Abstract
The chemolithoautotrophic bacterium Acidithiobacillus ferrooxidans has been known as an aerobe that respires on iron and sulfur. Here we show that the bacterium could chemolithoautotrophically grow not only on H2/O2 under aerobic conditions but also on H2/Fe3+, H2/S0, or S0/Fe3+ under anaerobic conditions. Anaerobic respiration using Fe3+ or S0 as an electron acceptor and H2 or S0 as an electron donor serves as a primary energy source of the bacterium. Anaerobic respiration based on reduction of Fe3+ induced the bacterium to synthesize significant amounts of a c-type cytochrome that was purified as an acid-stable and soluble 28-kDa monomer. The purified cytochrome in the oxidized form was reduced in the presence of the crude extract, and the reduced cytochrome was reoxidized by Fe3+. Respiration based on reduction of Fe3+ coupled to oxidation of a c-type cytochrome may be involved in the primary mechanism of energy production in the bacterium on anaerobic iron respiration.
Acidithiobacillus ferrooxidans is generally accepted to be an aerobic chemolithoautotroph that derives energy for growth from oxidative respiration involving the oxidation of ferrous iron or various sulfur compounds. Brock and Gustafson reported that the bacterium reduces Fe3+ in the presence of S0 (10). However, the reduction was not recognized as respiratory reactions since iron reduction did not permit growth of the bacterium (39). Therefore, it was accepted that coupling reduction of Fe3+ to oxidation of S0 was one of the steps in the sulfur metabolism by the bacterium (39). Pronk et al. (32, 33) and Das et al. (12) showed that the bacterium grew on the oxidation of S0 by Fe3+ under oxygen-limited conditions. Although these findings raised the possibility that A. ferrooxidans might be able to grow under anaerobic conditions, the aforementioned enzymatic activity was not accompanied by growth (39), and it is still unclear whether Fe3+ serves as an electron acceptor for anaerobic respiration. On the other hand, the bacterium would grow on hydrogen under aerobic conditions (14). In that case, H2 served as the electron donor enabling an oxidative respiratory chain to derive energy for chemolithoautotrophic growth.
On the other hand, in many facultative heterotrophs in both Archaea and Bacteria, anaerobic respiration involving reduction of Fe3+ or S0 is typically coupled to the oxidation of H2 (25, 26, 30, 38, 42). One of the principle roles of such respiration would have been to support energy for chemolithoautotrophy (34), a type of autotrophy that typically served as the growth mode of such facultative heterotrophs in Archaea and Bacteria as hyperthermophilic archaebacteria, sulfur-reducing bacteria, and primitive fermentative bacteria (18, 24, 36-38, 42, 44). However, little is known about the role played by anaerobic respiration involving Fe3+ or S0 reduction in the growth of typical, known chemolithoautotrophic bacteria, which include a variety of sulfur, iron, ammonia, and nitrite oxidizers.
We have found that anaerobic respiration using Fe3+ or S0 as an electron acceptor and H2 as an electron donor serves as a primary energy source for chemolithoautotrophic growth of A. ferrooxidans. Moreover, such anaerobic iron respiration induces A. ferrooxidans to synthesize significant amounts of a c-type cytochrome, which was responsible for the reduction of Fe3+. Anaerobic respiration based on reduction of Fe3+ coupled to oxidation of a c-type cytochrome may play an important role in the primary mechanism of energy production in the bacterium on anaerobic iron respiration.
MATERIALS AND METHODS
Microorganisms and medium.
Cultures of A. ferrooxidans strains were provided from several culture collections: ATCC 23270 was obtained from the American Type Culture Collection; JCM 3865, JCM 3863, and JCM 7811 were from the Japan Collection of Microorganisms; and IFO 14246 and IFO 14262 were from the Institute for Fermentation, Osaka, Japan. Each strain was purified by using the single-colony isolation method on a silica gel plate of Fe2+ medium. After purification, the strains were routinely maintained in 9K basal salts medium containing 160 mM Fe2+ in shake flasks and incubated under aerobic conditions at 30°C.
Anaerobic growth experiments.
