Abstract
Suilysin is a cholesterol-binding cytolysin encoded by sly in Streptococcus suis. DNA sequence determination of the sly locus in a strain lacking sly revealed the presence of another gene, designated orf102, in the place of sly. No transposable element or long-repeat sequence was found in the close vicinity. Except for six strains whose corresponding loci have been rearranged, all of the remaining 62 strains examined had either sly or orf102 at the same locus and their flanking regions were conserved. The genetic organizations having either sly or orf102 were found in the strains whose 16S rRNA sequences were identical. These results suggest that S. suis acquired sly or orf102 from a foreign source and that these genes subsequently spread among S. suis strains by homologous recombination.
Streptococcus suis is a gram-positive coccus that has been identified as a cause of meningitis, septicemia, arthritis, and sudden death in pigs (6). It can also cause human meningitis (2, 22). Thirty-five capsular serotypes have been described so far (11, 12, 17, 27), and some serotypes, especially serotype 2, are more frequently isolated from diseased pigs than others (6, 15, 16). However, not all strains of S. suis serotype 2 are virulent and there is variation in the degrees of virulence among the strains (40, 42). Comparisons between virulent and avirulent strains of S. suis have led to the proposal of several cellular and extracellular components as candidates for virulence markers (34, 41, 42). However, there are several variants of these markers and some S. suis isolates from diseased pigs do not possess one or more of them (1, 3, 4, 13, 19, 38, 41), indicating genetic heterogeneity with respect to these markers. Recently, it was shown that some S. suis strains possess a type II restriction-modification (R-M) system, designated SsuDAT1I, which is an isoschizomer of Moraxella bovis MboI (10), whereas some other strains lack the system (32). Nucleotide sequence comparison between strains having the SsuDAT1I system and those lacking the system revealed that the SsuDAT1I system was originally inserted into the S. suis chromosome from a foreign source by illegitimate recombination and was subsequently transferred among S. suis strains by homologous recombination (31, 32). These findings raise the question of whether a series of genetic exchanges, exemplified by the SsuDAT1I system, also occurred in other genes and is one of the typical processes involved in the evolution of this bacterium, which constitutes a population containing strains with various combinations of virulence markers.
Some strains of S. suis produce a hemolysin called suilysin, which is a member of the family of cholesterol-binding cytolysins (alternatively called thiol-activated cytolysins) (8, 14, 18). A gene encoding suilysin (sly) has been cloned and sequenced (30), and the absence of sly in some S. suis strains was demonstrated by PCR using different sets of primers and/or by Southern hybridization analysis using cloned or amplified sly as a probe (24, 30). In this study, using 40 field isolates and 28 serotype reference strains, we analyzed the sly region and the corresponding chromosomal region of the strains lacking sly in order to provide additional knowledge about the acquisition and intraspecies transfer of genes in this bacterium.
The S. suis strains used in this study are listed in Table 1. The Escherichia coli strains used were XL1 Blue MRF′ (Stratagene, La Jolla, Calif.), XLOLR (Stratagene), and DH5α (29). S. suis strains were grown in Todd-Hewitt broth or agar medium (Difco Laboratories, Detroit, Mich.) supplemented with 2% yeast extract at 37°C under 5% CO2. E. coli strains were cultured in Luria-Bertani broth or agar medium (Difco Laboratories) supplemented, when necessary, with ampicillin (50 μg/ml) and kanamycin (25 μg/ml) at 37°C. On the basis of our previous data (37), the sequences of the 5,545-bp sly region of strain DAT2 and the corresponding 4,257-bp chromosomal region of strain DAT1, which lacks the sly gene, were determined. The sequences were searched against current DNA databases by using either the blastn, blastp, blastx, tblastn, or tblastx program network services available at the National Center for Biotechnology Information, Bethesda, Md. (http://www.ncbi.nlm.nih.gov/). Further DNA comparisons were made with the preliminary sequence data released by genome sequencing projects at various institutions (University of Oklahoma, Norman, Okla. [http://www.genome.ou.edu/smutans.html]; Université Catholique de Louvain, Louvain-la-Neuve,Belgium [http://www.biol.ucl.ac.be/gene/genome/blast.html]; The Sanger Centre, Cambridge, United Kingdom [http://www.sanger.ac.uk/Projects/S_equi/]; The Institute for Genomic Research, Rockville, Md. [http://www.tigr.org/tdb/s_gordonii.shtml]).
TABLE 1.
