Abstract
We present the first description of a single-stranded DNA filamentous phage able to replicate in a gram-positive bacterium. Phage B5 infects Propionibacterium freudenreichii and has a genome consisting of 5,806 bases coding for 10 putative open reading frames. The organization of the genome is very similar to the organization of the genomes of filamentous phages active on gram-negative bacteria. The putative coat protein exhibits homology with the coat proteins of phages PH75 and Pf3 active on Thermus thermophilus and Pseudomonas aeruginosa, respectively. B5 is, therefore, evolutionarily related to the filamentous phages active on gram-negative bacteria.
Filamentous phages are flexible rods that contain circular single-stranded DNA (ssDNA) coding for about 10 genes. They infect gram-negative bacteria by using a unique strategy of virion morphogenesis. Progeny particles are continually extruded or secreted across the bacterial membranes, causing neither lysis nor cell death (for reviews see references 22 and 23). This mode of assembly and excretion is related to bacterial processes responsible for the assembly of type 4 pili and for type II protein secretion (30). Many different filamentous phages have been characterized, and the Escherichia coli Ff phage family (M13, fd, and f1) is the best studied. Previous studies led to a better understanding of the role of these phages in bacterial evolution and the of spread of virulence factors. They also led to important applications in DNA sequencing and phage display techniques. Until now, there have been only infrequent reports of Ff-related phage active on gram-positive bacteria. A filamentous virus-like particle has been isolated from Clostridium acetobutylicum (17). However, the infectivity of this particle was not demonstrated, and its DNA was not characterized. More recently, five phages with filamentous morphology were isolated during a survey of phages active on Propionibacterium freudenreichii (9), a bacterium widely used in Swiss-type cheese manufacture to produce the characteristic flavor and eyes. In this work, we characterized one of these phages, a phage designated B5. This phage contains a ssDNA consisting of 5,806 bases and has a genome organization similar to the genome organization of other filamentous phages. This is the first description of an ssDNA phage genome that replicates in a gram-positive bacterium.
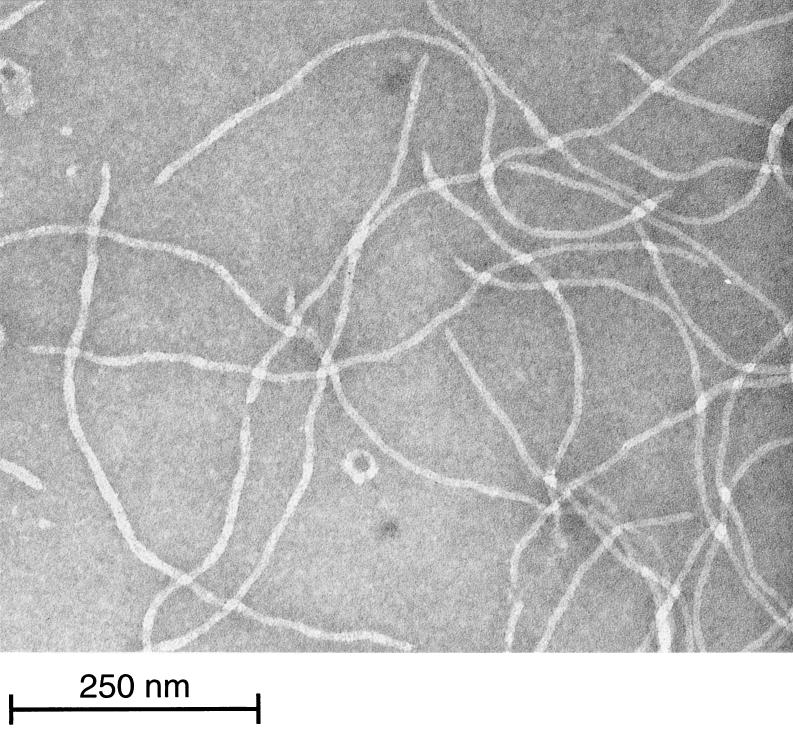
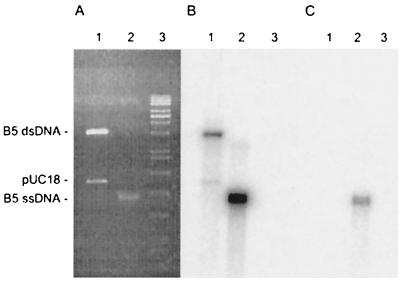
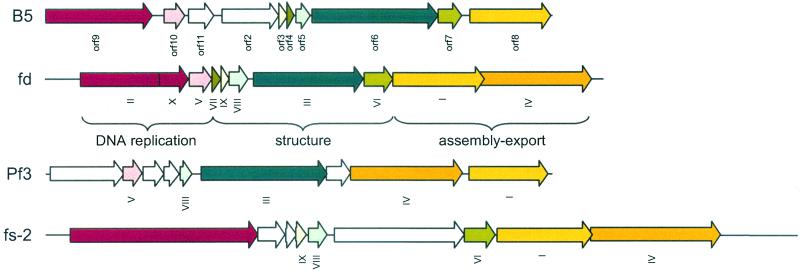
