Abstract
The pathogenesis of Clostridium perfringens-mediated gas gangrene or clostridial myonecrosis involves the extracellular toxins alpha-toxin and perfringolysin O. Previous studies (T. Shimizu, A. Okabe, J. Minami, and H. Hayashi, Infect. Immun. 59:137-142, 1991) carried out with Escherichia coli suggested that the perfringolysin O structural gene, pfoA, was positively regulated by the product of the upstream pfoR gene. In an attempt to confirm this hypothesis in C. perfringens, a pfoR-pfoA deletion mutant was complemented with isogenic pfoA+ shuttle plasmids that varied only in their ability to encode an intact pfoR gene. No difference in the ability to produce perfringolysin O was observed for C. perfringens strains carrying these plasmids. In addition, chromosomal pfoR mutants were constructed by homologous recombination in C. perfringens. Again no difference in perfringolysin O activity was observed. Since it was not possible to alter perfringolysin O expression by mutation of pfoR, it was concluded that the pfoR gene product is unlikely to have a role in the regulation of pfoA expression in C. perfringens.
The anaerobic human pathogen Clostridium perfringens is the primary cause of gas gangrene or clostridial myonecrosis. The pathogenesis of this disease involves the production of two extracellular toxins, alpha-toxin or phospholipase C and theta-toxin or perfringolysin O. The alpha-toxin is the more toxic of these proteins and is an essential factor in the disease process (1, 9, 22). Perfringolysin O is a pore-forming cytolysin that is able to lyse red blood cells in a cholesterol-dependent manner (21). Along with alpha-toxin, perfringolysin O modulates the host inflammatory response, causing leukocyte accumulation within blood vessels and inhibiting the normal influx of phagocytic cells into infected host tissue (2, 9, 20).
The production of both alpha-toxin and perfringolysin O, as well as collagenase and several housekeeping enzymes, is regulated at the transcriptional level by the VirS/VirR two-component signal transduction system (5, 12, 14, 18). The genes encoding these proteins are differentially regulated by the VirS/VirR network, implying that regulation is a complex process that involves different factors for each regulated gene (5, 6, 15). The perfringolysin O structural gene, pfoA, is the only target gene whose expression is totally dependent on the presence of a functional VirS/VirR system; mutation of either virS or virR totally eliminates the ability to produce perfringolysin O (12, 18). The VirR protein has previously been purified and shown to bind to two imperfect 12-bp directly repeated sequences, termed VirR boxes, that are located directly upstream of the pfoA promoter (7). There are no VirR boxes located near the alpha-toxin structural gene, plc, or the collagenase gene, colA, which is located 12 kb downstream of pfoA (13). The VirR-regulated hyp7 transcript has been shown previously to be involved in the regulation of the plc and colA genes (15) but not involved in the regulation of pfoA (5).
Previous studies showed that the pfoR gene was located 1.6 kb upstream of pfoA (19). Although these genes are transcribed from the same DNA strand, they are separated by a 590-bp intergenic region and do not comprise an operon. Deletion studies carried out exclusively with Escherichia coli led to the conclusion that the product of the pfoR gene was a cis-acting positive activator of pfoA expression. Comparative analysis of the putative 343-amino-acid PfoR protein did not reveal, and still does not reveal, any significant similarity to proteins of known function. It was reported (19) that PfoR had an N-terminal helix-turn-helix (HTH) domain, but our analysis of PfoR, by the standard algorithm of Dodd and Egan (8), failed to detect an HTH domain. It was also reported that deletion of the putative HTH-encoding region led to the elimination of the ability of PfoR to activate pfoA expression in E. coli (19).
Our studies were undertaken with a twofold objective, to confirm that in C. perfringens pfoR encoded a transcriptional activator of the pfoA gene and to determine how PfoR interacted with the VirS/VirR regulatory system. However, using two independent methods, we obtained clear evidence that mutation of pfoR did not alter perfringolysin O production in C. perfringens.
Complementation of a pfoR-pfoA deletion derivative.