Strains of S. suis used and the genetic organizations of their sly loci
| Type of genetic organizationa | Strain | Sero-type | Reference |
|---|---|---|---|
| DAT1 | Field isolates | ||
| 211, 212 | 1 | 32 | |
| NIAH11318 | 1/2 | 31 | |
| DAT1, 194, 195, 196, 197, 198, 199, 200, 202, 205, 220, 221, 222, 226, 227, 229, 230, 233, 234, 235, 236, 238, 239, 243, 244 | 2 | 32, 35 | |
| Reference strains | |||
| 4961 | 3 | 27 | |
| 2524 | 6 | 27 | |
| 8074 | 7 | 27 | |
| 22083 | 9 | 12 | |
| 4417 | 10 | 12 | |
| 12814 | 11 | 12 | |
| 8830 | 12 | 12 | |
| 2726 | 16 | 12 | |
| 89-3576-3 | 25 | 11 | |
| 89-5259 | 27 | 11 | |
| DAT2 | Field isolates | ||
| 203, 204 | 1 | 32 | |
| DAT2, 193, 207, 209, 210, 213, 223, 228, 246, 247 | 2 | 32, 36 | |
| Reference strains | |||
| NCTC10237 | 1 | 27 | |
| NCTC10234 | 2 | 27 | |
| 6407 | 4 | 27 | |
| 11538 | 5 | 27 | |
| 14636 | 8 | 27 | |
| 13730 | 14 | 12 | |
| NCTC10446 | 15 | 12 | |
| 93A | 17 | 12 | |
| NT77 | 18 | 12 | |
| 42A | 19 | 12 | |
| 89-2479 | 23 | 11 | |
| 89-590 | 28 | 11 | |
| Atypical | Reference strains | ||
| 10581 | 13 | 12 | |
| 86-5192 | 20 | 12 | |
| 14A | 21 | 12 | |
| 88-1861 | 22 | 12 | |
| 88-5299A | 24 | 11 | |
| 89-4109-1 | 26 | 11 |
Classification of the strains into the DAT1, DAT2, and atypical types was based on the results of PCR amplification and Southern hybridization as described in the text.
The sly region of strain DAT2 contained five putative open reading frames (ORFs) (Fig. 1). The five ORFs found in this region were carried on the same DNA strand. Two ORFs were located upstream of sly. The first ORF, designated ORF100, encoded a 148-amino-acid protein whose N-terminal end was truncated. The protein showed 65% identity with an ABC transporter homolog of Bacillus subtilis (accession no. H69828). The second ORF, designated ORF101, encoded a 236-amino-acid protein which showed 33% identity with a conserved hypothetical protein of Streptococcus pyogenes (accession no. AAK33575), but the gene was not preceded by a typical Shine-Dalgarno (SD) sequence. The ORF just downstream of sly encoded a 233-amino-acid protein which showed 73% identity with a putative N-acetylmannosamine-6-phosphate epimerase of S. pyogenes (accession no. AAK33327), and the gene was designated nanE. The remaining ORF encoded a 403-amino-acid protein whose C-terminal end was truncated. The protein showed 56% identity with phosphotransferase system II BC components of S. pneumoniae (accession no. AAK75763), and the gene was designated ptsG. The sly, nanE, and ptsG genes were not preceded by a typical SD sequence, although a conserved sequence, 5′-GAAAGGA-3′, was located 8 or 9 bp upstream of the putative start codons. The genes identified in this region were thus organized as shown in Fig. 1, and this genetic organization was designated the DAT2 type. The genetic organization of the DAT2 type was different from those of the pneumolysin gene (ply) region of S. pneumoniae strain TIGR4 (39) and the streptolysin O gene (slo) region of S. pyogenes strain SF370 (9). On the other hand, four genes, orf100, orf101, nanE, and ptsG, were also present in the corresponding chromosomal region of strain DAT1, although the orf101 homolog of strain DAT1 was 15 bp shorter than orf101 of strain DAT2. However, a putative ORF, designated ORF102, which was completely different from sly, was found in the place of sly, and thus sly was completely missing from strain DAT1. ORF102 encoded a 194-amino-acid protein which showed 70% identity with a conserved hypothetical protein of S. pneumoniae (accession no. AAK74572). The genes identified in this region were ordered as shown in Fig. 1, and the genetic organization was designated the DAT1 type.
FIG. 1.
Physical and genetic maps of the 5,519- and 4,232-bp chromosomal regions of S. suis DAT2 and DAT1, respectively, with putative genes indicated by arrows and boxes. Regions with high and relatively low identities are represented by lines drawn between the two physical maps, and the percentages of nucleotide identity are indicated in the spaces between the physical maps. In the line graphs, G+C contents scanned with a sliding window of 100 bp are shown in 25-bp increments; arrowheads between the line graphs and the physical maps depict the positions of primers used for PCR. The chromosomal regions shown correspond to nucleotides 1 to 5,519 and 26 to 4,257 of the sequences with accession no. AB055649 (DAT2) and AB071359 (DAT1), respectively.
