Abstract
The opportunistic fungal pathogen, Candida albicans, is reported to have several potential virulence factors. A potentially significant factor is the ability to undergo morphological transition from yeast to hypha. This alteration of form is accompanied by many changes within the cell, including alterations in gene expression and cell wall composition. We have isolated a gene that encodes a highly conserved serine/threonine kinase that appears to be involved in the regulation of proteins associated with the cell wall. We have assigned the designation CBK1 (cell wall biosynthesis kinase 1) to this gene. Mutants lacking CBK1 form large aggregates of round cells under all growth conditions and lack the ability to undergo morphological differentiation. Additionally, these mutants show an altered pattern of expression of several transcripts encoding proteins associated with the cell wall. The results suggest that the kinase encoded by CBK1 plays a general role in the maintenance and alteration of the cell wall of C. albicans in all morphologies.
Candida albicans is a member of the gastrointestinal normal microbiota of humans. This fungus is also a significant opportunistic pathogen capable of causing a wide range of infections in the compromised host (20). Advances in the understanding of the processes responsible for virulence in C. albicans have often come from the isolation and characterization of homologs responsible for similar processes in other fungi.
The corn pathogen Ustilago maydis alternates between the yeast and hyphal phases of growth during the infectious process. It was reported that a conserved serine/threonine kinase encoded by the UKC1 gene affected morphogenesis and virulence (7). Disruption of UKC1 resulted in strains that grew more slowly than wild-type strains, exhibited aberrant cellular morphology, produced more pigment, and had lost virulence. The homolog from Neurospora crassa, encoded by the cot-1 gene, is necessary for maintenance of normal cellular and colonial growth. Strains containing a temperature-sensitive cot-1 mutation showed compact growth, excessive branching, and lack of conidiation at the nonpermissive temperature (28). In the fission yeast, Schizosaccharomyces pombe, orb6 encodes this conserved kinase. orb6 is an essential gene in S. pombe that is involved in the maintenance of asymmetrical growth and reorganization of actin after mitosis (27).
We report here the isolation of the C. albicans homolog, which we have designated CBK1 (cell wall biosynthesis kinase) after the Saccharomyces cerevisiae homolog (22). CaCBK1 shows extensive homology to the other members of this family of kinases, a subgroup of the “AGC” kinases (11), within the defined kinase domain. cbk1 null mutants demonstrate aberrant morphology and show an altered pattern of expression of several genes encoding cell wall proteins and altered expression of two hypha-related transcripts. The results suggest that, like the other homologs, Cbk1p is involved in the maintenance and regulation of cellular morphology.
Isolation and identification of the UKC1 homolog.
Sequence data for C. albicans were obtained from the Stanford DNA Sequencing and Technology Center website (http://www-sequence.stanford.edu/group/candida/ [revision date, Jan. 2001; last date accessed, July 2001]). A TBLASTN search of the C. albicans genome sequence database with the U. maydis Ukc1p sequence identified a single homologous sequence. Homology searches were conducted using the BLAST algorithm (1). An 826-bp fragment beginning 213 bp 5′ to the start site of CBK1 was amplified by PCR, and this product was used as a probe for hybridization screening of a λGEM-12 genomic library (4). A 5.5-kb EcoRI insert containing the entire CBK1 open reading frame (ORF) was subcloned into pUC18, generating the plasmid pMM1. A partial sequence of the CBK1 ORF was determined by cycle sequencing with AmpliTaq DNA polymerase, the ABI Prism Ready Reaction Kit (Perkin-Elmer), and custom oligonucleotide primers. The sequence obtained from pMM1 was identical to the ORF designated YLN161 of Contig6-2278 that is available on the Stanford DNA Sequencing and Technology Center website (http://www-sequence.stanford.edu/group/candida/).
A BLASTP comparison with the GenBank database revealed that the predicted protein showed extensive homology with Cbk1p from S. cerevisiae (22), Ukc1p from U. maydis (7), orb6p from S. pombe (27), cot-1p from N. crassa (28), and multiple other members of the AGC family of eukaryotic kinases (5, 6, 11, 12).
