced number of origins per cell (ploidy). We found that the reduction in origins per cell exceeded that expected from the reduced growth rate and was primarily due to a decreased replication time. In addition, we found that the hns mutants showed a decreased origin concentration, despite a slightly higher-than-normal DnaA protein concentration. The effects on both ploidy and origin concentration were more pronounced in the hns206 mutant (insertion mutation in codon 37 [19]) than in the hns205 mutant (insertion at codon 93 [19]). Thus, the truncated H-NS protein produced in the hns205 strain (19) must have some residual activity. In many cases where H-NS acts as a transcriptional repressor it participates in thermoregulation, and inactivation of H-NS has more severe effects at lower temperature. Here we also found quantitatively more pronounced effects of the hns mutations at 30°C than at 37°C, with respect both to initiation and elongation of replication. The origin concentration, however, only decreased a little more going to 14°C, and the C time was nearly the same as that of the wild type at 14°C, indicating that the cold sensitivity of hns mutants (19) is not due to replication problems.
To determine whether H-NS plays a role in setting the initiation mass, we carried out a control analysis varying the H-NS concentration in the cell. Subnormal levels of H-NS gave an intermediate phenotype both with respect to origins per mass and ploidy, while higher than normal H-NS concentrations did not change the origin concentration. Thus, lack of H-NS limits initiation but H-NS cannot be considered to control initiation since increased H-NS concentrations, relative to that found under normal growth conditions, has no effect on initiation control. In a wild-type strain the H-NS concentration can be expected to be kept fairly constant throughout the cell cycle by the autoregulation of the hns gene (20), and the only reported environmental regulation of H-NS concentration is a moderate increase in stationary-phase cells and the threefold increase upon cold shock (see reference 4 for a review).
Lack of H-NS protein led to a decrease in ploidy, primarily due to a shorter C time, while higher-than-normal levels led to an increase in ploidy, probably due primarily to a longer D time. The increased D time could be caused by a negative effect of H-NS on transcription of cell division genes such as ftsZ, or it could be caused by an inhibitory effect on chromosome resolution and/or segregation by nonspecific binding of surplus H-NS that leads to nucleoid condensation (40).
The replication phenotypes of the hns mutants are very similar to those of ihf mutants (42). Both kinds of mutants have decreased origin concentration, normal or slightly increased DnaA concentrations, and a very short C time. The only phenotypic difference is that hns mutants do not exhibit the rifampin-resistant initiation phenotype and apparent asynchronous initiation. The very short replication times found in these mutants might be due to the increased mass present per replication fork, which could give a higher amount of processivity factors like Ssb protein, clamp loader complex, and a higher potential for deoxynucleoside triphosphate synthesis. It is probably not caused by increased synthesis of these factors in the hns mutants since the fast replication rate is also observed in the other mutants that have a decreased origin concentration, e.g., ihf (42), dnaA46 (12), other dnaA(Ts) mutant strains and in an fis mutant (T. Atlung and F. G. Hansen, unpublished data). The absence of H-NS (or IHF) might, however, also affect the replication rate directly by reducing the total number of nucleoid bound proteins that have to be cleared away by the replisome.
The decreased origin concentration in the hns mutants is not due to a decreased DnaA protein concentration. Thus, in the absence of H-NS it seems that more than twice the amount of DnaA per oriC is needed for initiation, in agreement with the suppression of the dnaA (cos) mutant over initiation and enhancement the dnaA(Ts) phenotypes by a hns mutation (28). That an hns mutant allows better replication than the wild type of minichromosomes with mutations in the right half of oriC (31) could be due to more available protein for binding to DnaA boxes. Alternatively, this might just be another example of the different behavior of minichromosomes and the chromosome (1).
The requirement for more DnaA protein per origin in the absence of H-NS might be because H-NS is needed for formation of a proper nucleoprotein structure for efficient binding of DnaA to low-affinity sites in oriC (e.g., DnaA boxes R3 and M). Two different sets of results, however, argue against this possibility. First, we did not observe any preferential binding of H-NS to fragments from the oriC region, to fragments encompassing the previously described bend (29), or to other fragments. Second, providing the cells with normal concentrations of H-NS by a burst of synthesis did not elicit an immediate round of initiation as expected if H-NS acted directly at oriC. The results of this shift experiment also argue against the hypothesis that the DnaA boxes located around the chromosome might be titrating more DnaA protein (17) in the absence of H-NS, i.e., boxes like that in the appY promoter, which is an in vitro high-affinity box (37) and is located close to a high-affinity H-NS binding site (7) but which does not titrate DnaA efficiently in vivo (Atlung and Hansen, unpublished). In this case we would also expect a sudden supply of H-NS to lead to a quick increase in the amount of free DnaA protein, followed by initiation in the majority of the cells. Therefore, we favor the alternative hypothesis that H-NS in some way indirectly increases the activity of the DnaA protein.