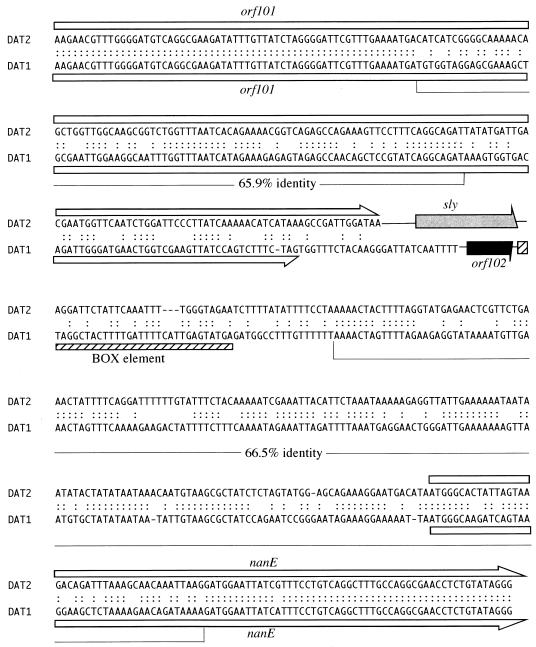
Nucleotide sequence comparison between DAT2- and DAT1-type organizations revealed that left- and right-hand ends of the regions were highly conserved (more than 98% identity), whereas the central regions were diverse (Fig. 1). sly and orf102 were bounded by regions which showed relatively low homologies (65.9 and 66.5% identities) and constituted mosaic structures with low- and high-homology segments (Fig. 1 and 2). The genetic regions with relatively low homologies overlapped the 3′ region of orf101 and the 5′ end of nanE (Fig. 1 and 2). The average G+C contents of sly (39.2%) and orf102 (43.6%), as well as those of other regions, were similar to that of the total genome of S. suis (39 to 41%) (20), whereas sly and orf102 were encompassed by segments of remarkably low G+C contents, and one segment located downstream of the genes coincided with a relatively low-homology region (Fig. 1). The codon usage patterns for the sly and orf102 genes were not anomalous compared to those previously reported for purine and cysteine biosynthetic genes (26, 32). No transposable element or long-repeat sequence was found in the 5,545 or 4,257-bp sequence. However, a 109-bp segment, which was similar to repeated DNA elements (BOX elements) found in S. pneumoniae (21, 23), was located 99 bp downstream of orf102 (Fig. 2). It was recently shown that similar DNA elements were located in the vicinity of genes encoding sortase-like proteins in S. suis strain NCTC10234 (25). The 109-bp segment located downstream of orf102 was one such homolog, suggesting that the BOX elements are scattered throughout the genome of S. suis, as was observed in S. pneumoniae (39). While one of the BOX elements was located downstream of ply in S. pneumoniae (23), no BOX element was found downstream of sly.
FIG. 2.
Nucleotide sequence comparison between sly-flanking region of DAT2 and orf102-flanking region of DAT1. Relatively low-homology regions and the percentages of nucleotide identity are indicated below the sequences. Colons, identical nucleotides; open boxes and arrows, orf101 and nanE; gray and black arrows, sly and orf102, respectively; hatched box, BOX element.
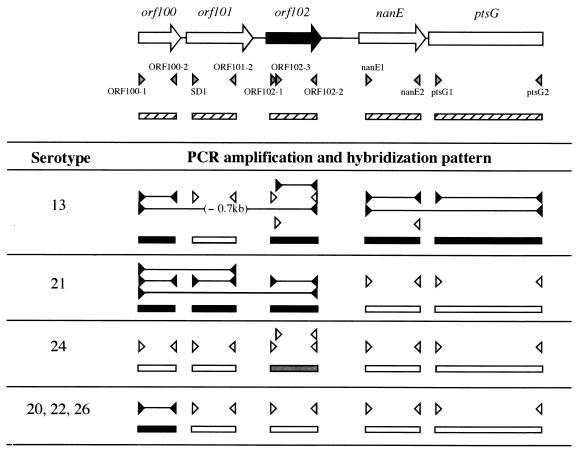
For the characterization of 68 S. suis strains with respect to the genetic organization of their sly loci, DNA fragments were amplified from the genomic DNAs of these strains by PCR with primers OS1 and OS2, which were complementary to highly conserved sequences in the sly-flanking regions (Fig. 1 and Table 2). The conditions of the PCR were essentially the same as described previously (36). The PCR products were analyzed by Southern hybridization with the sly and orf102 probes by procedures described previously (32), except that hybridization was carried out at 68°C. Genomic Southern hybridization was also performed using S. suis DNAs that had been digested with PstI, for which no cutting site is present in the sly or orf102 regions. For the preparation of sly and orf102 probes, the sly gene region was amplified from the genomic DNA of DAT2 with primers SL1 and SL4 (Table 2) and the orf102 gene region was amplified from the genomic DNA of DAT1 with primers ORF102-1 and ORF102-2 (Table 2), both of which were followed by cloning into pCR2.1 (Invitrogen, Groningen, The Netherlands). A 3.0-kb fragment was amplified with primers OS1 and OS2 from the genomic DNAs of 28 field isolates, including strain DAT1, as well as 10 reference strains. The amplified fragments were hybridized with the orf102 probe but not with the sly probe (data not shown). A DNA fragment that hybridized with the orf102 probe was also seen in the digested DNAs of the 28 field isolates and 10 reference strains, but no hybridizing fragment was seen when the sly