Abstract
Cell suspensions of Acetobacterium woodii prepared from cultures grown on fructose plus caffeate catalyzed caffeate reduction with electrons derived from molecular hydrogen. Hydrogen-dependent caffeate reduction was strictly Na+ dependent with a Km for Na+ of 0.38 mM; Li+ could substitute for Na+. The sodium ionophore ETH2120, but not protonophores, stimulated hydrogen-dependent caffeate reduction by 280%, indicating that caffeate reduction is coupled to the buildup of a membrane potential generated by primary Na+ extrusion. Caffeate reduction was coupled to the synthesis of ATP, and again, ATP synthesis coupled to hydrogen-dependent caffeate reduction was strictly Na+ dependent and abolished by ETH2120, but not by protonophores, indicating the involvement of a transmembrane Na+ gradient in ATP synthesis. The ATPase inhibitor N,N′-dicyclohexylcarbodiimide (DCCD) abolished ATP synthesis, and at the same time, hydrogen-dependent caffeate reduction was inhibited. This inhibition could be relieved by ETH2120. These experiments are fully compatible with a chemiosmotic mechanism of ATP synthesis with Na+ as the coupling ion during hydrogen-dependent caffeate reduction by A. woodii.
Homoacetogenic bacteria are a phylogenetically diverse group of strictly anaerobic bacteria which can use a wide variety of different substrates for fermentative growth under anaerobic conditions. Oxidation of carbohydrates such as hexose proceeds via the Embden-Meyerhof-Parnas pathway to pyruvate, which is split by the action of pyruvate ferredoxin oxidoreductase to acetyl coenzyme A (CoA), CO2, and reduced ferredoxin. Acetyl-CoA is further converted to acetate and CoA. The overall sum of the reaction is shown by equation:
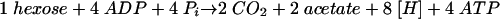 |
(1) |
The reducing equivalents are channeled to the CO2 produced, which is, thereby, converted to acetate in the Wood-Ljungdahl pathway (31, 32), according to equation:
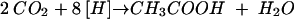 |
(2) |
The Wood-Ljungdahl pathway is also used by homoacetogens for chemolithoautotrophic growth according to equation:
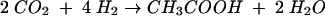 |
(3) |
In recent years it has turned out that the Wood-Ljungdahl pathway is coupled to a chemiosmotic mechanism of ATP synthesis, but the ways in which the ion gradients are established across the cytoplasmic membrane are different in different homoacetogens. Some organisms, the so-called proton organisms, Moorella thermoacetica being the primary example, have a cytochrome-containing (11), proton motive electron transport chain (4, 20); the electron donor can vary, but the electron acceptor is supposed to be methylene tetrahydrofolate, which is reduced to methyltetrahydrofolate (17, 18, 22). In the sodium ion organisms, which are devoid of cytochromes (29) but which have membrane-bound corrinoids (7), the pathway is strictly Na+ dependent, and experiments with Acetobacterium woodii established that the Wood-Ljungdahl pathway is coupled to the generation of a primary sodium ion potential, which in turn drives ATP synthesis via a Na+-translocating ATP synthase of the F1Fo type (2, 15, 16, 23, 26).
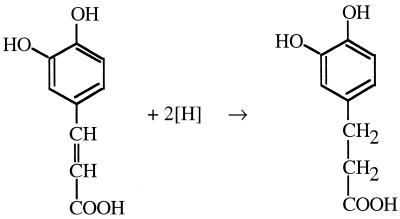
In recent years evidence has accumulated that homoacetogens can use not only CO2 but also alternative electron acceptors, including aromatic acrylate groups (3), fumarate (9, 10), dimethyl sulfoxide (P. S. Beaty and L. G. Ljungdahl, Abstr. 91st Gen. Meet. Am. Soc. Microbiol. 1991, abstr. K-131, p. 236, 1991), and nitrate (28). A. woodii is known to reduce the carbon-carbon double bond of phenylacrylate ethers such as caffeate according to the reaction shown in Fig. 1.