The medium used for the anaerobic growth experiments contained the following (per liter of distilled water): (NH4)2SO4, 133 mg; K2HPO4, 41 mg; MgSO4 · 7H2O, 490 mg; CaCl2 · 2H2O, 9 mg; KCl, 52 mg; ZnSO4 · 7H2O, 1 mg; CuSO4 · 5H2O, 2 mg; MnSO4 · H2O, 1 mg; NaMoO4 · 2H2O, 0.5 mg; CoCl2 · 6H2O, 0.5 mg; Na2SeO4 · 10H2O, 1 mg; and NiCl2 · 6H2O; 1 mg. To this basal salt medium, 2.5 g of Fe2(SO4)3 was added, and the pH was adjusted to 2.0 with 6 N H2SO4. The medium was then degassed by using a suction pump, after which it was bubbled with nitrogen gas for 1 h to reduce the level of dissolved oxygen. The deoxygenated medium was immediately stored in an anaerobic box under nitrogen overnight. Within the anaerobic box, 30 ml of the medium was then added to a 150-ml anaerobic culture bottle with filter sterilization, and the bottle was packed with a sterilized butyl stopper. The gas phase of the headspace in the packed bottle was then replaced with a H2 and CO2 mixture as follows. The nitrogen gas in the headspace was first removed by using a syringe needle inserted in the butyl stopper, after which the H2-CO2 (80:20) mixture was then used to replace the gas phase in the headspace. The suction and pressurization cycles were repeated at least three times until the mixed gas in the headspace finally reached a pressure of 250 kPa. When the culture experiments were carried out with a H2-O2 gas phase, 15 ml of air was added to the bottle by using the syringe. In all of the experiments, the inoculation volume was 1 ml per 30 ml of the medium. Cultivation was carried out at 30°C in an incubator with shaking.
Soluble iron.
The concentrations of total soluble iron and of Fe2+ were determined by using the phenanthroline method. Samples (1 ml) of culture medium were taken at each culture time and passed through a membrane filter (pore size, 0.2 μm). Aliquots (100 μl) from each filtered sample were then added to 2.5 ml of the buffer containing 68.0 g of sodium acetate and 28.8 ml of acetic acid per liter of distilled water (pH 4.6). A 2.5-ml sample of 0.2% phenanthroline solution was then added to the sample mixture to determine the Fe2+ concentrations. In the case of the total iron determination, 1 ml of 10% NH2OH · HCl was added to the buffer before sample mixing to reduce the Fe3+ to Fe2+. The concentrations of Fe3+ and total iron ion were determined from a calibration curve plotting iron concentration in the sample as a function of the absorbance at 510 nm.
Cell numbers.
Cell numbers were determined by a counting chamber under a phase-contrast microscope at ×400 magnification. The cell densities were then calculated as an average of more than 25 determinations of each culture sample.
Purification of cytochrome.
Cells grown aerobically or anaerobically on several growth modes such as H2/O2, Fe2+/O2, H2/Fe3+, H2/S0, and S0/Fe3+ were separately harvested and were resuspended in sulfuric acid solution at pH 2.0. A series of cells were subsequently broken by sonication, and insoluble fractions were removed twice by centrifugation at 100,000 × g for 1 h. The supernatants were used for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by Coomassie brilliant blue (CBB) staining. The supernatant of cells grown anaerobically on H2 or Fe3+ was then applied to the purification step of cytochrome. The purification steps were carried out as described in a previous report (11) with the modifications indicated. An ammonium sulfate was added to the supernatant at 20% of saturation, and the precipitation was removed by centrifugation at 10,000 × g for 30 min. The concentration of ammonium sulfate was increased to 60% of saturation, and the red pellet after centrifugation was obtained as the cytochrome fraction. The pellet was resuspended in 50 mM methyleneethanesulfinic acid (MES) buffer (pH 4.5), and the resupension was dialyzed against the same buffer. After the dialysis, the suspension was applied to a carboxymethyl cellulose column equilibrated in the 50 mM MES buffer (pH 4.5). The absorbed proteins in the column were eluted with a linear gradient of up to 500 mM NaCl. The fraction exhibiting red color was obtained at 320 mM NaCl. After desalting with a membrane concentrator, the fraction was applied to a MonoQ column equibrated in the 50 mM MES buffer (pH 4.5). The proteins were then eluted with a linear gradient up to 500 mM NaCl. The red fraction was obtained at 380 mM NaCl. The elution was concentrated with a membrane concentrator to <0.5 ml. The concentrated proteins were applied to a Sephacryl S-100 column equibrated in 0.01 N sulfuric acid solution (pH 2.0). The red protein was obtained as the fraction exhibiting a band at 28 kDa with a calibration with molecular size markers. The purified cytochrome was then used to determine absorbance spectra in 0.01 N sulfuric acid (pH 2.0). The cytochrome was purified as the oxidized form; to determine the spectrum of the reduced form, samples containing the oxidized cytochrome and an excess of Na2S2O4 were incubated for 20 min under nitrogen gas.