probe was used (data not shown), indicating that the DAT1-type genetic organization was conserved in these strains (Table 1). On the other hand, a 4.3-kb fragment was amplified from genomic DNAs of the remaining 12 field isolates, including strain DAT2, as well as 12 reference strains (data not shown). The amplified fragments and a DNA fragment of the digested DNAs from the 12 field isolates and 12 reference strains were hybridized with the sly probe but not with the orf102 probe (data not shown), indicating that the DAT2-type genetic organization was conserved in these strains (Table 1). However, no DNA fragment was amplified with primers OS1 and OS2 from the genomic DNAs of the remaining six reference strains of serotypes 13, 20, 21, 22, 24, and 26. The genomic DNAs of these strains did not show a hybridizing fragment with the sly probe (data not shown). The strains of serotypes 13, 21, and 24 provided a DNA fragment that hybridized with the orf102 probe, although the hybridization signal in the strain of serotype 24 was weak (Fig. 3). No fragment hybridizing with the orf102 probe appeared in the digested DNAs of the remaining three reference strains of serotypes 20, 22, and 26 (Fig. 3). These results indicate that the six reference strains had different genetic organizations with respect to the sly locus, and the six strains were collectively grouped into the atypical type of genetic organization (Table 1). Genomic Southern hybridization and PCR with various combinations of probes and primers (Fig. 3) were performed to examine the genetic organizations of the six atypical strains. As summarized in Fig. 3, the results indicated that genetic rearrangements in the sly loci had occurred in these strains. Consequently, six atypical-type strains could be divided into four minor types (Fig. 3); their genetic organizations are represented in Fig. 4. The sly and orf102 genes of several selected strains were amplified by PCR with primers SD1 and SD2 (Table 2) and directly sequenced. Comparison of the sly sequences among strains DAT2 and 203 and reference strains of serotypes 1, 4, 8, 19, 23, and 28 showed striking similarities (99.4 to 100% identity), and the deduced amino acid sequences were completely identical with the exception of one amino acid difference found in the serotype 1 reference strain. On the other hand, the identities among the orf102 sequences of strains DAT1 and 226 and reference strains of serotypes 6, 7, 9, and 12 ranged from 96.1 to 100% and several amino acid differences occurred among the ORF102 proteins of these strains.
TABLE 2.
Oligonucleotide primers used
| Primer | Sequence (5′-3′) | Location or description |
|---|---|---|
| OS1 | AAGCAACTTCTCATATTGATACGGAGACGG | 5′ region of truncated orf100 |
| OS2 | CCACGCTTGATCCAATACAGGAAATTGTGC | 5′ region of truncated ptsG |
| SL1 | TACATTGATAATCCGCCAGC | 5′ region of sly |
| SL4 | AAACTGTTCTCCACCATTCC | 3′ region of sly |
| ORF102-1 | ACGAGAAAACCTTGCGACTG | 5′ region of orf102 |
| ORF102-2 | CTGGATTGATAGGAGTGTTG | 3′ region of orf102 |
| ORF102-3 | GTCAAGAAAAATAATGGCGG | Just downstream of ORF102-1 |
| ORF100-1 | CTATCTCTTTACAGGGACGA | 5′ region of truncated orf100 |
| ORF100-2 | ACACCTTTGCTTGAATCTCA | 3′ region of truncated orf100 |
| SD1 | AGGTGAATTCGTTTGAACGTGCTTTGG | 5′ region of orf101 |
| ORF101-2 | AACGTTCTTCCATTAGTTGA | 3′ region of orf101 |
| nanE1 | TTTCCTGTCAGGCTTTGCCA | 5′ region of nanE |
| nanE2 | TTCCTTTGGACGTGTGATCG | 3′ region of nanE |
| ptsG1 | TGTTGCTGGTCTCTTACTGG | 5′ region of truncated ptsG |
| ptsG2 | CCATACCAGGAATTAGCACGTGAAAT | 3′ region of truncated ptsG |
| SD2 | CGCAGGATCCAATACAGGAAATTGT | 5′ region of truncated ptsG |
| F1 | GAGTTTGATCCTGGCTCAG | 5′ region of 16S rRNA |
| R13 | AGAAAGGAGGTGATCCAGCC | 3′ region of 16S rRNA |
FIG. 3.