FIG. 1.
Reduction of caffeate to hydrocaffeate as carried out by A. woodii.
The electrons can be derived from various donors such as fructose, methanol, or hydrogen. Cell yield measurements with cells grown on fructose or methyl-group-containing substrates gave evidence not only that caffeate is used as an electron sink but in addition that caffeate reduction is coupled to energy conservation (29). Very clear evidence for ATP synthesis being coupled to caffeate reduction was obtained with resting cells of A. woodii in which hydrogen-dependent caffeate reduction was accompanied by synthesis of ATP (13). However, the coupling mechanism was not analyzed in detail. We have followed up these studies and will present evidence here that reduction of caffeate with hydrogen as the electron donor by resting cells of A. woodii is coupled to a chemiosmotic mechanism with Na+ as the coupling ion.
MATERIALS AND METHODS
Organism and cultivation.
A. woodii (DSMZ 1030) was cultivated at 30°C in 1.2-liter infusion flasks (Müller-Krempel, Bülach, Switzerland). The medium was prepared according to anaerobic techniques as described previously (6, 19). The medium contained, under an atmosphere of N2, the following: KH2PO4, 1.76 g/liter; K2HPO4, 8.44 g/liter; NH4Cl, 1.0 g/liter; cysteine hydrochloride, 0.5 g/liter; MgSO4 · 7H2O, 0.33 g/liter; NaCl, 2.9 g/liter; yeast extract, 2.0 g/liter; trace element solution SL 9, 1.0 ml/liter; selenite-tungstate solution, 1.0 ml/liter; and vitamin solution (DSMZ 141), 2.0 ml/liter. The pH was adjusted to 7.1 to 7.2 with HCl. Fructose was used as a carbon source to a final concentration of 8 mM. Growth was monitored by measuring the optical density at 600 nm (A600).
Preparation of cell suspensions.
Cells were grown up to an A600 of 0.15 to 0.25. Then, caffeate was added from an 0.1 M stock solution to induce the cells' ability to reduce caffeate. Cultures were harvested anaerobically at the end of the exponential growth phase by centrifugation (2,700 × g, 10 min, 4°C) and washed three times with imidazole-HCl buffer (20 mM imidazole-HCl, 20 mM MgSO4, 5 mM dithioerythritol, 1 mg of resazurin per liter, pH 7). The cells were resuspended in the same buffer to a final protein concentration of 11 to 16 mg/ml under an atmosphere of N2-H2 (95:5 [vol/vol]). This suspension was stored on ice and used immediately for the experiments. The protein concentration of the cell suspension was determined as described previously (27). All manipulations were done under strictly anaerobic conditions in an anaerobic chamber (Coy, Grass Lake, Mich.).
Experiments with cell suspensions.
All experiments were performed in 58-ml bottles. They contained, in a final volume of 10 ml, 9 ml of imidazole-HCl buffer, 1 ml of the concentrated cell suspension, and NaCl as indicated. After the suspensions were gassed with H2 for 30 min at 30°C in a shaking water bath at 180 rpm, caffeate was added as indicated in the figure legends from an 0.1 M stock solution. The ionophores N,N,N′,N′-tetracyclohexyl-1,2-phenylenedioxydiacetamide (ETH2120), tetrachlorosalicylanilide (TCS), 2-(3,5-di-tert-butyl-4-hydroxy-benzylidene)-malononitrile (SF6487), and the ATPase inhibitor N,N′-dicyclohexylcarbodiimide (DCCD) were added as ethanolic solutions as indicated in the figure legends; controls received the solvent only.
Determination of caffeate.
Samples (0.5 ml) were withdrawn by syringe and freed of cells by centrifugation at 20,000 × g. The supernatant was diluted 100-fold with imidazole-HCl buffer. The concentration of caffeate was determined in a photometric assay using the absorption maximum of caffeate at 312 nm. The caffeate concentration was calculated with the help of a calculation curve established with standards of known caffeate content.