Phage B5 was propagated on P. freudenreichii strain TIL18 in Yel medium (12) at 30°C and was enumerated by using the soft agar layer method (1). One-step growth experiments (1) revealed a latent period of about 11 h that corresponded to the generation time of the host. Phage B5 particles were filamentous, and the average size was 620 nm long by 12 nm wide (Fig. 1). Phage DNA, extracted as previously described (8), was resistant to all restriction enzymes but was degraded by S1 nuclease. These results suggested that like the genomes of all filamentous phages described so far, the phage B5 genome consists of ssDNA. Therefore, upon infection phage DNA must be converted to a double-stranded replicative form (RF) by the host enzymes (23). In order to isolate such a RF, DNA was extracted from strain TIL18 7 h after infection with phage B5. A 5.5-kb plasmid, which was absent from the parental strain, was isolated, purified, and transferred into strain TL18 by electroporation (7). A control without DNA was included in the analysis. Transfected cells were spread on a lawn of indicator strain TIL18. Following transfection with 100 ng of DNA, 104 PFU was obtained, indicating that the plasmid DNA extracted from infected cells was indeed a RF of phage B5. Southern hybridization experiments revealed that as expected for ssDNA, the packaged B5 DNA was transferred to a nitrocellulose membrane without denaturation (Fig. 2). In order to sequence the phage B5 genome, BamHI-cleaved RF DNA was cloned in the pUC18 plasmid. Sequencing was carried out by chromosome walking. The sequence was determined by a cycle extension reaction with dye terminator cycle sequencing or by dGTP Big Dye terminator ready reactions (Applied Biosystems) by using a 377 DNA sequencer (Applied Biosystems). A sequence consisting of 5,806 bases with a minimum redundancy of six was obtained, and each region was sequenced at least once on both strands. The total G+C content of the B5 phage genome is 64%, which is similar to the G+C content of the P. freudenreichii host (5). The genome organization (Fig. 3) is very similar to the genome organization of other filamentous phages in which three genes that are required for DNA synthesis are followed by five genes coding for structural proteins of the virion and three genes involved in assembly and export (30).
FIG. 1.
Electron micrograph of phage B5 purified on a cesium chloride gradient and negatively stained with 2% uranyl acetate.
FIG. 2.
Characterization of phage B5 DNA. (A) Agarose gel electrophoresis gel stained with ethidium bromide; (B and C) Southern hybridization with an oligonucleotide complementary to the phage DNA. In order to differentiate ssDNA from double-stranded DNA (dsDNA), DNA was transferred with (B) and without (C) an alkaline denaturation treatment (19). Lane 1, control with the double-stranded DNA RF of phage B5 cloned in pUC18 and digested with BamHI; lane 2, packaged form of B5 DNA; lane 3, RAOUL molecular weight marker (Appligene; Qbiogene, Strasbourg, France).
FIG. 3.
Genome organization of P. freudenreichii phage B5 (this study) compared to genome organization of E. coli phage fd (2), P. aeruginosa phage Pf3 (18, 27, 31), and Vibrio cholerae phage fs-2 (14). Open reading frames with similar functions are indicated by the same color.