Schematic representations of the results of systematic PCR and Southern hybridization analyses of atypical-type reference strains. Positions of primers and probes used relative to the DAT1-type genetic organization are indicated at the top by gray arrowheads and hatched boxes, respectively. orf100, orf101, orf102, nanE, and ptsG probes were amplified by PCR from the genomic DNA of strain DAT1 with primers ORF100-1 and ORF100-2, SD1 and ORF101-2, ORF102-1 and ORF102-2, nanE1 and nanE2, and ptsG1 and ptsG2, respectively. The genomic DNAs of the six reference strains were digested with PstI and XhoI. Solid lines between the closed arrowheads, regions amplified by PCR; open arrowheads facing each other, primers that did not amplify any PCR fragment; closed and gray boxes, probes that gave strong and weak hybridization signals, respectively; open boxes, probes that did not give any hybridization signals. When the length of the amplified fragment was different from that expected from the sequence of DAT1, the size difference is indicated in parentheses.
FIG. 4.
Distribution of genetic organization types in S. suis strains whose positions are shown on a 16S rRNA-based tree constructed by the neighbor-joining method (28). The tree was rooted using the S. pyogenes 16S rRNA sequence as the outgroup. The numbers at the nodes of branches indicate the bootstrap values based on 1,000 resamplings. Reference strains of each serotype are referred to by the serotype number sandwiched between “S” and “ref.” S. suis strains were divided into three clusters on the basis of their distances from the 16S rRNA sequence of the serotype 1 reference strain (NCTC10237 [S1ref]). The distances were calculated by using the DNADIST program (PHYLIP) with the Maximum Likelihood option as the model of nucleotide substitution. Scale bar, sequence dissimilarity; ○, DAT2 type; •, DAT1 type.
A phylogenetic tree was constructed on the basis of the sequence discrepancies in the 16S rRNA genes of 15 field isolates and reference strains of serotypes 1 to 28. The 16S rRNA genes of the 15 field isolates of S. suis were amplified using the previously reported primers F1 and R13 (Table 2) (7) and sequenced. The 16S rRNA gene sequences retrieved from the GenBank database were for S. pyogenes NCDO2381 (accession no. X59029) and S. suis reference strains of serotypes 1 and 2 to 28 (accession no. AF009475 and AF009477 to AF009503, respectively). The tree was constructed by usingCLUSTAL W (http://www.ddbj.nig.ac.jp/E-mail/clustalw-e.html) and the programs of the Phylogeny Inference Package (PHYLIP, version 3.573c, 1995) as described previously (5), except that sequence similarities for S. suis and S. pyogenes strains were determined only for a region corresponding to nucleotides 28 to 1,473 of the E. coli 16S rRNA sequence, and unambiguous parts of the aligned sequence were cut and concatenated to a single data matrix. The topology of the tree obtained generally resembles that of the previously reported tree (5). S. suis strains were divided into three clusters on the basis of their distances from the 16S rRNA sequence of the serotype 1 reference strain, as previously performed by Chatellier et al. (5). Strain 226 and the reference strains of serotypes 7 and 9 belonged to cluster II (distances between 0.0126 and 0.0190). Reference strains of serotypes 20, 22, and 26 belonged to cluster III (distances between 0.0219 and 0.0291). Other S. suis strains were grouped into cluster I (distances between 0 and 0.0105) (Fig. 4). From these results, there were four principal findings. First, there were both DAT1- and DAT2-type genetic organizations among the strains in which the 16S rRNA sequences were identical (e.g., reference strains of serotypes 12 and 14 or 6 and 18). Second, all the strains of DAT2-type genetic organization were classified into cluster I, whereas strains of DAT1-type genetic organization were widely distributed. Third, three reference strains of serotypes 20, 22, and 26, which had an atypical type of organization containing only orf100, belonged to cluster III, whereas other atypical-type strains of serotypes 13, 21, and 24 were grouped into cluster I, where the strains of serotypes 13 and 21 formed a sister group. And lastly, the 16S rRNA sequence divergence between strain DAT1 (cluster I) and the serotype 7 reference strain (cluster II) was significantly large (distance, 0.0112), although the orf102 sequences of these two strains were completely identical to each other.