Determination of intracellular ATP content.
ATP was determined by the luciferin-luciferase assay. Samples (0.5 ml) were withdrawn by syringe and incubated for 90 min in 3 M perchloric acid on ice. After neutralization by addition of aliquots of a saturated solution of K2CO3 and Na-TES [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid] buffer, pH 7.4, samples were centrifuged to remove the KClO4. Ten to fifteen microliters of the supernatant was transferred to a Lumacuvette (Celsis-Lumac, Landgraaf, The Netherlands), containing 250 μl of an ATP determination buffer (5 mM NaHAsO4, 4 mM MgSO4, 20 mM glycylglycine, pH 8) according to the description in reference 21. After the addition of 20 μl of firefly lantern crude extract, light emission was measured in a Luminometer (Celsis-Lumac). Calibration was done with standards of known ATP content.
Chemicals and gases.
Chemicals were purchased from Roth (Karlsruhe, Germany) and Merck (Ismaning, Germany), and firefly lantern crude extract was from Sigma (Taufkirchen, Germany). TCS was kindly provided by P. Smigan (University of Bratislava, Bratislava, Slovakia). Gases were from Messer-Griesheim (Kassel, Germany).
RESULTS
Na+ dependence of caffeate reduction.
Cells of A. woodii that had been grown on fructose plus caffeate in CO2-depleted medium were resuspended in buffer and incubated under an atmosphere of hydrogen at 30°C. After addition of caffeate to a final concentration of 10 mM, caffeate reduction started immediately and proceeded with a constant rate over a period of up to 140 min, until caffeate was completely reduced (data not shown). The ability to reduce caffeate was strictly dependent on the presence of caffeate in the growth medium, which is evidence that the enzymes involved in caffeate reduction have to be induced.
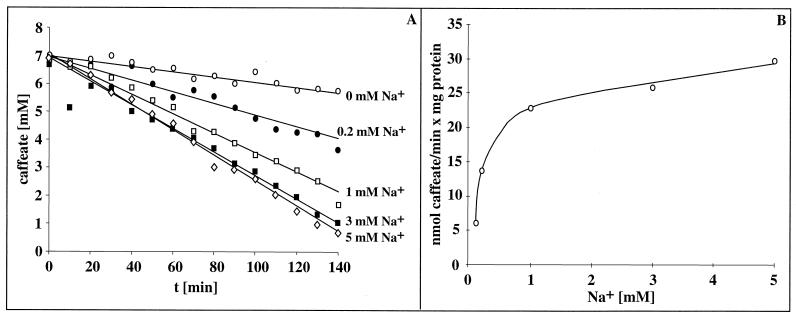
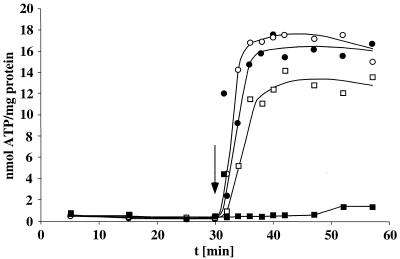
Acetate formation from H2 plus CO2 by A. woodii is strictly Na+ dependent and coupled to the generation of a transmembrane Na+ gradient across the cytoplasmic membrane. Because an initial indication for the presence of a sodium motive enzyme can be obtained by analyzing the effect of Na+ on a given reaction sequence, we determined the effect of Na+ on hydrogen-dependent caffeate reduction as carried out by resting cells of A. woodii. As can be seen from Fig. 2, caffeate reduction was largely impaired in buffers depleted of Na+; the residual activity was due to contaminating amounts of Na+ (100 μM) in the buffer used. However, caffeate reduction increased with increasing extracellular Na+ concentrations; half-maximal activity was obtained at 0.38 mM Na+, and saturation was obtained at 5 mM Na+. Addition of Na+ to a Na+-free cell suspension resulted in an immediate onset of caffeate reduction (data not shown). The same stimulation was observed with sodium chloride, sodium sulfate, sodium gluconate, and sodium nitrate, but potassium chloride did not stimulate caffeate reduction (data not shown), which is clear evidence that hydrogen-dependent caffeate reduction depends on Na+ for activity. Li+ could substitute for Na+.