Previous studies carried out in this laboratory involved the isolation of pfoA mutants by Tn916 mutagenesis (3). One such mutant, JIR4228, carries a large deletion that extends throughout the entire pfoR-pfoA-colA region. No hybridization is observed when DNA from JIR4228 is probed with pfoR-, pfoA-, or colA-specific probes. However, the plc gene, which is not located in this gene region, is present. Therefore, JIR4228 is considered ideal for use in pfoA complementation studies. If pfoR encodes a cis-acting activator of pfoA, then a JIR4228 derivative harboring a shuttle plasmid that carries the wild-type pfoR-pfoA gene region should have a higher level of perfringolysin O activity than an isogenic derivative that carries the wild-type pfoA gene and a deleted derivative of pfoR.
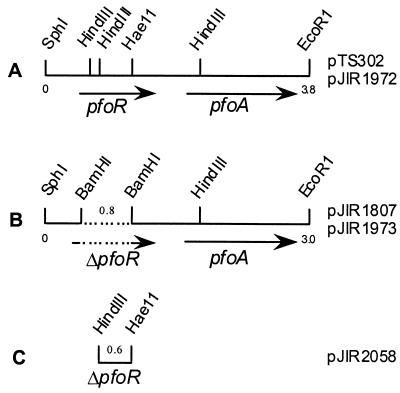
A 3.8-kb SphI-EcoRI fragment from pTS302 (19), which contains both pfoR and pfoA (Fig. 1), was cloned into the SphI-EcoRI sites of the C. perfringens-E. coli shuttle vector pJIR751 (4) to construct pJIR1972 (Table 1). A derivative of pTS302 that contains an 0.8-kb deletion within the pfoR gene was then constructed by T4 polymerase synthesis using outward-firing oligonucleotides that each contained a BamHI site. After BamHI digestion and ligation, the 3.0-kb SphI-EcoRI fragment from the recircularized derivative, pJIR1807 (Fig. 1), was cloned into pJIR751 to construct pJIR1973. This plasmid was identical to pJIR1972 except for the internal pfoR deletion, which encompassed codons 21 to 287 and included all of the putative HTH domain deleted in the previous study (19). Since the deletion involves a frameshift, the pfoR gene on pJIR1973 would encode only the first 21 amino acids of the putative PfoR protein.
FIG. 1.
Schematic representation of the pfoR-pfoA gene region. Fragments contained within pTS302 and pJIR1972 (A), pJIR1807 and pJIR1973 (B), and pJIR2058 (C) are shown. The dotted line represents a deletion of 0.8 kb within the pfoR gene. Fragment sizes are indicated in kilobases.
TABLE 1.
Plasmid properties
| Plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| pTS302 | AprpfoR+pfoA+ | 19 |
| pTOX6 | Aprplc+ | 16 |
| pVB101 | Tcr | V. Burdett (Duke University) |
| pJIR751 | oriEC oriCP rep erm(B)+ (Emr) | 4 |
| pJIR817 | pTS302ΔpfoA(EcoRI-SpeI; 0.5 kb) | 1 |
| pJIR872 | pJIR751 virR+virS+ | 12 |
| pJIR1318 | pUK21(NruI)Ω pJIR235(EcoRI-XbaI; 1.25 kb; catQ)(T4 polymerase) | This study |
| pJIR1457 | pJIR751 oriT (Emr) | 11 |
| pJIR1620 | pJIR1457Δ(AatII-SpeI; 2.7 kb) | This study |
| pJIR1791 | pJIR1620(SmaI)Ω pTS302(DraI; 0.79 kb) (ΔpfoR) | This study |
| pJIR1807 | pTS302(T4 polymerase; Δ0.8 kb) (ΔpfoR nucleotides 1405 to 2203)a | This study |
| pJIR1955 | pJIR1620(SmaI-HindIII)Ω pJIR1791[HaeII(T4)-HindIII; 0.6 kb](ΔpfoR) | This study |
| pJIR1972 | pJIR751(EcoRI-SphI)Ω pTS302(EcoRI-SphI; 3.8 kb)(pfoR+pfoA+) | This study |
| pJIR1973 | pJIR751(EcoRI-SphI)Ω pJIR1807(EcoRI-SphI; 3.0 kb)(ΔpfoR pfoA+) | This study |
| pJIR2058 | pJIR1955Δ(XmnI; 0.25 kb)[Δerm(B)]Ω pVB101[BamHI(T4); 3.0 kb][tet(M)] | This study |
Nucleotides were designated previously (19).