Iron-reducing activity.
Cells grown anaerobically on H2/Fe3+ were harvested and resuspended in sulfuric acid solution at pH 2.0. The cell suspension was immediately added to an anaerobic culture bottle containing a nitrogen gas. The suspension was then taken by a syringe and was injected into a glass vessel with sulfuric acid solution at pH 2.0 for volume adjustment. The solution was left for 20 min under conditions in which a hydrogen gas continuously flowed into the vessel. Then, the pH 2.0 solution containing Fe2(SO4)3 at various concentrations was added to the vessel to start iron reduction. Next, 0.2-ml portions of the sample were taken at various time intervals by using a syringe. After the filtration of the sample to remove the cells, the concentration of the produced Fe2+ in the sample was determined by the method described above. The iron-reducing activity was calculated as the produced Fe2+ (in moles per minute per cell) from the total cell number and the rate of Fe2+ production in the reaction mixture. The inhibition experiments of the reductive activities were carried out with the same method in the presence of the respiratory inhibitors.
Optical spectroscopy.
Spectrophotometric measurement was performed with a JASCO spectrophotometer model V-560 (Tokyo, Japan). The crude extract of the cells grown anaerobically on H2/Fe3+ was prepared by sonication of the intact cells and the subsequent centrifugation at 100,000 × g for 1 h in an anaerobic box containing a nitrogen gas. The prepared extract was kept in an anaerobic culture bottle in the presence of hydrogen before the use. The oxidized form of the purified cytochrome was also kept in the bottle with hydrogen. The reduction of the cytochrome by the extract was recorded with a mixture of 25 μl of sulfuric acid solution at pH 2.0 containing the oxidized cytochrome at 350 μg/ml and 25 μl of the extract. The reoxidation of the reduced cytochrome was also recorded with the addition of 4 mM of Fe3+ to the mixture of the cytochrome and the extract.
Reduction potential.
The redox potential of the purified cytochrome was measured with a Bioanalytical Systems electrochemical analyzer model 100B (West Lafayette, Ind.) by the modified electrode (15).
RESULTS
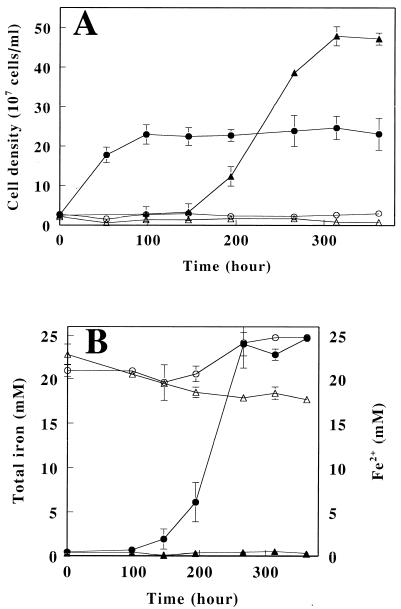
In order to investigate growth on H2 under aerobic conditions, a series of cultivation experiments was carried out with six strains of A. ferrooxidans. Each strain was subcultured on Fe2+-containing medium under aerobic conditions and then inoculated into H2 medium and incubated under aerobic conditions for 17 days. Cell numbers before and after cultivation for each strain are summarized in Table 1. No significant growth was observed in five of the six strains. Only strain IFO 14262 grew chemolithoautotrophically on H2 under aerobic conditions, with cell numbers increasing >15-fold from 0.89 × 107 to 1.59 × 108 cells/ml. In order to confirm that the observed growth was mediated by H2, time-dependent changes in cell density of IFO 14262 were characterized when H2 was supplied as the sole electron donor (Fig. 1A). Under these conditions, we found that the cell density increased from 2.56 × 107 to 2.29 × 108 cells/ml after 98 h of incubation, whereas no growth occurred when the same size inoculation was performed in the absence of H2. Based on these results, it was concluded that A. ferrooxidans strain IFO 14262 is able to utilize H2 as an electron donor and O2 as an electron acceptor to provide energy for chemolithoautotrophic growth.