FIG. 2.
Na+ dependence of hydrogen-dependent caffeate reduction by A. woodii. (A) Cell suspensions of A. woodii (1.54 mg of protein/ml) grown on fructose plus caffeate were prepared and incubated under an atmosphere of hydrogen at 30°C in a shaking water bath in a buffer containing NaCl as indicated. After preincubation for 30 min, caffeate was added from a stock solution. At the time points indicated, samples were withdrawn and analyzed for caffeate as described in Materials and Methods. Panel B displays the caffeate reduction rates as a function of the external Na+ concentration.
Respiratory control during hydrogen-dependent caffeate reduction.
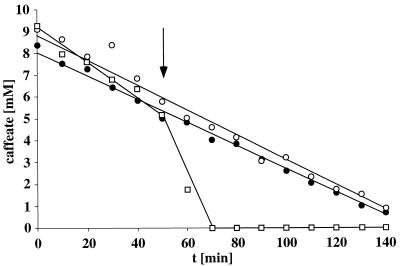
The experiments described so far clearly established a Na+ dependence of hydrogen-dependent caffeate reduction which can be interpreted as resulting from a membrane-bound sodium motive enzyme. If such an enzyme is present, its activity (i.e., vectorial Na+ export) will lead to the generation of an electrochemical Na+ potential (Δμ̃Na+) across the cytoplasmic membrane. The Δμ̃Na+ established will create a thermodynamic backup pressure which in turn will slow down further Na+ export and, concomitantly, caffeate reduction. If this backup pressure is relieved by the action of sodium ionophores, enzymatic activity will be restored. This phenomenon is called respiratory control and was first observed for mitochondria (5). As can be seen in Fig. 3, resting cells of A. woodii reduced caffeate at a rate of 46 μmol/min · mg of protein. However, upon addition of the Na+ ionophore ETH2120, the Na+ gradient was dissipated (14), and at the same time, caffeate reduction was stimulated 3.8-fold to 175 μmol/min · mg of protein. This experiment is clear evidence for the generation of a membrane potential during caffeate reduction in A. woodii. From the Na+ dependence of the reaction, the obvious stimulation of caffeate reduction by the Na+ ionophore ETH2120, and the inability of protonophores (which are active in A. woodii [14, 16]) to exert such stimulation, it can be concluded that the membrane potential is generated by primary Na+ extrusion coupled to the reduction of caffeate.
FIG. 3.
Stimulation of hydrogen-dependent caffeate reduction by sodium ionophores. Cell suspensions (1.25 mg of protein/ml) were treated as described in the legend to Fig. 2. At the time indicated by the arrow, one cell suspension received the protonophore SF6847 (○) (final concentration, 27 μM), and another received the sodium ionophore ETH2120 (□) (final concentration, 27 μM). The control (•) received the solvent only.
Na+ dependence of ATP synthesis coupled to hydrogen-dependent caffeate reduction.