With the exception of atypical-type strains in which the corresponding chromosomal regions have been rearranged, all the S. suis strains used in this study had either a sly or an orf102 gene at the same location in the genomes between orf101 and nanE, and the sly-flanking regions were conserved irrespective of the presence of sly. The mutually exclusive localization of sly and orf102 at the same place suggests that at least one of them was horizontally transferred into S. suis from a foreign source and was replaced with the gene that had existed between orf101 and nanE. Although natural transformation has not yet been demonstrated for S. suis, the presence of at least two genes which showed homology to competence-related genes has been indicated (33) and we have occasionally found several competence-related genes from shotgun sample sequencing data of the S. suis NCTC10234 genomic library (unpublished observations). Therefore, it is plausible that the sly or orf102 gene may be delivered into a recipient S. suis strain via a transformation event. No vestiges of the sequences affecting their integration, such as long-repeat DNA sequences or remnants of translocatable elements, were found in the vicinity of sly and orf102, while a BOX-like element was found downstream of the orf102 in DAT1 (Fig. 2). Unique structures found in the flanking region, which showed relatively low homology (Fig. 1 and 2), may suggest that the original incorporation of sly or orf102 into the S. suis genome has occurred via a unique mechanism of gene transfer rather than by the insertion of a mobile genetic element. Phylogenetic analysis suggests that the sly and the orf102 gene regions were also transferred among S. suis strains, and hence the incorporation of DNA was apparently mediated by homologous recombination via conserved flanking regions. Alternatively, the results, especially those obtained for strain DAT1 and the reference strain of serotype 7, raise the possibility that the 16S rRNA gene region could also be transferred among the strains.
An R-M system can work as a barrier to the incorporation of foreign DNA; however, the genetic exchange could occur within an appropriate combination of the strains, i.e., between strains carrying the same R-M system and between strains lacking an R-M system or from the former to the latter. Therefore, our findings about the genetic structures of sly loci and their distribution in the S. suis population, together with the findings of previous reports (31, 32), suggest that a series of gene transfers, in which a foreign gene is acquired by a certain mechanism and subsequently spread among the strains, is a common occurrence in S. suis and that such genomic conversions may contribute to the heterogeneity of the population.
Nucleotide sequence accession numbers.
The nucleotide sequences determined in this study have been deposited in the DDBJ, EMBL, and GenBank databases, and their accession numbers are listed in Table 3.
TABLE 3.
Nucleotide sequence accession numbers
| Gene or region | S. suis straina | Accession no. |
|---|---|---|
| 16S rRNA | DAT1 | AB071336 |
| DAT2 | AB071337 | |
| NIAH11318 | AB071338 | |
| 193 | AB071339 | |
| 194 | AB071340 | |
| 203 | AB071341 | |
| 209 | AB071342 | |
| 210 | AB071343 | |
| 211 | AB071344 | |
| 220 | AB071345 | |
| 222 | AB071346 | |
| 226 | AB071347 | |
| 228 | AB071348 | |
| 235 | AB071349 | |
| 243 | AB071350 | |
| sly region | DAT2 | AB055649 |
| sly | 203 | AB071351 |
| NCTC10237 (serotype 1) | AB071353 | |
| 6407 (serotype 4) | AB071354 | |
| 14636 (serotype 8) | AB071355 | |
| 42A (serotype 19) | AB071356 | |
| 89-2479 (serotype 23) | AB071357 | |
| 89-590 (serotype 28) | AB071358 | |
| orf102 region | DAT1 | AB071359 |
| orf102 | 226 | AB071360 |
| 2524 (serotype 6) | AB071361 | |
| 8074 (serotype 7) | AB071362 | |
| 22083 (serotype 9) | AB071363 | |
| 8830 (serotype 12) | AB071364 | |
| nanE with upstream region | 10581 (serotype 13) | AB071365 |
Only the serotypes of the reference strains are indicated.
Acknowledgments
We are grateful to Yoshihiro Shimoji for a critical review of the manuscript and helpful discussions. We thank Yasushi Kataoka for providing us with the S. suis strains and Mitoyo Takahashi for excellent technical assistance.