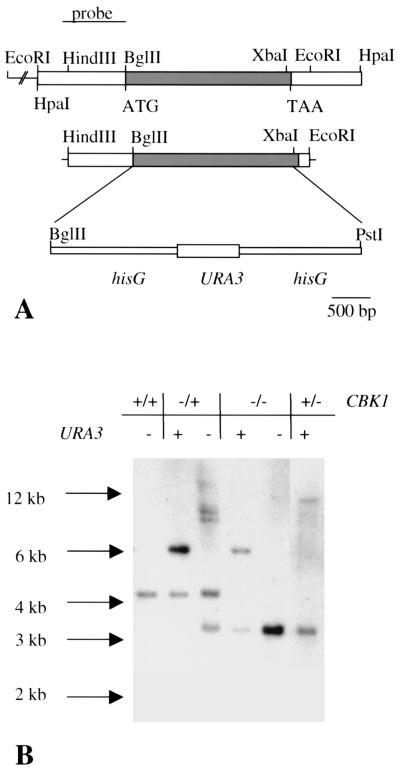
To determine the role CBK1 plays in C. albicans, a cbk1 null mutant was constructed by replacing a 2,167-bp segment of the CBK1 coding region with a hisG-URA3-hisG cassette (Fig. 1A). A 3.5-kb EcoRI-HindIII fragment from pMM1 was subcloned into like sites in pUC18. The resulting plasmid, pMM2, was digested with XbaI and filled in using Klenow DNA polymerase to create a blunt end. The blunt-ended linearized plasmid was then digested with BglII. This resulted in the deletion of the CBK1 ORF from position −1 to 2161. This region was replaced with a BglII-PstI fragment from pMB7 (9), containing the hisG-URA3-hisG cassette. The PstI end was made flush with Klenow DNA polymerase prior to ligation. This plasmid, pMM3, was cut with XmnI and BbsI to liberate the disruption cassette with 151 bp 5′ and 166 bp 3′ of CBK1. Eight micrograms of digested plasmid DNA was used to transform C. albicans strain CAI4 (ura3Δ::imm434/ura3Δ::imm434) by using a lithium acetate-mediated procedure (10). Reconstitution of the cbk1 null strain was achieved by integration of plasmid pMM4. This plasmid was constructed by cloning an AatI-NarI fragment from pSMS44 (24), containing the entire URA3 ORF and surrounding sequences, into pMM2 digested with the same enzymes. This plasmid was linearized at a BsrGI site located 566 bp upstream of the CBK1 ORF and it was used to transform strain CAMM 292-27.
FIG. 1.
Disruption of C. albicans CBK1. (A) Partial restriction map of the CBK1 locus. The HindIII/EcoRI fragment was subcloned into pUC18, generating pMM1. The BglII/XbaI fragment of the CBK1 locus was replaced with the BglII/PstI hisG-URA3-hisG fragment to construct the disruption cassette. (B) Southern blot of genomic DNA digested with HpaI and hybridized with a CBK1-specific probe. Genotypes of the strains are indicated above each lane.
A BbsI-XmnI fragment containing the cassette flanked by portions of CBK1 was used to transform strain CAI4 (9) to uridine prototrophy. One representative transformant, termed CAMM-29 (ura3Δ::imm434/ura3Δ::imm434/cbk1-Δ1::hisG-URA3-hisG/CBK1), was plated on 5-fluoroorotic acid (5-FOA) to select for intrachromosomal recombination between the repetitive hisG sequences, resulting in the loss of the URA3 selectable marker (9). The resulting strain, CAMM-292 (ura3Δ::imm434/ura3Δ::imm434/cbk1-Δ1::hisG/CBK1), was transformed with the disruption cassette to delete the second allele. The cbk1 null mutant strain was named CAMM-292-4 (ura3Δ::imm434/ura3Δ::imm434/cbk1-Δ1::hisG/cbk1-Δ1::hisG-URA3-hisG). This strain was plated on 5-FOA to isolate the Uri− null mutant strain, CAMM-8 (ura3Δ::imm434/ura3Δ::imm434/cbk1-Δ1::hisG/cbk1-Δ1::hisG). Proper integration events were verified in each strain by Southern blot analysis. Southern blot analysis was performed by standard methods (2). Blots were washed twice with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.1% sodium dodecyl sulfate (SDS) (5 and 10 min) and twice with 0.1× SSC, 0.1% SDS (15 min). All washes and hybridizations were performed at 68°C.