Both pJIR1972 and pJIR1973 were used to transform (17) C. perfringens strain JIR4228 to erythromycin resistance. Both plasmids restored the ability to produce perfringolysin O, as detected on horse blood agar. Quantitative analysis of perfringolysin O activity was carried out using a doubling dilution assay as previously described (1). The results showed that the same amount of extracellular perfringolysin O activity was detected in JIR4228 derivatives carrying pJIR1972 and those carrying pJIR1973 (Table 2).
TABLE 2.
Perfringolysin O activity of pfoR constructs
| Species and strain | Genotype | Perfringolysin Oa (log2[titer]) |
|---|---|---|
| C. perfringens | ||
| JIR4564 | JIR4228(pJIR751) | <1 |
| JIR4565 | JIR4228(pJIR1972) | 5.4 ± 0.5 |
| JIR4566 | JIR4228(pJIR1973) | 5.4 ± 0.4 |
| JIR325 | Rifr Nalr | 7.8 ± 0.1 |
| JIR4583 | JIR325 pfoRΩpJIR2058 | 7.7 ± 0.1 |
| JIR4588 | JIR325 pfoRΩpJIR2058 | 7.7 ± 0.1 |
| E. coli | ||
| JIR2492 | DH5α(pTS302) | 10.8 ± 0.1 |
| JIR2518 | DH5α(pJIR817) | <1.0 |
| JIR7521 | DH5α(pJIR1807) | 10.9 ± 0.1 |
Perfringolysin O activity was determined as previously described (1), by a doubling dilution hemolytic assay with horse red blood cells.
Since the only difference between these plasmids is the internal pfoR deletion in pJIR1973, these data suggest that pfoR has no effect on pfoA activity. However, these experiments were undertaken with genes carried on a low-copy-number plasmid rather than on the chromosome and utilized a host strain that contained a significant chromosomal deletion. Accordingly, it was decided to construct a chromosomal pfoR mutant.
Construction and analysis of a chromosomal pfoR mutant.
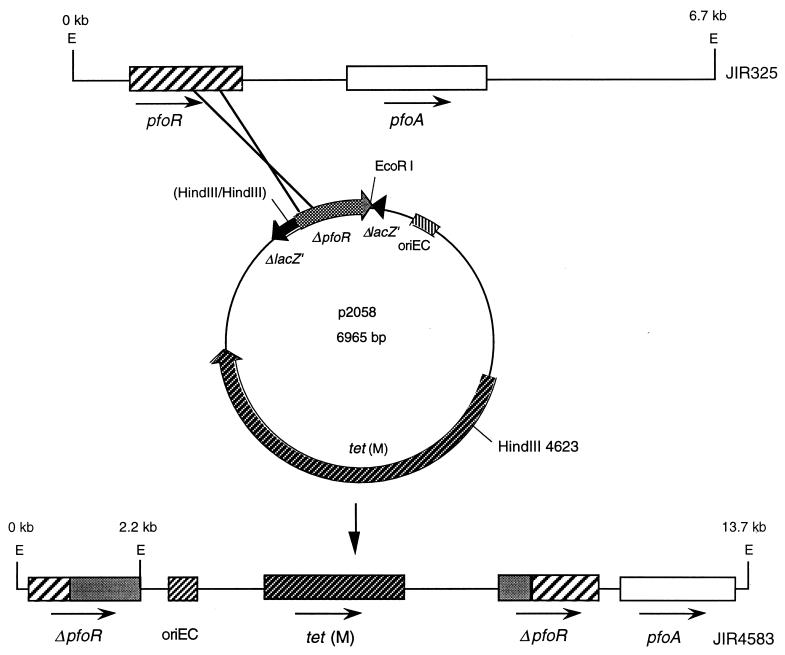
A pfoR suicide vector was constructed by using an internal 0.6-kb HaeII (T4-filled)-HindIII pfoR fragment (Fig. 1), which was isolated from a derivative of pTS302, to construct pJIR2058 (Fig. 2), a tet(M)-containing suicide plasmid that was unable to replicate in C. perfringens. Since an internal pfoR fragment was present in the vector, a single crossover event would lead to the inactivation of the chromosomal pfoR gene.