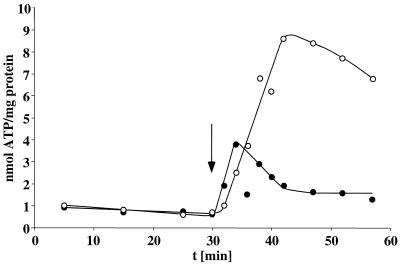
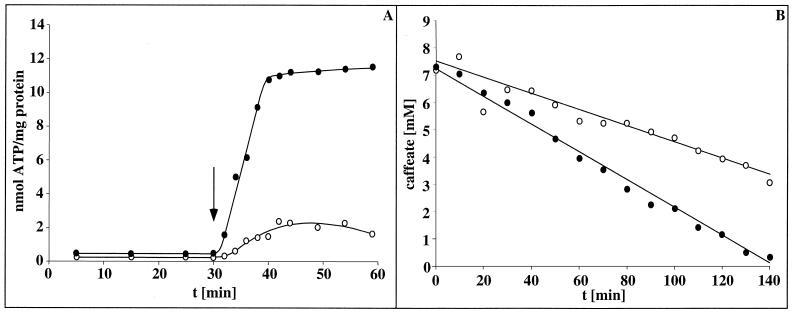
Next, it was tested whether the transmembrane Na+ gradient is coupled to ATP synthesis. The intracellular ATP content of cells incubated in the presence of hydrogen but the absence of an electron acceptor was rather low (1 nmol/mg of protein). Upon addition of caffeate, the intracellular ATP content increased immediately to 8.6 nmol/mg of protein, as shown before (13). However, in the absence of supplemental Na+ (presence of only contaminating amounts) ATP synthesis was largely impaired (Fig. 4). This experiment clearly indicates an involvement of Na+ in ATP synthesis. Although the rate of ATP synthesis is difficult to resolve in these experiments, a first approximation gives a ratio of 0.2 mol of ATP formed per mol of caffeate reduced.
FIG. 4.
Na+ dependence of ATP synthesis coupled to hydrogen-dependent caffeate reduction. Cell suspensions of A. woodii (1.33 mg of protein/ml) were incubated in buffer in the absence of supplemental Na+ (•) or in the presence of 10 mM Na+ (○). Caffeate was added to a final concentration of 10 mM at the time indicated by the arrow. At time points indicated, samples were withdrawn and analyzed for cellular ATP content as described in Materials and Methods.
Next, ionophore studies were performed to unravel the nature of the Na+ dependence of ATP synthesis. Preincubation of cells with the protonophore TCS or SF6847 had little or no effect on the intracellular ATP content. However, preincubation of the cells with the Na+ ionophore ETH2120 not only stimulated caffeate reduction (data not shown; cf. Fig. 3), but completely abolished ATP synthesis (Fig. 5). Addition of ETH2120 to cells in the steady state of caffeate reduction immediately dissipated the intracellular ATP level (data not shown). This is evidence that ATP synthesis is not dependent on the presence of Na+ per se but on a transmembrane Na+ gradient.
FIG. 5.
Inhibition of ATP synthesis coupled to hydrogen-dependent caffeate reduction by the sodium ionophore ETH2120. Cell suspensions of A. woodii (1.54 mg of protein/ml) were preincubated under a hydrogen atmosphere in the presence of 10 mM Na+ and 20 μM TCS (□), 20 μM SF6847 (•), or 20 μM ETH2120 (▪). A control received the solvent only (○). Caffeate was added to a final concentration of 10 mM at the time point indicated by the arrow. At time points indicated, samples were withdrawn and analyzed for cellular ATP content as described in Materials and Methods.
Inhibition of ATP synthesis and caffeate reduction by DCCD.
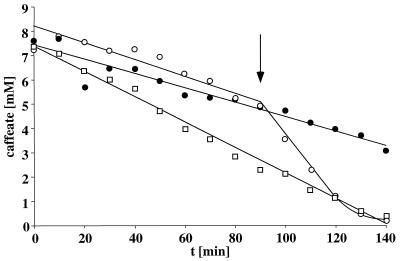
The experiments described so far are fully compatible with the following sequence of events: caffeate reduction → generation of a transmembrane Na+ gradient → generation of ATP. Inhibition of the ATPase should therefore inhibit both ATP synthesis and, subsequently, caffeate reduction. To test this, resting cells of A. woodii were incubated under a hydrogen atmosphere with DCCD, a potent inhibitor of the Na+-F1Fo-ATPase of A. woodii. As can be seen in Fig. 6A, DCCD effectively inhibited ATP synthesis coupled to caffeate reduction. At the same time, caffeate reduction was inhibited (Fig. 6B) by producing a thermodynamic backup pressure on the caffeate reduction pathway. However, upon addition of the Na+ ionophore ETH2120 to cells previously inhibited by DCCD, the thermodynamic backup pressure was relieved, and subsequently, caffeate reduction was not only restored to control levels but stimulated as seen before in the absence of DCCD (Fig. 7).