Genomic DNA was isolated from all representative isolates, digested with HpaI, and hybridized with a HindIII-BglII fragment upstream of CBK1 (Fig. 1B). The parental strain displayed a single hybridizing band of approximately 4.3 kb. The heterozygous Uri+ cbk1/CBK1 strain exhibited one band of 4.3 kb and a second band of approximately 6.3 kb, the predicted size of the replaced allele. One hybridizing band of approximately 3.1 kb, corresponding to the cbk1::hisG allele, and a second band of 6.3 kb were exhibited in the null mutant strain. The cbk1/cbk1 Uri− strain, CAMM-8, was transformed with BsrGI-linearized plasmid pMM4 to reintroduce a wild-type allele of CBK1. The revertant strain was named CAMM-81 and exhibited the predicted 11.1-kb hybridizing band indicative of a proper integration event. Lack of expression of CBK1 message in the null mutant and detection of a hybridizing band in the revertant strain, determined by Northern blot analysis, confirmed the validity of the Southern blot analyses (data not shown).
CBK1 is required for maintenance of normal cellular morphology.
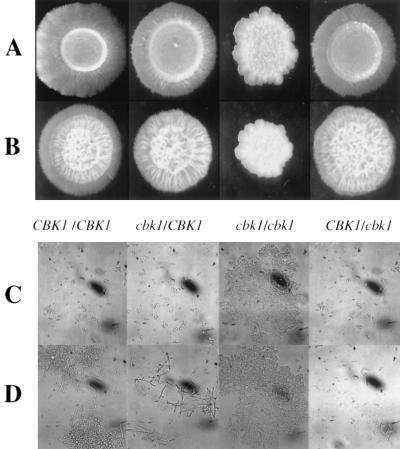
The effects of deletion of CBK1 were analyzed using various liquid and agar-solidified media. Cells were grown to stationary phase in yeast-peptone-dextrose (YPD) at 30°C, and 106 cells were spotted on agar plates. The null mutant lacked the ability to form lateral hyphae at 37°C on medium 199 buffered at pH 7.0 and on Lee's medium (14) plates (Fig. 2). The heterozygous strain showed a decrease in hyphal radiation under these conditions (Fig. 2). Filamentation by the null mutant was also absent on spider medium (15) and 10% serum plates incubated at 37°C (data not shown). In addition to lacking hyphae, colonies of the cbk1 strain had irregular borders and a rough appearance. This phenotype was noted on all media mentioned above at both 30 and 37°C (data not shown). In liquid Lee's medium at both 30 and 37°C, the null mutant strain formed large aggregations of cells (Fig. 2). This aggregation phenotype was also noted at 37°C in liquid medium 199 at pH 7.0, 10% serum, and YPD and at 25°C in YPD, 10% serum, and medium 199 at pH 4.0 (data not shown). These aggregates could not be disrupted by the addition of SDS or EDTA, suggesting a physical attachment between cells. When grown under conditions that normally lead to yeast growth, the heterozygous strain grew essentially normally; however, more pseudohyphae were present at 37°C than in the control strain (Fig. 2). Reintroduction of the wild-type gene into the null mutant reverted all associated phenotypes. These results were reproduced with a set of two independently constructed mutants.
FIG. 2.
Effects of CBK1 deletion on colonial and cellular morphology. (A and B) Strains were spotted on medium 199 (A) or Lee's medium (B) plates and incubated at 37°C for 5 days. (C and D) Strains were grown overnight in YPD at 25°C and then inoculated into the Lee's medium at 25°C (C) or 37°C (D) for 4 h.
Altered expression pattern of cell wall- and hypha-related genes.