TABLE 1.
Aerobic growth of A. ferrooxidans upon oxidation of H2 by O2
| Strain | Mean cell density (107 cells/ml) ± SDa
|
|
|---|---|---|
| Initial | Final | |
| ATCC 23270 | 0.79 ± 0.05 | 1.14 ± 0.38 |
| JCM 3863 | 0.70 ± 0.32 | 0.75 ± 0.40 |
| JCM 3865 | 1.06 ± 0.05 | 2.77 ± 0.83 |
| JCM 7811 | 0.76 ± 0.27 | 3.20 ± 1.60 |
| IFO 14246 | 0.85 ± 0.05 | 3.98 ± 0.96 |
| IFO 14262 | 0.89 ± 0.04 | 15.91 ± 3.60 |
Initial and final indicate cultivation times before and after 17 days of incubation, respectively. Values are expressed as the average of three cultures.
FIG. 1.
Chemolithoautotrophic growth of A. ferrooxidans on aerobic and anaerobic respiration with H2 as the electron donor. (A) Time-dependent changes in cell density of strain IFO 14262 aerobically respired in the presence (•) or absence (○) of H2 and those of JCM 7811 anaerobically respired using Fe3+ as an electron acceptor in the presence (▴) or absence (▵) of H2. (B) Time-dependent changes in the concentrations of total iron (open symbols) and Fe2+ (closed symbols) in the presence (○ and •) or absence (▵ and ▴) of bacteria; in this case, strain JCM 7811 anaerobically respired by using Fe3+ as an electron acceptor. The datum points are averages of three independent determinations with the standard deviations.
A series of anaerobic cultivation experiments was then carried out with the same six strains of A. ferrooxidans with medium containing Fe3+ as the electron acceptor and H2 as the electron donor. The cell numbers and iron concentrations before and after 17 days of incubation are summarized in Table 2. Bacterial growth was clearly observed with strains JCM 7811, IFO 14246, and IFO 14262. The cell density of each strain increased a minimum of >15-fold, reaching 2.18 × 108 to 4.95 × 108 cells/ml. Strain JCM 3865 also grew under these conditions, but the final cell density was lower than for the other strains. The reduction of Fe3+ to Fe2+ was strongly related to the bacterial growth. As such, Fe2+ accumulated in the medium of strains JCM 3865, JCM 7811, IFO 14246, and IFO 14262 but not in the medium of the two strains that did not grow or in the medium serving as a chemical control and which was not inoculated with cells at all (data not shown). We selected strain JCM 7811 to confirm that the observed growth was driven by the anaerobic reduction of Fe3+ by H2 (Fig. 1A). The cell density reached 4.78 × 108 cells/ml after 314 h of incubation, and again growth was correlated with the accumulation of Fe2+ (Fig. 1B). By the time growth reached the stationary phase, the amount of the accumulated Fe2+ accounted for 99% of the soluble iron in the medium. In contrast, there was no increase in cell density in the absence of Fe3+ or H2, nor was there accumulation of Fe2+ without the inoculation. A. ferrooxidans strain JCM 7811 is able to grow autotrophically under anaerobic conditions with H2 as the electron donor and Fe3+ as the electron acceptor.
TABLE 2.