Acknowledgments
We thank Anne Nielsen, Søs Koefoed, and Kirsten Olesen for expert technical assistance and Erhard Bremer for the generous gift of H-NS antibody.
This work was supported by a grant from the Danish Natural Science Research Council.
REFERENCES
- 1.Asai, T., D. B. Bates, E. Boye, and T. Kogoma. 1998. Are minichromosomes valid model systems for DNA replication control? Lessons learned from Escherichia coli. Mol. Microbiol. 29:671-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atlung, T., and F. G. Hansen. 1993. Three distinct chromosome replication states are induced by increasing concentrations of DnaA protein in Escherichia coli. J. Bacteriol. 175:6537-6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atlung, T., and F. G. Hansen. 1999. Low-temperature-induced DnaA protein synthesis does not change initiation mass in Escherichia coli K-12. J. Bacteriol. 181:5557-5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atlung, T., and H. Ingmer. 1997. H-NS: a modulator of environmentally regulated gene expression. Mol. Microbiol. 24:7-17. [DOI] [PubMed] [Google Scholar]
- 5.Atlung, T., A. Nielsen, L. J. Rasmussen, L. J. Nellemann, and F. Holm. 1991. A versatile method for integration of genes and gene fusions into the λ attachment site of Escherichia coli. Gene 107:11-17. [DOI] [PubMed] [Google Scholar]
- 6.Atlung, T., H. V. Nielsen, and F. G. Hansen. 2002. Characterisation of the allelic variation in the rpoS gene in 13 K12 and six other nonpathogenic Escherichia coli strains. Mol. Gen. Genomics 266:873-881. [DOI] [PubMed]
- 7.Atlung, T., S. Sund, K. Olesen, and L. Brøndsted. 1996. The histone-like protein H-NS acts as a transcriptional repressor for expression of the anaerobic and growth phase activator AppY of Escherichia coli. J. Bacteriol. 178:3418-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azam, T. A., A. Iwata, A. Nishimura, S. Ueda, and A. Ishihama. 2000. Growth phase-dependent variation in protein composition of the Escherichia coli nucleoid. J. Bacteriol. 181:6361-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachmann, B. J. 1990. Linkage map of Escherichia coli K-12, 8th ed. Microbiol. Rev. 54:130-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barth, M., C. Marschall, A. Muffler, D. Fischer, and R. Hengge-Aronis. 1995. Role for the histone-like protein H-NS in growth phase-dependent and osmotic regulation of σS and many σS-dependent genes in Escherichia coli. J. Bacteriol. 177:3455-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boye, E., A. Lyngstadaas, A. Løbner-Olesen, K. Skarstad, and S. Wold. 1992. Regulation of DNA replication in Escherichia coli, p. 15-26. In E. Fanning, R. Knippers, and E.-L.Winnacker (ed.), Colloquium Mosbach-DNA replication and the cell cycle. Springer-Verlag, Berlin, Germany.
- 12.Boye, E., T. Stokke, N. Kleckner, and K. Skarstad. 1996. Coordinating DNA replication initiation with cell growth: differential roles for DnaA and SeqA proteins. Proc. Natl. Acad. Sci. USA 93:12206-12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun, R. E., K. O'Day, and A. Wright. 1985. Autoregulation of the DNA replication gene dnaA in E. coli. Cell 40:159-169. [DOI] [PubMed] [Google Scholar]
- 14.Brøndsted, L., and T. Atlung. 1994. Anaerobic regulation of the hydrogenase 1 (hya) operon of Escherichia coli. J. Bacteriol. 176:5423-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brøndsted, L., and T. Atlung. 1996. Effect of growth conditions on expression of the acid phosphatase (cyx-appA) operon and the appY gene, which encodes a transcriptional activator of Escherichia coli. J. Bacteriol. 178:1556-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang, A. C. Y., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the p15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christensen, B. B., T. Atlung, and F. G. Hansen. 1999. DnaA boxes are important elements in setting the initiation mass of Escherichia coli. J. Bacteriol. 181:2683-2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark, D. J., and O. Maaløe. 1967. DNA replication and the division cycle in Escherichia coli. J. Mol. Biol. 23:99-112. [Google Scholar]
- 19.Dersch, P., S. Kneip, and E. Bremer. 1994. The nucleoid-associated DNA-binding protein H-NS is required for the efficient adaptation of Escherichia coli K-12 to a cold environment. Mol. Gen. Genet. 245:255-259. [DOI] [PubMed] [Google Scholar]
- 20.Dersch, P., K. Schmidt, and E. Bremer. 1993. Synthesis of the Escherichia coli K-12 nucleoid-associated DNA-binding protein H-NS is subjected to growth-phase control and autoregulation. Mol. Microbiol. 8:875-889. [DOI] [PubMed] [Google Scholar]