FIG. 2.
Construction of a pfoR mutant by homologous recombination. A single crossover event between the pfoR gene on the JIR325 chromosome and an internal pfoR gene region located on the suicide vector pJIR2058 resulted in the construction of the pfoR mutant JIR4583 as shown.
Strain JIR325 (12), a rifampin- and nalidixic acid-resistant derivative of the C. perfringens wild-type strain 13, was transformed to tetracycline resistance with pJIR2058. Tetracycline-resistant transformants were obtained at a frequency of 9.8 × 10−6 transformants per pJIR751-derived transformant. Several of these derivatives were examined by PCR and had profiles consistent with that of the expected pfoR mutant. One mutant, JIR4583, was selected for further study and was found to be rifampin (10 μg/ml), nalidixic acid (10 μg/ml) and tetracycline (5 μg/ml) resistant, as expected. Note that several attempts to isolate a pfoR mutant by using a vector that would result in a double crossover event were unsuccessful.
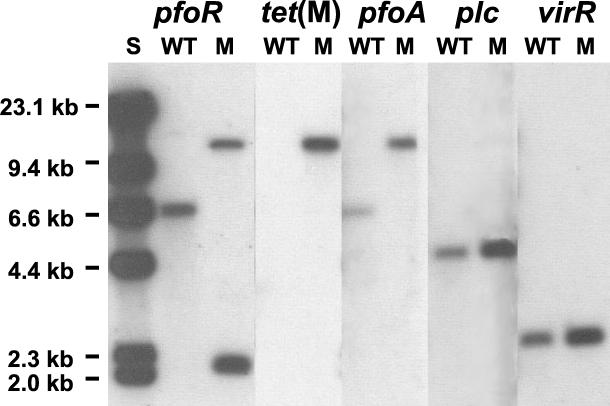
Southern hybridization analysis confirmed that JIR4583 had the profile predicted from the insertion of pJIR2058 into the chromosomal pfoR gene of JIR325 (Fig. 3). As expected, chromosomal JIR325 DNA probed with an internal pfoR-derived 0.5-kb PCR fragment hybridized to a 6.7-kb band, whereas 2.2-kb and ca. 11.5-kb bands were observed from JIR4583 (Fig. 3). The 0.9-kb SpeI-HindIII pfoA-specific fragment from pTS302 hybridized with the same 6.7- and 11.5-kb bands from JIR325 and JIR4583 DNA, respectively, but not with the 2.2-kb JIR4583 band, as predicted. Probing with a 3.0-kb BamHI tet(M)-specific fragment isolated from pVB101 (V. Burdett, unpublished data) was used to confirm that the 11.5-kb band carried the tet(M) gene from pJIR2058. To further confirm the pfoR specificity of the chromosomal rearrangement in JIR4583, both the wild-type and mutant digests were probed with an 0.95-kb BamHI-HindIII plc-specific fragment from pTOX6 (16) and an 0.4-kb HpaI virR-specific digoxigenin-labeled fragment from pJIR872 (12). In both experiments, the wild-type and mutant chromosomes hybridized to the same-sized fragments, a 4.6- and a 2.5-kb band, respectively (Fig. 3), confirming that these genes were unaltered. PCR analysis was used to confirm the chromosomal rearrangement present in JIR4583 (data not shown). Finally, an independent pfoR mutant, JIR4588, was isolated by the same procedure and also shown by Southern blotting and PCR analysis to be derived from a single crossover into the pfoR gene (data not shown).
FIG. 3.