FIG. 6.
Inhibition of ATP synthesis and hydrogen-dependent caffeate reduction by the ATPase inhibitor DCCD. Cell suspensions of A. woodii (1.54 mg of protein/ml) were preincubated under a hydrogen atmosphere in the presence of 3 mM Na+ and absence (•) or presence (○) of 100 μM DCCD for 30 min. Caffeate was added at the time point indicated by the arrow (A) or at zero time to a final concentration of 10 mM (B). At time points indicated, samples were withdrawn and analyzed for cellular ATP content (A) or caffeate concentration (B) as described in Materials and Methods.
FIG. 7.
Inhibition of hydrogen-dependent caffeate reduction by the ATPase inhibitor DCCD and relief of DCCD inhibition by the sodium ionophore ETH2120. Cell suspensions of A. woodii (1.54 mg of protein/ml) were preincubated under a hydrogen atmosphere in the presence of 3 mM Na+ and absence (□) or presence (○ and •) of 100 μM DCCD for 30 min. Caffeate was added to a final concentration of 10 mM at zero time. At the time point indicated by the arrow one suspension received the sodium ionophore ETH2120 (○) at a final concentration of 36 μM. Samples were withdrawn and analyzed for caffeate concentration as described in Materials and Methods.
DISCUSSION
Although it has been reported before that resting cells of A. woodii (strain NZvA16) couple caffeate reduction to the synthesis of ATP (13), the coupling mechanism was not elucidated. We have followed up these studies with strain DSMZ 1030, and the experiments described here gave clear evidence for a chemiosmotic mechanism of ATP synthesis during hydrogen-dependent caffeate reduction in A. woodii. Most interestingly, like CO2 reduction, hydrogen-dependent caffeate reduction and ATP synthesis coupled to caffeate reduction are strictly Na+ dependent, and the latter is dependent on a transmembrane Na+ gradient. The studies described here are fully compatible with the following sequence of events: caffeate reduction → generation of a transmembrane Na+ gradient → generation of ATP by the Na+-F1Fo-ATP synthase.
It is likely that the electrons are channeled from hydrogen to caffeate via a membrane-bound electron transport chain. Oxidation of hydrogen is catalyzed by a hydrogenase, and in earlier studies a hydrogenase was purified from A. woodii. Because more than 99% of the activity was found in the cytoplasm, the enzyme was described as being a soluble, cytoplasmic enzyme (25). This would argue for an additional electron carrier such as NAD+ or ferredoxin to transport the electrons to the membrane and would require a membrane-bound NADH dehydrogenase or reduced ferredoxin dehydrogenase. On the other hand, it cannot be excluded that a membrane-bound hydrogenase might have been overlooked in earlier studies. In light of our results, a careful reexamination of the cellular localization of hydrogenase activities in A. woodii is important.
After oxidation of the electron donor the electrons are transferred to the acceptor, caffeate. The components involved in the electron transport are still obscure. It should be remembered that, for A. woodii, even after growth in the presence of caffeate, cytochromes or quinones were not detected (29). However, the electron transport chain could contain yet-unknown electron carriers. Methanosarcinales were shown some 25 years ago to be devoid of quinones (24), but recently, a novel membrane-bound electron carrier, methanophenazine, was discovered in the archaeon Methanosarcina mazei Gö1 (1). On the other hand, it should be noted in this connection that some fumarate reductase systems do not contain cytochromes (12) and that Methanobacteriales catalyze an electron transport from hydrogenase to the heterodisulfide in the absence of cytochromes and quinones (8). In addition, Ruminobacter amylophilus catalyzes fumarate reduction in the absence of cytochromes, and its reduction was shown previously to be stimulated by Na+ (30). The unraveling of this interesting electron transport system in A. woodii leading from hydrogen to caffeate, the identification of its components including the primary Na+ pump, and its regulation are the subjects of further studies in our laboratory.