Phage DNA replication.
The double-stranded RF of filamentous phage DNA replicates by a rolling circle mechanism (23). Two proteins needed for this replication are putatively encoded by the phage B5 genome. Orf9 exhibits 27 to 35% identity with rolling circle mechanism plasmid replication proteins of the pIJ101/pIV1 family, and there are five conserved domains (3, 13, 15). Orf10 has in its C-terminal region a motif consisting of aromatic and positively charged residues similar to those proposed for ssDNA-binding proteins (10, 34). Replication could initiate just upstream of orf9 at several strong secondary structures present at the 3′ end of orf8.
Phage structural proteins.
Filamentous phages are tubes formed by many copies of the major coat protein (pVIII), and a few copies of four minor proteins are located at the ends. Two of these proteins, pVII and pIX, are necessary for efficient particle assembly; the other two, pVI and pIII, are required for particle stability and phage infectivity (29, 31). All five structural proteins are anchored in the inner membrane prior to incorporation into phage particles (4, 6, 35). Amino acid homologies and structural properties allowed us to identify putative equivalents of these five structural proteins on the B5 phage genome. According to the Smith-Waterman algorithm (24, 32), Orf5 exhibits 41 and 31% identity with the coat proteins of filamentous phages PH75and Pf3 active on Thermus thermophilus (25) and Pseudomonas aeruginosa (18), respectively (Fig. 4). These proteins lack a leader sequence present in E. coli Ff phages, but the Pf3 coat protein has been shown to insert spontaneously into the cell membrane (16). Analysis of phage B5 proteins revealed a major ca. 6.0-kDa protein (9), which is consistent with the Orf5 calculated molecular mass. Moreover, Orf5, Orf6, Orf7, Orf4, and Orf3 are similar in terms of size and structure to structural proteins of E. coli phage fd (Fig. 5).
FIG. 4.
Alignment of coat proteins from P. aeruginosa phage Pf3 (accession number M19377) (18), T. thermophilus phage PH75 (accession number P82889) (25), and P. freudenreichii phage B5 (accession number AF428260) (this study). Conserved amino acids are indicated in boldface type.
FIG. 5.
Comparison of P. freudenreichii phage B5 proteins (Orf5, Orf4, Orf3, Orf7, Orf6) (this study) and structural proteins of E. coli phage fd (pVIII, pVII, pIX, pVI, pIII) (2). Charged residues are indicated by red (positive charge) or blue (negative charge) boxes. Hydrophobic helices and leader peptide sequences are indicated by green and yellow rectangles, respectively. Repeats are indicated by arrowheads. Transmembrane helices of phage B5 proteins have been predicted by using the PHD program (28).
Phage assembly and export.
Two proteins (pI and pIV) are required for filamentous phage assembly and export (30). pI is most probably involved in formation of a specialized assembly site or exit complex in the inner membrane (29). All filamentous phages that have been studied have a pI equivalent. However, the only detectable feature shared by pI proteins is a motif common to ATP-binding proteins (33). This motif, which is present in the N-terminal part of Orf8, suggests that this protein could be a pI equivalent. pIV, which plays the role of an outer membrane phage-conducting channel (20, 21), had no equivalent in phage B5. It is likely that B5 does not require this protein, since it is active on a gram-positive host that lacks an outer membrane.
Conclusions.
The morphology of phage B5, the organization of its ssDNA genome, and structural properties of its proteins indicate that it is related to filamentous phages and, most probably, has the same life cycle. Except for very closely related E. coli Ff phages and the IKe phages that are about 50% identical at the nucleotide level (26), there is no sequence similarity between filamentous phages (29). However, amino acid homologies were detected for the coat proteins of phages B5, PH75, and Pf3 active on phylogenetically distantly related hosts. Therefore, as proposed for double-stranded DNA phages (11), filamentous phages could be derived from a common ancestral pool of genes. Establishment of evolutionary relationships among filamentous phages will rely on characterization of isolates active on different hosts, particularly on gram-positive bacteria. Furthermore, the relationship of the replication regions of ssDNA phages and plasmids is interesting. Quite interestingly, the former predominate in gram-negative bacteria, whereas the latter are overwhelmingly dominant in gram-positive bacteria. This may reflect a profound difference in the physiologies of the two bacterial groups which remains to be determined.