Anaerobic growth of A. ferrooxidans upon reduction of Fe3+ by H2a
| Strain | Mean cell density (107 cells/ml) ± SD
|
Mean iron concn (mM) ± SD
|
||||
|---|---|---|---|---|---|---|
| Initial | Final | Initial
|
Final
|
|||
| Total | Fe2+ | Total | Fe2+ | |||
| ATCC 23270 | 1.59 ± 0.15 | 2.32 ± 0.56 | 21.11 ± 0.11 | 1.22 ± 0.20 | 20.91 ± 0.13 | 0.87 ± 0.08 |
| JCM 3863 | 0.87 ± 0.42 | 2.53 ± 0.15 | 21.74 ± 0.29 | 1.06 ± 0.07 | 22.33 ± 0.10 | 0.89 ± 0.03 |
| JCM 3865 | 1.34 ± 0.05 | 8.95 ± 0.25 | 21.77 ± 0.14 | 1.80 ± 0.23 | 22.26 ± 0.06 | 4.99 ± 0.74 |
| JCM 7811 | 0.97 ± 0.19 | 49.51 ± 5.00 | 22.02 ± 0.24 | 1.73 ± 0.09 | 22.32 ± 0.10 | 22.32 ± 0.03 |
| IFO 14246 | 1.03 ± 0.19 | 26.00 ± 3.20 | 22.17 ± 0.17 | 2.26 ± 0.07 | 22.45 ± 0.26 | 22.47 ± 0.22 |
| IFO 14262 | 1.25 ± 0.08 | 21.81 ± 1.96 | 22.06 ± 0.09 | 1.23 ± 0.26 | 21.29 ± 0.11 | 21.03 ± 0.24 |
Initial and final indicate the cultivation times before and after 17 days of incubation, respectively. The values are expressed as an average of three cultures.
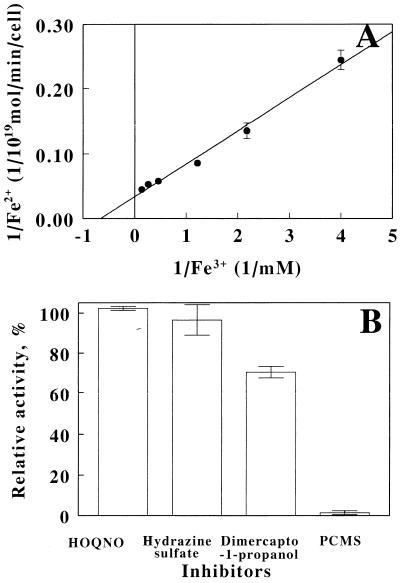
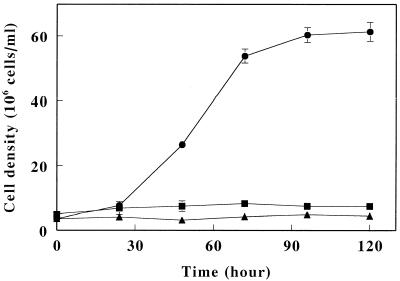
The iron-reducing activity on hydrogen was investigated with intact cells of strain JCM 7811 grown on anaerobic iron respiration. The rates of iron reduction were measured with reaction mixtures containing the cells at the fixed concentration and Fe3+ at the varied concentrations under anaerobic condition with H2. The measured rates were plotted with the corresponding initial Fe3+ concentrations (Fig. 2A). No reduction of Fe3+ occurred in the absence of the cells or H2 (data not shown). In addition, no oxidation of Fe2+ by the cells was observed in the presence of O2 or H2 (data not shown). Under these conditions, Km and Vmax were calculated as 1.51 mM Fe3+ and 29.8 × 10−19 mol of Fe2+ production/min/cell, respectively. Next, the effects of several inhibitors on iron reduction in intact cells were investigated (Fig. 2B). 2-Heptyl-4-hydroxyquinoline-N-oxide (HOQNO), hydrazine sulfate, 2,3-dimercapto-1-propanol, and p-chloromercuriphenylsulfonic acid (PCMS) were selected as inhibitors for the respiratory chain. More than 90% of Fe3+ reduction activity remained in the presence of 100 μM HOQNO or 100 μM hydrazine sulfate when the reduction without the inhibitors was 100%, whereas 34% of the activity was inhibited by 100 μM 3-dimercapto-1-propanol and the activity was completely blocked by 100 μM PCMS. These results suggested that iron reduction occurred as a result of anaerobic iron respiration on H2. We next examined the growth of A. ferrooxidans JCM 7811 in the presence of H2 and S0 under anaerobic conditions (Fig. 3). We found that indeed this strain was able to grow chemolithoautotrophically with H2 as the electron donor and S0 as the electron acceptor, yielding final cell densities of 6.1 × 107 cells/ml after 120 h of incubation. No growth occurred in the absence of either S0 or H2. Finally, the capacity of A. ferrooxidans JCM 7811 to grow anaerobically using S0 as the electron donor and Fe3+ as the acceptor was examined. The cell numbers and iron concentrations before and after cultivation are summarized in Table 3. Under these conditions, the cell density increased >12-fold, reaching 1.05 × 108 cells/ml after 14 days of incubation. During that period, Fe3+ added to the medium was reduced to Fe2+, and again there was no accumulation in the absence of inoculation. Thus, strain JCM 7811 is able to grow anaerobically on the oxidation of S0 by Fe3+.