REFERENCES
- 1.Allgaier, A., R. Goethe, H. J. Wisselink, H. E. Smith, and P. Valentin-Weigand. 2001. Relatedness of Streptococcus suis isolates of various serotypes and clinical backgrounds as evaluated by macrorestriction analysis and expression of potential virulence traits. J. Clin. Microbiol. 39:445-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arends, J. P., and H. C. Zanen. 1988. Meningitis caused by Streptococcus suis in humans. Rev. Infect. Dis. 10:131-137. [DOI] [PubMed] [Google Scholar]
- 3.Berthelot-Hérault, F., H. Morvan, A.-M. Kéribin, M. Gottschalk, and M. Kobisch. 2000. Production of muraminidase-released protein (MRP), extracellular factor (EF) and suilysin by field isolates of Streptococcus suis capsular types 2, 1/2, 9, 7 and 3 isolated from swine in France. Vet. Res. 31:473-479. [DOI] [PubMed] [Google Scholar]
- 4.Chatellier, S., M. Gottschalk, R. Higgins, R. Brousseau, and J. Harel. 1999. Relatedness of Streptococcus suis serotype 2 isolates from different geographic origins as evaluated by molecular fingerprinting and phenotyping. J. Clin. Microbiol. 37:362-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatellier, S., J. Harel, Y. Zhang, M. Gottschalk, R. Higgins, L. A. Devriese, and R. Brousseau. 1998. Phylogenetic diversity of Streptococcus suis strains of various serotypes as revealed by 16S rRNA gene sequence comparison. Int. J. Syst. Bacteriol. 48:581-589. [DOI] [PubMed] [Google Scholar]
- 6.Clifton-Hadley, F. A. 1983. Streptococcus suis type 2 infections. Br. Vet. J. 139:1-5. [DOI] [PubMed] [Google Scholar]
- 7.Dorsch, M., and E. Stackebrandt. 1992. Some modifications in the procedure of direct sequencing of PCR amplified 16S rDNA. J. Microbiol. Methods 16:271-279. [Google Scholar]
- 8.Feder, I., M. M. Chengappa, B. Fenwick, M. Rider, and J. Staats. 1994. Partial characterization of Streptococcus suis type 2 hemolysin. J. Clin. Microbiol. 32:1256-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferretti, J. J., W. M. McShan, D. Ajdic, D. J. Savic, G. Savic, K. Lyon, C. Primeaux, S. Sezate, A. N. Suvorov, S. Kenton, H. S. Lai, S. P. Lin, Y. Qian, H. G. Jia, F. Z. Najar, Q. Ren, H. Zhu, L. Song, J. White, X. Yuan, S. W. Clifton, B. A. Roe, and R. McLaughlin. 2001. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. USA 98:4658-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelinas, R. E., P. A. Myers, and R. J. Roberts. 1977. Two sequence-specific endonucleases from Moraxella bovis. J. Mol. Biol. 114:169-179. [DOI] [PubMed] [Google Scholar]
- 11.Gottschalk, M., R. Higgins, M. Jacques, M. Beaudoin, and J. Henrichsen. 1991. Characterization of six new capsular types (23 through 28) of Streptococcus suis. J. Clin. Microbiol. 29:2590-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottschalk, M., R. Higgins, M. Jacques, K. R. Mittal, and J. Henrichsen. 1989. Description of 14 new capsular types of Streptococcus suis. J. Clin. Microbiol. 27:2633-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottschalk, M., A. Lebrun, H. Wisselink, J. D. Dubreuil, H. Smith, and U. Vecht. 1998. Production of virulence-related proteins by Canadian strains of Streptococcus suis capsular type 2. Can. J. Vet. Res. 62:75-79. [PMC free article] [PubMed] [Google Scholar]
- 14.Gottschalk, M. G., S. Lacouture, and J. D. Dubreuil. 1995. Characterization of Streptococcus suis capsular type 2 haemolysin. Microbiology 141:189-195. [DOI] [PubMed] [Google Scholar]
- 15.Higgins, R., and M. Gottschalk. 1996. Distribution of Streptococcus suis capsular types in 1995. Can. Vet. J. 37:242.. [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins, R., M. Gottschalk, M. Beaudoin, and S. A. Rawluk. 1992. Distribution of Streptococcus suis capsular types in Quebec and western Canada. Can. Vet. J. 33:27-30. [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins, R., M. Gottschalk, M. Boudreau, A. Lebrun, and J. Henrichsen. 1995. Description of six new capsular types (29-34) of Streptococcus suis. J. Vet. Diagn. Investig. 7:405-406. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs, A. A. C., P. L. W. Loeffen, A. J. G. van den Berg, and P. K. Storm. 1994. Identification, purification, and characterization of a thiol-activated hemolysin (suilysin) of Streptococcus suis. Infect. Immun. 62:1742-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs, A. A. C., A. J. G. van den Berg, J. C. Baars, B. Nielsen, and L. W. Johannsen. 1995. Production of suilysin, the thiol-activated haemolysin of Streptococcus suis, by field isolates from diseased pigs. Vet. Rec. 137:295-296. [DOI] [PubMed] [Google Scholar]
- 20.Kilpper-Bälz, R., and K. H. Schleifer. 1987. Streptococcus suis sp. nov., nom. rev. Int. J. Syst. Bacteriol. 37:160-162. [Google Scholar]