Nucleotide sequence accession number.
The nucleotide sequence of the phage B5 genome has been deposited in the GenBank database under accession number AF428260.
Acknowledgments
We thank Françoise Michel for electron microscopy, Alla Lapidus for advice concerning sequencing high-G+C-content DNA, and Alain Chopin for help with preparation of the manuscript.
REFERENCES
- 1.Adams, M. H. 1959. Bacteriophages. Interscience Publishers, Inc., New York, N.Y.
- 2.Beck, E., and B. Zink. 1981. Nucleotide sequence and genome organisation of filamentous bacteriophages f1 and fd. Gene 16:35-58. [DOI] [PubMed] [Google Scholar]
- 3.Billington, S. J., B. H. Jost, and J. G. Songer. 1998. The Arcanobacterium (Actinomyces) pyogenes plasmid pAP1 is a member of the pIJ/pJV1 family of rolling circle replication plasmids. J. Bacteriol. 180:3233-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boeke, J. D., and P. Model. 1982. A prokaryotic membrane anchor sequence: carboxyl terminus of bacteriophage f1 gene III protein retains it in the membrane. Proc. Natl. Acad. Sci. USA 79:5200-5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carvalho, A. F. 1994. Ph.D. thesis. E. N. S. A. de Rennes, Rennes, France.
- 6.Endemann, H., and P. Model. 1995. Location of filamentous phage minor coat proteins in phage and in infected cells. J. Mol. Biol. 250:496-506. [DOI] [PubMed] [Google Scholar]
- 7.Gautier, M., A. Rouault, and R. Lemée. 1995. Electrotransfection of Propionibacterium freudenreichii TL110. Lett. Appl. Microbiol. 20:125-129. [Google Scholar]
- 8.Gautier, M., A. Rouault, P. Sommer, and R. Briandet. 1995. Occurrence of Propionibacterium freudenreichii bacteriophages in Swiss cheese. Appl. Environ. Microbiol. 61:2572-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gautier, M., A. Rouaut, C. Hervé, P. Sommer, V. Leret, G. Jan, J. M. Fraslin, F. Prévot, and A. Coste. 1999. Bacteriophage of dairy propionibacteria. Lait 79:93-104. [Google Scholar]
- 10.Gutiérrez, C., G. Martin, J. M. Sogo, and M. Salas. 1991. Mechanism of stimulation of DNA replication by bacteriophage phi29 single-stranded DNA-binding protein p5. J. Biol. Chem. 266:2104-2111. [PubMed] [Google Scholar]
- 11.Hendrix, R. W., M. C. M. Smith, R. N. Burns, M. E. Ford, and G. F. Hatfull. 1999. Evolutionary relationships among diverse bacteriophages and prophages: all the world's a phage. Proc. Natl. Acad. Sci. USA 96:2192-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hettinga, D. H., E. R. Vedamuthu, and G. W. Reinbold. 1968. Pouch method for isolating and numerating propionibacteria. J. Dairy Sci. 51:1707-1709. [DOI] [PubMed] [Google Scholar]
- 13.Iiyina, T. V., and E. V. Koonin. 1992. Conserved sequence motifs in the initiator proteins for rolling circle DNA replication encoded by diverse replicons from eubacteria, eucaryotes and archaebacteria. Nucleic Acids Res. 20:3279-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikema, M., and Y. Honma. 1998. A novel filamentous phage, fs-2, of Vibrio cholerae O139. Microbiology 144:1901-1906. [DOI] [PubMed] [Google Scholar]