Acknowledgments
This study was supported by a grant from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Abken, H. J., M. Tietze, J. Brodersen, S. Baumer, U. Beifuss, and U. Deppenmeier. 1998. Isolation and characterization of methanophenazine and function of phenazines in membrane-bound electron transport of Methanosarcina mazei Gö1. J. Bacteriol. 180:2027-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aufurth, S., H. Schägger, and V. Müller. 2000. Identification of subunits a, b, and c1 from Acetobacterium woodii Na+-F1F0-ATPase. Subunits c1, c2, and c3 constitute a mixed c-oligomer. J. Biol. Chem. 275:33297-33301. [DOI] [PubMed] [Google Scholar]
- 3.Bache, R., and N. Pfennig. 1981. Selective isolation of Acetobacterium woodii on methoxylated aromatic acids and determination of growth yields. Arch. Microbiol. 130:255-261. [Google Scholar]
- 4.Baronofsky, J. J., and W. J. A. Schreurs. 1984. Uncoupling by acetic acid limits growth of and acetogenesis by Clostridium thermoaceticum. Appl. Environ. Microbiol. 48:1134-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beechey, R. B., A. M. Roberton, C. T. Holloway, and I. G. Knight. 1967. The properties of dicyclohexylcarbodiimide as an inhibitor of oxidative phosphorylation. Biochemistry 6:3867-3879. [DOI] [PubMed] [Google Scholar]
- 6.Bryant, M. P. 1972. Commentary on the Hungate technique for culture of anaerobic bacteria. Am. J. Clin. Nutr. 25:1324-1328. [DOI] [PubMed] [Google Scholar]
- 7.Dangel, W., H. Schulz, G. Diekert, H. König, and G. Fuchs. 1987. Occurrence of corrinoid-containing membrane proteins in anaerobic bacteria. Arch. Microbiol. 148:52-56. [Google Scholar]
- 8.Deppenmeier, U., V. Müller, and G. Gottschalk. 1996. Pathways of energy conservation in methanogenic archaea. Arch. Microbiol. 165:149-163. [Google Scholar]
- 9.Dorn, M., J. R. Andreesen, and G. Gottschalk. 1978. Fermentation of fumarate and l-malate by Clostridium formicoaceticum. J. Bacteriol. 133:26-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorn, M., J. R. Andreesen, and G. Gottschalk. 1978. Fumarate reductase of Clostridium formicoaceticum. A peripheral membrane protein. Arch. Microbiol. 119:7-11. [DOI] [PubMed] [Google Scholar]
- 11.Gottwald, M., J. R. Andreesen, J. LeGall, and L. G. Ljungdahl. 1975. Presence of cytochrome and menaquinone in Clostridium formicoaceticum and Clostridium thermoaceticum. J. Bacteriol. 122:325-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hägerhäll, C., and L. Hederstedt. 1996. A structural model for the membrane-integral domain of succinate: quinone oxidoreductases. FEBS Lett. 389:25-31. [DOI] [PubMed] [Google Scholar]
- 13.Hansen, B., M. Bokranz, P. Schönheit, and A. Kröger. 1988. ATP formation coupled to caffeate reduction by H2 in Acetobacterium woodii NZva16. Arch. Microbiol. 150:447-451. [Google Scholar]
- 14.Heise, R., V. Müller, and G. Gottschalk. 1993. Acetogenesis and ATP synthesis in Acetobacterium woodii are coupled via a transmembrane primary sodium ion gradient. FEMS Microbiol. Lett. 112:261-268. [Google Scholar]
- 15.Heise, R., V. Müller, and G. Gottschalk. 1992. Presence of a sodium-translocating ATPase in membrane vesicles of the homoacetogenic bacterium Acetobacterium woodii. Eur. J. Biochem. 206:553-557. [DOI] [PubMed] [Google Scholar]