Southern hybridization analysis. Chromosomal DNA was prepared from the wild-type strain JIR325 (WT) and the pfoR mutant JIR4583 (M), digested with EcoRI, separated by agarose gel electrophoresis, and probed with digoxigenin-labeled DNA fragments specific for the pfoR, tet(M), pfoA, plc, and virR genes as indicated. Molecular size standards are as indicated (S).
Quantitative perfringolysin O assays were carried out to determine the effect of the pfoR mutation. The results showed that indistinguishable levels of perfringolysin O were produced by the wild-type strain JIR325 and the pfoR mutants JIR4583 and JIR4588 (Table 2). These data provide independent evidence that under these conditions the pfoR gene product has no observable effect on the expression of the pfoA gene.
To see if similar results were obtained in E. coli, periplasmic extracts were prepared (10) from 100-ml cultures of E. coli DH5α strains carrying pTS302 or its pfoR deletion derivative, pJIR1807. The results (Table 2) showed that deletion of the 0.8-kb internal pfoR fragment, which removes almost all of the PfoR-encoding capacity, had no effect on perfringolysin O expression in E. coli. As expected, a DH5α strain carrying pJIR817, a pTS302 derivative with an 0.6-kb internal pfoA deletion, produced no detectable levels of perfringolysin O (Table 2).
Based on the use of complementation studies with the deletion mutant JIR4228, and by the analysis of chromosomal pfoR mutants constructed by homologous recombination, it is concluded that mutation of the upstream pfoR gene has no effect on pfoA expression in C. perfringens. Therefore, it appears that pfoR does not encode a cis-active positive regulator of perfringolysin O expression as previously postulated (19). Although we cannot rule out the possibility that under different experimental conditions, such as in an infected lesion, the pfoR gene does have a regulatory role, there is no experimental evidence to support such a role. It is not clear why the previous studies showed that in E. coli mutation of pfoR did affect pfoA expression (19). However, our results do point out the importance of carrying out regulation studies with the original bacterial host.
Acknowledgments
This research was supported by grants to J.I.R. from the Australian National Health and Medical Research Council.
REFERENCES
- 1.Awad, M. M., A. E. Bryant, D. L. Stevens, and J. I. Rood. 1995. Virulence studies on chromosomal α-toxin and θ-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of α-toxin in Clostridium perfringens-mediated gas gangrene. Mol. Microbiol. 15:191-202. [DOI] [PubMed] [Google Scholar]
- 2.Awad, M. M., D. M. Ellemor, R. L. Boyd, J. J. Emmins, and J. I. Rood. 2001. Synergistic effects of alpha-toxin and perfringolysin O in Clostridium perfringens-mediated gas gangrene. Infect. Immun. 69:7904-7910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awad, M. M., and J. I. Rood. 1997. Isolation of α-toxin, θ-toxin and κ-toxin mutants of Clostridium perfringens by Tn916 mutagenesis. Microb. Pathog. 22:275-284. [DOI] [PubMed] [Google Scholar]
- 4.Bannam, T. L., and J. I. Rood. 1993. Clostridium perfringens-Escherichia coli shuttle vectors that carry single antibiotic resistance determinants. Plasmid 29:223-235. [DOI] [PubMed] [Google Scholar]
- 5.Banu, S., K. Ohtani, H. Yaguchi, T. Swe, S. T. Cole, H. Hayashi, and T. Shimizu. 2000. Identification of novel VirR/VirS-regulated genes in Clostridium perfringens. Mol. Microbiol. 35:854-864. [DOI] [PubMed] [Google Scholar]