FIG. 2.
Iron-reducing activity of A. ferrooxidans strain JCM 7811 grown on anaerobic iron respiration. (A) Lineweaver-Burk plot of iron-reducing activity of the cells on hydrogen. (B) Effects of respiratory inhibitors on iron-reducing activity of the cells. The rate of iron reduction at 2.0 mM of Fe3+ in the presence of HOQNO, hydrazine sulfate, 2,3-dimercapto-1-propanol, or PCMS at 100 μM was expressed as relative activity of control without the inhibitors. The datum points are averages of three independent determinations with the standard deviations.
FIG. 3.
Chemolithoautotrophic growth of A. ferrooxidans strain JCM 7811 on anaerobic S0 respiration with H2 as the electron donor. Shown are time-dependent changes in cell density in the presence of H2 (•) or N2 (▴) with S0 and in the presence of H2 without S0 (▪). The datum points are averages of three independent determinations with the standard deviations.
TABLE 3.
Anaerobic growth of strain JCM 7811 upon reduction of Fe3+ by S0a
| Medium | Mean cell density (107 cells/ml) ± SD
|
Mean iron concn (mM) ± SD
|
||||
|---|---|---|---|---|---|---|
| Initial | Final | Initial
|
Final
|
|||
| Total | Fe2+ | Total | Fe2+ | |||
| S0 + Fe3+ | - | - | 24.66 ± 0.33 | 1.22 ± 0.20 | 22.91 ± 0.57 | 0.37 ± 0.02 |
| S0 | 0.80 ± 0.10 | 0.06 ± 0.04 | - | - | - | - |
| S0 + Fe3+ | 0.84 ± 0.32 | 10.46 ± 2.81 | 23.79 ± 0.13 | 0.59 ± 0.07 | 23.45 ± 0.05 | 23.26 ± 0.17 |
Initial and final indicate the cultivation times before and after 17 days of incubation, respectively. The values are expressed as the average of three cultures.
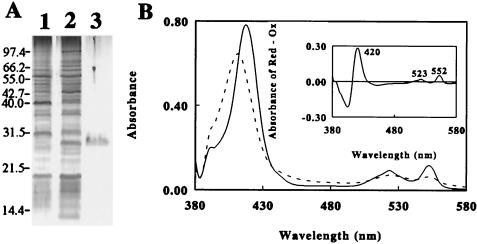
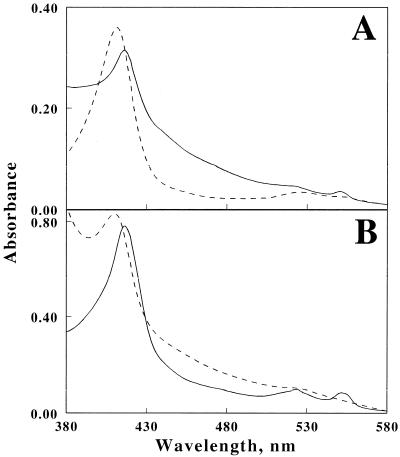
To identify the components involved in the reduction of Fe3+, soluble proteins present in crude extracts of cells grown aerobically on Fe2+/O2 or anaerobically on H2/Fe3+ were compared by SDS-PAGE. The profiles of the two cell groups differed significantly in that the anaerobes contained higher levels of a 28-kDa protein (Fig. 4A, lanes 1 and 2). After sodium sulfate and acid precipitation, this protein was purified to electrophoretic homogeneity by ion and gel chromatography (Fig. 4A, lane 3), yielding a product that was stable against acidity and highly soluble in solution at pH 2.0. The molecular mass of the purified protein was determined to be 27.4 kDa on gel filtration. This red protein exhibited a broad absorbance peak at 411 nm (dotted line in Fig. 4B), and reduction of the protein by using a reducing reagent produced new absorbance peaks at 552, 523, and 418 nm (solid line). The inset of Fig. 4B shows the difference spectrum for the oxidized and reduced forms, which was found to be typical of a c-type cytochrome.