- 21.Diederich, L., A. Roth, and W. Messer. 1994. A versatile plasmid vector system for the regulated expression of genes in Escherichia coli. BioTechniques 16:916-923. [PubMed] [Google Scholar]
- 22.Dixon, N. E., and A. Kornberg. 1984. Protein HU in the enzymatic replication of the chromosomal origin of Escherichia coli. Proc. Natl. Acad. Sci. USA 81:424-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen, F. G., T. Atlung, R. E. Braun, A. Wright, P. Hughes, and M. Kohiyama. 1991. Initiator (DnaA) protein concentration as a function of growth rate in Escherichia coli and Salmonella typhimurium. J. Bacteriol. 173:5194-5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helmstetter, C. E. 1996. Timing of synthetic activities in the cell cycle, p. 1627-1639. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 25.Herrick, J., M. Kohiyama, T. Atlung, and F. G. Hansen. 1996. The initiation mess? Mol. Microbiol. 19:659-666. [DOI] [PubMed] [Google Scholar]
- 26.Higgins, C. F., C. J. Dorman, D. A. Stirling, L. Waddell, I. R. Booth, G. May, and E. Bremer. 1988. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell 52:569-584. [DOI] [PubMed] [Google Scholar]
- 27.Kaidow, A., M. Wachi, J. Nakamura, J. Magae, and K. Nagai. 1995. Anucleate cell production by Escherichia coli Δhns mutant lacking a histone-like protein, H-NS. J. Bacteriol. 177:3589-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katayama, T., M. Takata, and K. Sekimizu. 1996. The nucleoid protein H-NS facilitates chromosome DNA replication in Escherichia coli dnaA mutants. J. Bacteriol. 178:5790-5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimura, T., T. Asai, M. Imai, and M. Takanami. 1989. Methylation strongly enhances DNA bending in the replication origin region of the Escherichia coli chromosome. Mol. Gen. Genet. 219:69-74. [DOI] [PubMed] [Google Scholar]
- 30.Lange, R., and R. Hengge-Aronis. 1991. Identification of a central regulator of stationary phase gene expression in Escherichia coli. Mol. Microbiol. 5:49-59. [DOI] [PubMed] [Google Scholar]
- 31.Langer, U., S. Richter, A. Roth, C. Weigel, and W. Messer. 1996. A comprehensive set of DnaA box mutations in the replication origin, oriC, of Escherichia coli. Mol. Microbiol. 21:301-311. [DOI] [PubMed] [Google Scholar]
- 32.La Teana, A., A. Brandi, M. Falconi, R. Spurio, C. L. Pon, and C. O. Gualerzi. 1991. Identification of a cold shock transcriptional enhancer of the Escherichia coli gene encoding nucleoid protein H-NS. Proc. Natl. Acad. Sci. USA 88:10907-10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Løbner-Olesen, A., K. Skarstad, F. G. Hansen, K. von Meyenburg, and E. Boye. 1989. The DnaA protein determines the initiation mass of Escherichia coli K-12. Cell 57:881-889. [DOI] [PubMed] [Google Scholar]
- 34.Messer, W., and C. Weigel. 1996. Initiation of chromosome replication, p. 1578-1601. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 35.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratories, Cold Spring Harbor, N.Y.
- 36.Rasmussen, L. J., P. L. Møller, and T. Atlung. 1991. Carbon metabolism regulates expression of the pfl (pyruvate formate-lyase) gene in Escherichia coli. J. Bacteriol. 173:6390-6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roth, A., and W. Messer. 1998. High-affinity binding sites for the initiator protein DnaA on the chromosome of Escherichia coli. Mol. Microbiol. 28:395-401. [DOI] [PubMed] [Google Scholar]
- 38.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Skarstad, K., H. B. Steen, and E. Boye. 1985. Escherichia coli DNA distributions measured by flow cytometry and compared with theoretical computer simulations. J. Bacteriol. 163:661-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spurio, R., M. Dürrenberger, M. Falconi, A. La Teana, C. L. Pon, and C. O. Gualerzi. 1992. Lethal overproduction of the Escherichia coli nucleoid protein H-NS: ultramicroscopic and molecular autopsy. Mol. Gen. Genet. 231:201-211. [DOI] [PubMed] [Google Scholar]
- 41.Studier, F. W., and B. A. Moffat. 1986. Use of the bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189:113-120. [DOI] [PubMed] [Google Scholar]
- 42.von Freiesleben, U., K. V. Rasmussen, T. Atlung, and F. G. Hansen. 2000. Rifampicin resistant initiation of chromosome replication from oriC in ihf mutants. Mol. Microbiol. 37:1087-1093. [DOI] [PubMed] [Google Scholar]
- 43.Yamada, H., T. Yoshida, K.-I. Tanaka, C. Sasakawa, and T. Mizuno. 1991. Molecular analysis of the Escherichia coli hns gene encoding a DNA-binding protein, which preferentially recognizes curved DNA sequences. Mol. Gen. Genet. 230:332-336. [DOI] [PubMed] [Google Scholar]