- 16.Heise, R., J. Reidlinger, V. Müller, and G. Gottschalk. 1991. A sodium-stimulated ATP synthase in the acetogenic bacterium Acetobacterium woodii. FEBS Lett. 295:119-122. [DOI] [PubMed] [Google Scholar]
- 17.Hugenholtz, J., D. M. Ivey, and L. G. Ljungdahl. 1987. Carbon monoxide-driven electron transport in Clostridium thermoautotrophicum membranes. J. Bacteriol. 169:5845-5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hugenholtz, J., and L. G. Ljungdahl. 1989. Electron transport and electrochemical proton gradient in membrane vesicles of Clostridium thermoaceticum. J. Bacteriol. 171:2873-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hungate, R. E. 1969. A roll tube method for cultivation of strict anaerobes. Methods Microbiol. 3b:117-132. [Google Scholar]
- 20.Ivey, D. M., and L. G. Ljungdahl. 1986. Purification and characterization of the F1-ATPase from Clostridium thermoaceticum. J. Bacteriol. 165:252-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimmich, G. A., J. Randles, and J. S. Brand. 1975. Assay of picomole amounts of ATP, ADP, and AMP using the luciferase enzyme system. Anal. Biochem. 169:187-206. [DOI] [PubMed] [Google Scholar]
- 22.Ljungdahl, L. G. 1994. The acetyl-CoA pathway and the chemiosmotic generation of ATP during acetogenesis, p. 63-87. In H. L. Drake (ed.), Acetogenesis. Chapman & Hall, New York, N.Y.
- 23.Müller, V., S. Aufurth, and S. Rahlfs. 2001. The Na+ cycle in Acetobacterium woodii: identification and characterization of a Na+ translocating F1FO-ATPase with a mixed oligomer of 8 and 16 kDa proteolipids. Biochim. Biophys. Acta 1505:108-120. [DOI] [PubMed] [Google Scholar]
- 24.Müller, V., M. Blaut, and G. Gottschalk. 1993. Bioenergetics of methanogenesis, p. 360-406. In J. G. Ferry (ed.), Methanogenesis. Chapman & Hall, New York, N.Y.
- 25.Ragsdale, S. W., and L. G. Ljungdahl. 1984. Hydrogenase from Acetobacterium woodii. Arch. Microbiol. 139:361-365. [DOI] [PubMed] [Google Scholar]
- 26.Reidlinger, J., and V. Müller. 1994. Purification of ATP synthase from Acetobacterium woodii and identification as a Na+-translocating F1F0-type enzyme. Eur. J. Biochem. 223:275-283. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt, K., S. Liaanen-Jensen, and H. G. Schlegel. 1963. Die Carotinoide der Thiorodaceae. Arch. Mikrobiol. 46:117-126. [PubMed] [Google Scholar]
- 28.Seifritz, C., S. L. Daniel, A. Gossner, and H. L. Drake. 1993. Nitrate as a preferred electron sink for the acetogen Clostridium thermoaceticum. J. Bacteriol. 175:8008-8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tschech, A., and N. Pfennig. 1984. Growth yield increase linked to caffeate reduction in Acetobacterium woodii. Arch. Microbiol. 137:163-167. [Google Scholar]
- 30.Wetzstein, H. G., and G. Gottschalk. 1985. A sodium-stimulated membrane-bound fumarate reductase in Bacteroides amylophilus. Arch. Microbiol. 143:157-162. [DOI] [PubMed] [Google Scholar]
- 31.Wood, H. G. 1991. Life with CO or CO2 and H2 as a source of carbon and energy. FASEB J. 5:156-163. [DOI] [PubMed] [Google Scholar]
- 32.Wood, H. G., S. W. Ragsdale, and E. Pezacka. 1986. The acetyl-CoA pathway of autotrophic growth. FEMS Microbiol. Rev. 39:345-362. [Google Scholar]