FIG. 4.
Expression of a soluble, acid-stable cytochrome in strain JCM 7811 anaerobically cultured with H2 as the electron donor and Fe3+ as the electron acceptor. (A) CBB-stained SDS-polyacrylamide gel. Lanes 1 and 2 were loaded with the respective supernatants from crude extracts of cells grown with Fe2+/O2 and H2/Fe3+; lane 3 shows the cytochrome purified from the anaerobic extract loaded into lane 2. (B) Absorbance spectra of the oxidized (Ox; dashed line) and reduced (Red; solid line) forms of the cytochrome. The inset shows the difference spectrum representing the absolute spectrum of the cytochrome obtained by subtracting the spectrum of the oxidized form from that of the reduced form.
To investigate redox responses of the cytochrome, the spectrum was recorded with the crude extract of strain JCM 7811 grown on H2/Fe3+. A peak was observed at 418 nm, indicating the existence of the cytochrome as the reduced form in the extract (solid line in Fig. 5A). The oxidized form of the purified cytochrome exhibiting an absorbance peak at 411 nm (dashed line in Fig. 5A) was then mixed with the extract. The spectrum of the mixture showed an absorbance peak at 418 nm (solid line in Fig. 5B). The peak shift from 411 to 418 nm ensured that the oxidized form of the cytochrome was converted into the reduced form in the presence of the extract. However, the spectrum of the mixture with the addition of 4 mM of Fe3+ showed a peak at 411 nm (dashed line in Fig. 5B). Upon exposure to Fe3+, the reduced form of the cytochrome was immediately reoxidized by Fe3+. In addition, the production of Fe2+ by the oxidation of the reduced cytochrome was confirmed by an increase in absorbance at 510 nm, corresponding to chelating reactions of Fe2+ with phenanthroline (data not shown). From these results, this c-type cytochrome was thus able to serve as an electron acceptor for the oxidation of the extract and as an electron donor for the reduction of Fe3+.
FIG. 5.
Redox response of the cytochrome in cell extract and Fe3+. (A) Absorbance spectra of the crude extract of the cells grown on H2/Fe3+(solid line) and the oxidized form of the purified cytochrome (dashed line). (B) Absorbance spectra of the mixture containing the crude extract and the oxidized form of the purified cytochrome with (dashed line) or without (solid line) Fe3+.
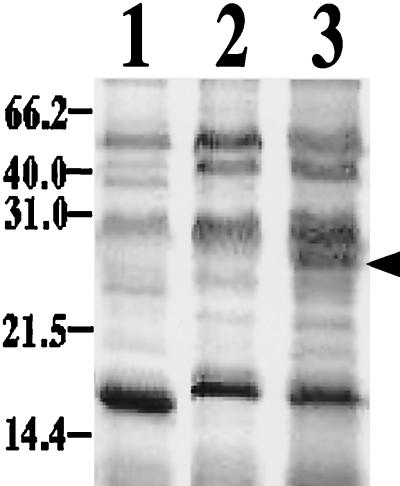
To clarify the relationship between the purified cytochrome and anaerobic iron respiration, soluble proteins present in crude extracts of cells grown on H2/O2, H2/S0 or S0/Fe3+ were compared by SDS-PAGE (Fig. 6). A large amount of the 28-kDa protein was detected in the extracts of the cells grown on S0/Fe3+ but not in extracts of cells grown on H2/O2 or H2/S0. That the 28-kDa protein was highly expressed in the cells anaerobically respiring on H2/Fe3+ or S0/Fe3+ (Fig. 4) suggests that expression of the protein was induced when the cells respired with Fe3+ as an electron acceptor, irrespective of the donor.
FIG. 6.
CBB-stained SDS-polyacrylamide gel showing the levels of the cytochrome in crude extracts of cells grown with H2/O2 (lane 1), H2/S0 (lane 2), or S0/Fe3+ (lane 3). The arrow indicates the 28-kDa protein of the cytochrome.