- 15.Khan, S. A. 1997. Rolling-circle replication of bacterial plasmids. Microbiol. Mol. Biol. Rev. 61:442-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiefer, D., and A. Khun. 1999. Hydrophobic forces drive spontaneous membrane insertion of the bacteriophage Pf3 coat protein without topological control. EMBO J. 18:6299-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, A. Y., and H. P. Blaschek. 1991. Isolation and characterization of a filamentous viruslike particle from Clostridium acetobutylicum NCIB 6444. J. Bacteriol. 173:530-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luiten, R. G., D. G. Putterman, J. G. Schoenmakers, R. N. Konings, and L. A. Day. 1985. Nucleotide sequence of the genome of Pf3, an IncP-1 plasmid-specific filamentous bacteriophage of Pseudomonas aeruginosa. J. Virol. 56:268-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 20.Marciano, D. K., M. Russel, and S. M. Simon. 1999. An aqueous channel for filamentous phage export. Science 284:1516-1519. [DOI] [PubMed] [Google Scholar]
- 21.Marciano, D. K., M. Russel, and S. M. Simon. 2001. Assembling filamentous phage occlude pIV channels. Proc. Natl. Acad. Sci. USA 98:9359-9364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marvin, D. A. 1998. Filamentous phage structure, infection and assembly. Curr. Opin. Struct. Biol. 8:150-158. [DOI] [PubMed] [Google Scholar]
- 23.Model, P., and M. Russel. 1988. Filamentous bacteriophage, p. 375-456. In R. Calendar (ed.), The bacteriophages. Plenum Press, New York, N.Y.
- 24.Pearson, W. R. 1991. Searching protein sequence libraries: comparison of the sensitivity and selectivity of the Smith-Waterman and FASTA algorithms. Genomics 11:635-650. [DOI] [PubMed] [Google Scholar]
- 25.Pederson, D. M., L. C. Welsh, D. A. Marvin, M. Sampson, R. N. Perham, M. Yu, and M. R. Slater. 2001. The protein capsid of filamentous bacteriophage PH75 from Thermus thermophilus. J. Mol. Biol. 309:401-421. [DOI] [PubMed] [Google Scholar]
- 26.Peeters, B. P., R. M. Peters, J. G. Schoenmakers, and R. N. Konings. 1985. Nucleotide sequence and genetic organization of the genome of the N-specific filamentous bacteriophage IKe. Comparison with the genome of the F-specific filamentous phages M13, fd and f1. J. Mol. Biol. 181:27-39. [DOI] [PubMed] [Google Scholar]
- 27.Putterman, D. G., A. Casadevall, P. D. Boyle, H. Yang, B. Frangione, and L. A. Day. 1984. Major coat protein and single-stranded DNA-binding protein of filamentous virus Pf3. Proc. Natl. Acad. Sci. USA 81:699-703. [DOI] [PMC free article] [PubMed]
- 28.Rost, B., R. Casadio, P. Fariselli, and C. Sander. 1995. Transmembrane helices predicted at 95% accuracy. Protein Sci. 4:521-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russel, M. 1991. Filamentous phage assembly. Mol. Microbiol. 5:1607-1613. [DOI] [PubMed] [Google Scholar]
- 30.Russel, M. 1995. Moving through the membrane with filamentous phages. Trends Microbiol. 3:223-228. [DOI] [PubMed] [Google Scholar]
- 31.Russel, M., N. A. Linderoth, and A. Sali. 1997. Filamentous phage assembly: variation on a protein export theme. Gene 192:23-32. [DOI] [PubMed] [Google Scholar]
- 32.Smith, T. F., and M. S. Waterman. 1981. Identification of common molecular subsequences. J. Mol. Biol. 147:195-197. [DOI] [PubMed] [Google Scholar]
- 33.Walker, J. E., M. Saraste, M. J. Runswick, and N. J. Gay. 1982. Distantly related sequences in the α- and β-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang, Y., and J. D. Hall. 1990. Characterization of a major DNA-binding domain in the herpes simplex virus type 1 DNA-binding protein (ICP8). J. Virol. 64:2082-2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webster, R. E., and J. S. Cashman. 1973. Abortive infection of Escherichia coli with bacteriophage f1: cytoplasmic membrane proteins and the f1 DNA-gene 5 protein complex. Virology 55:20-38. [DOI] [PubMed] [Google Scholar]