The phenotype associated with disruption of CBK1 in C. albicans is similar to that caused by disruption of the CBK1 gene in S. cerevisiae. It has been reported that in S. cerevisiae cbk1 mutants, expression of the gene encoding chitinase, CTS1, is altered (22). Furthermore, disruption of CTS1 results in strains that form aggregations of cells under all culture conditions (13). C. albicans has three genes potentially encoding chitinases, but only two, CHT2 and CHT3, have been shown to be expressed (16, 17). The possibility that altered expression of these two genes is at least partially responsible for the cbk1 null mutant phenotype was examined by Northern blot analysis.
RNA was prepared by growing cultures to stationary phase at 30°C in YPD. Cells from these cultures were used to inoculate 500 ml of medium 199 buffered at pH 7.0 or pH 4.0, prewarmed to either 25 or 37°C, at an initial density of 4 × 106 cells per ml. Cultures were incubated at either 25 or 37°C for 3 h on a rotary shaker at 180 rpm. RNA was extracted from the cultures as previously described (2). Blotting and hybridization were conducted as described above for Southern blot analyses. Blots were hybridized with one of the following probes: a 1,063-bp AatII-NdeI fragment from within the ORF of PHR1; a 1,257-bp BamHI-NheI fragment of PHR2; a 1,078-bp PCR product from within the ORF of CHT2; an 804-bp PCR product from the ORF of CHT3; a 984-bp StyI-XbaI fragment of the CBK1 ORF; a 1.6-kb BamHI-EcoRV fragment of the ECE1 ORF; or a 4.3-kb EcoRI fragment containing the entirety of the HWP1 ORF. All probes were labeled by random priming using [α-32P]dCTP and Ready-to-go DNA labeling beads (Amersham Pharmacia Biotech).
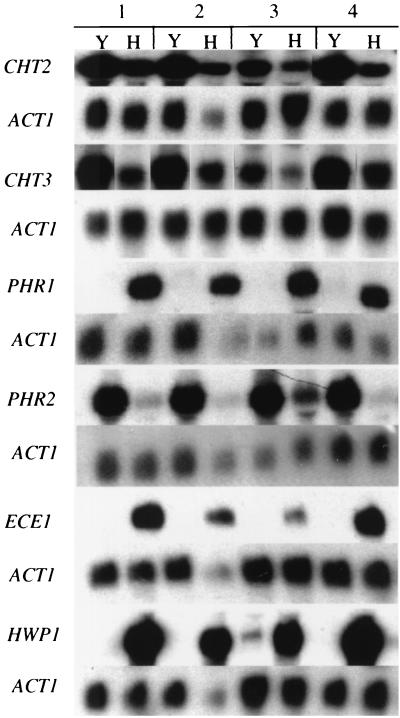
The CBK1 wild-type control strain showed the expected pattern of CHT2 and CHT3 expression (Fig. 3). The cbk1 null mutant strain, CAMM-292-4, showed decreased expression of both genes under these conditions compared to the control strain. The cbk1/CBK1 strain showed a level of expression of both genes similar to that of the control strain. While there was a reduction in the level of expression of the two chitinase-encoding genes in the cbk1 null mutant strain, abundant mRNA for both was apparent.
FIG. 3.
Effect of CBK1 mutations on gene expression. Total RNAs were isolated from CAI-4, CAMM-29, CAMM-292-4, and CAMM-81 (lane groupings 1 to 4, respectively) as mentioned in Materials and Methods. Cultures were incubated under conditions which favor yeast (medium 199 at 25°C and pH 4.0) (Y) or hyphae (medium 199 at 37°C and pH 7.0) (H). Hybridization probes are listed on the left.