- 21.Koeuth, T., J. Versalovic, and J. R. Lupski. 1995. Differential subsequence conservation of interspersed repetitive Streptococcus pneumoniae BOX elements in diverse bacteria. Genome Res. 5:408-418. [DOI] [PubMed] [Google Scholar]
- 22.Lutticken, R., N. Temme, G. Hahn, and E. W. Bartelheimer. 1986. Meningitis caused by Streptococcus suis: case report and review of the literature. Infection 14:181-185. [DOI] [PubMed] [Google Scholar]
- 23.Martin, B., O. Humbert, M. Camara, E. Guenzi, J. Walker, T. Mitchell, P. Andrew, M. Prudhomme, G. Alloing, R. Hakenbeck, D. A. Morrison, G. J. Boulnois, and J.-P. Claverys. 1992. A highly conserved repeated DNA element located in the chromosome of Streptococcus pneumoniae. Nucleic Acids Res. 20:3479-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okwumabua, O., O. Abdelmagid, and M. M. Chengappa. 1999. Hybridization analysis of the gene encoding a hemolysin (suilysin) of Streptococcus suis type 2: evidence for the absence of the gene in some isolates. FEMS Microbiol. Lett. 181:113-121. [DOI] [PubMed] [Google Scholar]
- 25.Osaki, M., D. Takamatsu, Y. Shimoji, and T. Sekizaki. 2002. Characterization of the Streptococcus suis genes encoding proteins homologous to sortase of gram-positive bacteria. J. Bacteriol. 184:971-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osaki, M., D. Takamatsu, N. Tsuji, and T. Sekizaki. 2000. Cloning and characterization of the gene encoding O-acetylserine lyase from Streptococcus suis. Curr. Microbiol. 40:67-71. [DOI] [PubMed] [Google Scholar]
- 27.Perch, B., K. B. Pedersen, and J. Henrichsen. 1983. Serology of capsulated streptococci pathogenic for pigs: six new serotypes of Streptococcus suis. J. Clin. Microbiol. 17:993-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.Segers, R. P. A. M., T. Kenter, L. A. M. de Haan, and A. A. C. Jacobs. 1998. Characterisation of the gene encoding suilysin from Streptococcus suis and expression in field strains. FEMS Microbiol. Lett. 167:255-261. [DOI] [PubMed] [Google Scholar]
- 31.Sekizaki, T., M. Osaki, D. Takamatsu, and Y. Shimoji. 2001. Distribution of the SsuDAT1I restriction-modification system among different serotypes of Streptococcus suis. J. Bacteriol. 183:5436-5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sekizaki, T., Y. Otani, M. Osaki, D. Takamatsu, and Y. Shimoji. 2001. Evidence for horizontal transfer of SsuDAT1I restriction-modification genes to the Streptococcus suis genome. J. Bacteriol. 183:500-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith, H. E., H. Buijs, R. de Vries, H. J. Wisselink, N. Stockhofe-Zurwieden, and M. A. Smits. 2001. Environmentally regulated genes of Streptococcus suis: identification by the use of iron-restricted conditions in vitro and by experimental infection of piglets. Microbiology 147:271-280. [DOI] [PubMed] [Google Scholar]
- 34.Staats, J. J., B. L. Plattner, J. Nietfeld, S. Dritz, and M. M. Chengappa. 1998. Use of ribotyping and hemolysin activity to identify highly virulent Streptococcus suis type 2 isolates. J. Clin. Microbiol. 36:15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takamatsu, D., M. Osaki, and T. Sekizaki. 2000. Sequence analysis of a small cryptic plasmid isolated from Streptococcus suis serotype 2. Curr. Microbiol. 40:61-66. [DOI] [PubMed] [Google Scholar]
- 36.Takamatsu, D., M. Osaki, and T. Sekizaki. 2001. Construction and characterization of Streptococcus suis-Escherichia coli shuttle cloning vectors. Plasmid 45:101-113. [DOI] [PubMed] [Google Scholar]
- 37.Takamatsu, D., M. Osaki, and T. Sekizaki. 2001. Thermosensitive suicide vectors for gene replacement in Streptococcus suis. Plasmid 46:140-148. [DOI] [PubMed] [Google Scholar]
- 38.Tarradas, C., C. Borge, A. Arenas, A. Maldonado, R. Astorga, A. Miranda, and I. Luque. 2001. Suilysin production by Streptococcus suis strains isolated from diseased and healthy carrier pigs in Spain. Vet. Rec. 148:183-184. [DOI] [PubMed] [Google Scholar]
- 39.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 40.Vecht, U., J. P. Arends, E. J. van der Molen, and L. A. M. G. van Leengoed. 1989. Differences in virulence between two strains of Streptococcus suis type II after experimentally induced infection of newborn germ-free pigs. Am. J. Vet. Res. 50:1037-1043. [PubMed] [Google Scholar]
- 41.Vecht, U., H. J. Wisselink, M. L. Jellema, and H. E. Smith. 1991. Identification of two proteins associated with virulence of Streptococcus suis type 2. Infect. Immun. 59:3156-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vecht, U., H. J. Wisselink, J. E. van Dijk, and H. E. Smith. 1992. Virulence of Streptococcus suis type 2 strains in newborn germfree pigs depends on phenotype. Infect. Immun. 60:550-556. [DOI] [PMC free article] [PubMed] [Google Scholar]