- 6.Ba-Thein, W., M. Lyristis, K. Ohtani, I. T. Nisbet, H. Hayashi, J. I. Rood, and T. Shimizu. 1996. The virR/virS locus regulates the transcription of genes encoding extracellular toxin production in Clostridium perfringens. J. Bacteriol. 178:2514-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung, J. K., and J. I. Rood. 2000. The VirR response regulator from Clostridium perfringens binds independently to two imperfect direct repeats located upstream of the pfoA promoter. J. Bacteriol. 182:57-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodd, I., and J. B. Egan. 1990. Improved detection of helix-turn-helix DNA binding motifs in protein sequences. Nucleic Acids Res. 18:5019-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellemor, D. M., R. N. Baird, M. M. Awad, R. L. Boyd, J. I. Rood, and J. J. Emmins. 1999. Use of genetically manipulated strains of Clostridium perfringens reveals that both alpha-toxin and theta-toxin are required for vascular leukostasis to occur in experimental gas gangrene. Infect. Immun. 67:4902-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ludwig, A., S. Bauer, R. Benz, B. Bergmann, and W. Goebel. 1999. Analysis of the SlyA-controlled expression, subcellular localization and pore-forming activity of a 34 kDa haemolysin (ClyA) from Escherichia coli K-12. Mol. Microbiol. 31:557-567. [DOI] [PubMed] [Google Scholar]
- 11.Lyras, D., and J. I. Rood. 1998. Conjugative transfer of RP4-oriT shuttle vectors from Escherichia coli to Clostridium perfringens. Plasmid 39:160-164. [DOI] [PubMed] [Google Scholar]
- 12.Lyristis, M., A. E. Bryant, J. Sloan, M. M. Awad, I. T. Nisbet, D. L. Stevens, and J. I. Rood. 1994. Identification and molecular analysis of a locus that regulates extracellular toxin production in Clostridium perfringens. Mol. Microbiol. 12:761-777. [DOI] [PubMed] [Google Scholar]
- 13.Ohtani, K., M. Bando, T. Swe, S. Banu, M. Oe, H. Hayashi, and T. Shimizu. 1997. Collagenase gene (colA) is located in the 3′-flanking region of the perfringolysin O (pfoA) locus in Clostridium perfringens. FEMS Microbiol. Lett. 146:155-159. [DOI] [PubMed] [Google Scholar]
- 14.Ohtani, K., H. Takamura, H. Yaguchi, H. Hayashi, and T. Shimizu. 2000. Genetic analysis of the ycgJ-metB-cysK-ygaG operon negatively regulated by the VirR/VirS system in Clostridium perfringens. Microbiol. Immunol. 44:525-528. [DOI] [PubMed] [Google Scholar]
- 15.Rood, J. I. 1998. Virulence genes of Clostridium perfringens. Annu. Rev. Microbiol. 52:333-360. [DOI] [PubMed] [Google Scholar]
- 16.Saint-Joanis, B., T. Garnier, and S. T. Cole. 1989. Gene cloning shows the alpha toxin of Clostridium perfringens to contain both sphingomyelinase and lecithinase activities. Mol. Gen. Genet. 219:453-460. [DOI] [PubMed] [Google Scholar]
- 17.Scott, P. T., and J. I. Rood. 1989. Electroporation-mediated transformation of lysostaphin-treated Clostridium perfringens. Gene 82:327-333. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu, T., W. Ba-Thein, M. Tamaki, and H. Hayashi. 1994. The virR gene, a member of a class of two-component response regulators, regulates the production of perfringolysin O, collagenase, and hemagglutinin in Clostridium perfringens. J. Bacteriol. 176:1616-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimizu, T., A. Okabe, J. Minami, and H. Hayashi. 1991. An upstream regulatory sequence stimulates expression of the perfringolysin O gene of Clostridium perfringens. Infect. Immun. 59:137-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens, D. L., R. Tweten, M. M. Awad, J. I. Rood, and A. E. Bryant. 1997. Clostridial gas gangrene: evidence that α and θ toxins differentially modulate the immune response and induce acute tissue necrosis. J. Infect. Dis. 176:189-195. [DOI] [PubMed] [Google Scholar]
- 21.Tweten, R. K. 1997. The thiol-activated clostridial toxins, p. 211-221. In J. I. Rood, B. A. McClane, G. S. Songer, and R. W. Titball (ed.), The clostridia: molecular biology and pathogenesis. Academic Press, London, United Kingdom.
- 22.Williamson, E. D., and R. W. Titball. 1993. A genetically engineered vaccine against the alpha-toxin of Clostridium perfringens protects against experimental gas gangrene. Vaccine 11:1253-1258. [DOI] [PubMed] [Google Scholar]