It has been noted in S. cerevisiae strains lacking a functional CBK1 gene that the expression of more than 50 genes is altered by more than 2.2-fold (3). Because the decrease in the level of expression of CHT2 and CHT3 was not considered likely to be the sole cause of the complex morphological phenotype seen in the cbk1 strain, the expression of several genes encoding known cell wall proteins was analyzed. PHR1 and PHR2 are regulated by external pH and are expressed in a reciprocal pattern to each other (19, 24). It has recently been suggested that the homologous proteins encoded by PHR1 and PHR2 are putative glycosidases necessary for proper construction of the cell wall (8, 18). The cbk1 null mutant strain showed a pattern of expression of PHR1 identical to that of the control strain (Fig. 3). However, the cbk1/cbk1 strain showed an increased level of expression of PHR2 at pH 7.0 when compared to the control strain (Fig. 3). Deletion of CBK1 had no effect on the expression of PRR1 and RIM101/PRR2, two known regulators of PHR1 and PHR2 expression (data not shown) (21, 23). Additionally, CBK1 expression is unaffected in prr1 and rim101/prr2 null mutant strains (data not shown).
Expression of the hypha-associated transcripts, ECE1 (4) and HWP1 (25, 26), was also analyzed in the cbk1 null mutant. Both ECE1 and HWP1 are expressed under conditions that lead to hyphal growth. The cellular location of Ece1p has not been identified, but Hwp1p has been shown to be a cell wall protein (26). Under conditions that led to hyphal growth of the control strain, both ECE1 and HWP1 showed a high level of induction (Fig. 3). The cbk1 strain showed a lack of complete induction of ECE1 at pH 7.0 and 37°C but, like the control strain, it lacked expression at pH 4.0 and 25°C. HWP1 expression in the cbk1 null mutant strain grown at pH 7.0 and 37°C was essentially identical to that of the control strain, but it showed a lack of complete repression under normally noninducing conditions. Reintroduction of a functional copy of CBK1 in the revertant strain, CAMM-81, restored a normal pattern of expression of CHT2, CHT3, ECE1, HWP1, and PHR2. Whether Cbk1p affects expression of these genes directly or indirectly is not known, and further analysis will be necessary to elucidate its function in the maintenance of normal morphological development.
Acknowledgments
This work was supported by Public Health Service grant GM47727 from the National Institutes of Health.
We recognize the valuable assistance of Christopher Herbert.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1993. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 3.Bidlingmaier, S., E. L. Weiss, C. Seidel, D. G. Drubin, and M. Snyder. 2001. The Cbk1p pathway is important for polarized cell growth and cell separation in Saccharomyces cerevisiae. Mol. Cell. Biol. 21:2449-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birse, C. E., M. Y. Irwin, W. A. Fonzi, and P. S. Sypherd. 1993. Cloning and characterization of ECE1, a gene expressed in association with cell elongation of the dimorphic pathogen Candida albicans. Infect. Immun. 61:3648-3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brook, J. D., M. E. McCurrach, H. G. Harley, A. J. Buckler, D. Church, H. Aburatani, K. Hunter, V. P. Stanton, J. P. Thirion, T. Hudson, et al. 1992. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell 68:799-808. [DOI] [PubMed] [Google Scholar]
- 6.Buhr, T. L., S. Oved, G. M. Truesdell, C. Huang, O. Yarden, and M. B. Dickman. 1996. A kinase-encoding gene from Colletotrichum trifolii complements a colonial growth mutant of Neurospora crassa. Mol. Gen. Genet. 251:565-572. [DOI] [PubMed] [Google Scholar]
- 7.Dürrenberger, F., and J. Kronstad. 1999. The UKC1 gene encodes a protein kinase involved in morphogenesis, pathogenicity and pigment formation in Ustilago maydis. Mol. Gen. Genet. 261:281-289. [DOI] [PubMed] [Google Scholar]
- 8.Fonzi, W. A. 1999. PHR1 and PHR2 of Candida albicans encode putative glycosidases required for proper cross-linking of beta-1,3- and beta-1,6-glucans. J. Bacteriol. 181:7070-7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain constructions and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geitz, D., A. St. Jean, R. A. Woods, and R. H. Scheistl. 1992. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20:1425.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunter, T., and G. D. Plowman. 1997. The protein kinases of budding yeast: six score and more. Trends Biochem. Sci. 22:18-22. [DOI] [PubMed] [Google Scholar]
- 12.Justice, R. W., O. Zilian, D. F. Woods, M. Noll, and P. J. Bryant. 1995. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 9:534-546. [DOI] [PubMed] [Google Scholar]
- 13.Kuranda, M. J., and P. W. Robbins. 1987. Cloning and heterologous expression of glycosidase genes from Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 84:2585-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee, K. L., H. R. Buckley, and C. C. Campbell. 1975. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida albicans. Sabouraudia 13:148-153. [DOI] [PubMed] [Google Scholar]
- 15.Liu, H., J. Kohler, and G. R. Fink. 1994. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science 266:1723-1726. [DOI] [PubMed] [Google Scholar]
- 16.McCreath, K. J., C. A. Specht, and P. W. Robbins. 1995. Molecular cloning and characterization of chitinase genes from Candida albicans. Proc. Natl. Acad. Sci. USA 92:2544-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCreath, K. J., C. A. Specht, Y. Liu, and P. W. Robbins. 1996. Molecular cloning of a third chitinase gene (CHT1) from Candida albicans. Yeast 12:501-504. [DOI] [PubMed] [Google Scholar]
- 18.Mouyna, I., T. Fontaine, M. Vai, M. Monod, W. A. Fonzi, M. Diaquin, L. Popolo, R. P. Hartland, and J. P. Latge. 2000. Glycosylphosphatidylinositol-anchored glucanosyltransferases play an active role in the biosynthesis of the fungal cell wall. J. Biol. Chem. 275:14882-14889. [DOI] [PubMed] [Google Scholar]
- 19.Mühlschlegel, F. A., and W. A. Fonzi. 1997. PHR2 of Candida albicans encodes a functional homolog of the pH-regulated gene PHR1 with an inverted pattern of pH-dependent expression. Mol. Cell. Biol. 17:5960-5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odds, F. C. 1988. Candida and candidosis. A review and bibliography, 2nd ed. Bailliere Tindal, London, United Kingdom.
- 21.Porta, A., A. M. Ramon, and W. A. Fonzi. 1999. PRR1, a homolog of Aspergillus nidulans palF, controls pH-dependent gene expression and filamentation in Candida albicans. J. Bacteriol. 181:7516-7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Racki, W. J., A. M. Becam, F. Nasr, and C. J. Herbert. 2000. Cbk1p, a protein similar to the human myotonic dystrophy kinase, is essential for normal morphogenesis in Saccharomyces cerevisiae EMBO J. 19:4524-4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramon, A. M., A. Porta, and W. A. Fonzi. 1999. Effect of environmental pH on morphological development of Candida albicans is mediated via the PacC-related transcription factor encoded by PRR2. J. Bacteriol. 181:7524-7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saporito-Irwin, S. M., C. E. Birse, P. S. Sypherd, and W. A. Fonzi. 1995. PHR1, a pH-regulated gene of Candida albicans is required for morphogenesis. Mol. Cell. Biol. 15:601-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharkey, L. L., M. D. McNemar, S. M. Saporito-Irwin, P. S. Sypherd, and W. A. Fonzi. 1999. HWP1 functions in the morphological development of Candida albicans downstream of EFG1, TUP1, and RBF1. J. Bacteriol. 181:5273-5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staab, J. F., and P. Sundstrom. 1998. Genetic organization and sequence analysis of the hypha-specific cell wall protein gene HWP1 of Candida albicans. Yeast 14:681-686. [DOI] [PubMed] [Google Scholar]
- 27.Verde, F., D. J. Wiley, and P. Nurse. 1988. Fission yeast orb6, a ser/thr protein kinase related to mammalian rho kinase and myotonic dystrophy kinase, is required for maintenance of cell polarity and coordinates cell morphogenesis with the cell cycle. Proc. Natl. Acad. Sci. USA 95:7526-7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yarden, O., M. Plamann, D. J. Ebbole, and C. Yanofsky. 1992. cot-1, a gene required for hyphal elongation in Neurospora crassa, encodes a protein kinase. EMBO J. 11:2159-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]