DISCUSSION
It is well known that A. ferrooxidans can aerobically respire using Fe2+ or S0 as the electron donor. This study provided evidence that A. ferrooxidans can utilize four different forms of respiration, including H2/Fe3+, H2/S0, and S0/Fe3+ for anaerobic growth and H2/O2 for aerobic growth (Fig. 1 and 3 and Tables 1 to 3). Although some of the forms were identical to those known for hyperthermophilic archaebacteria (36), it is notable that the same bacterium exhibited several different forms for aerobic or anaerobic respiration using iron, sulfur, and hydrogen. On all respiration forms, the bacterium could grow autotrophically. This chemolithoautotrophy of the bacterium was confirmed by additional growth experiments without CO2 in the gas phase. No significant growth occurred without CO2 in the anaerobic respiratory mode of H2/Fe3+, H2/S0, and S0/Fe3+ and in the aerobic mode of H2/O2 (data not shown). No significant growth was also observed in the absence of an electron donor or an electron acceptor (Fig. 1 and 3 and Table 3). These results ensured the autotrophy of the bacterium on several forms of respiration.
On the other hand, four of six strains were able to anaerobically respire on H2/Fe3+ (Fig. 1 and Table 2). In addition, one strain was also able to anaerobically respire on S0/Fe3+ and H2/S0 (Fig. 3 and Table 3), whereas only one of six strains was able to aerobically respire on H2 (Fig. 1 and Table 1). Many strains could not use O2 as an electron acceptor for H2 even though the bacterium is clearly able to use O2 on iron. The disappearance of the potential to use O2 was also observed in the cells grown on H2/Fe3+. The iron-oxidizing activity of intact cells completely disappeared (data not shown). The expression or activities of enzymes for the reduction of O2 seemed to be negatively regulated in the bacterium in the presence of H2 as an electron donor. However, it is unclear at present how the bacterium is able to select useable donors and acceptors for respiration along the conditions. To understand a mechanism for the regulation of respiratory phenotype, more information about the corresponding genes to respiratory enzymes is needed. Although the genes for electron transfer enzymes such as rusticyanin and cytochrome oxidase for aerobic respiration have been reported (4, 5, 7), additional information is not yet available.
Several c-type cytochromes in membrane-bound and soluble form have been purified from aerobically grown A. ferrooxidans cells (4, 11, 16, 20, 35, 40, 41, 43). The present study is the first to purify a cytochrome from anaerobically grown A. ferrooxidans cells. The purified soluble protein, which has a molecular mass of 28 kDa, differs in size from the previously described cytochromes (4, 11, 16, 20, 35, 40, 41, 43). Moreover, the sequence of N-terminal 30 amino acids of the purified protein was preliminarily determined (data not shown) and found to be dissimilar to the known sequences of either the soluble or membrane-bound cytochromes (11, 16, 20, 43). This is not surprising, however, given that this cytochrome is involved in Fe3+ reduction, whereas the others are involved in Fe2+ oxidation. One of remarkable properties of this soluble cytochrome was a redox potential. The midpoint redox potentials of several c-type cytochromes from A. ferrooxidans typically ranged between +330 and +360 mV at pH 7.0 and between +610 and +660 mV at pH 3.5 (4, 11, 16, 20, 35, 40, 41, 43). The potential of the new cytochrome was approximately +560 mV at pH 2.0. The potential of the new cytochrome was different from the potential of the other cytochromes of this bacterium. However, the potential ensured that the cytochrome was able to reduce Fe3+ at low pH. The other remarkable property of the new cytochrome was stability at a low pH at which typical c-type cytochromes could be autooxidized. It was reported that the reduced c-type cytochrome could be reoxidized by oxygen dissolved in the solution at pH 2.7 (11). However, >90% of the reduced cytochrome in pH 2.0 solution was retained after several hours of incubation under aerobic conditions, and the retained cytochrome could also reduce Fe3+ after the incubation (data not shown). Thus, we characterized the protein found in this study as a new cytochrome expressed in the bacterium.
In the cells, the relationship between iron reduction and respiratory activity was significant based on the rate parameters for iron reduction and a decrease of activity by the respiratory inhibitors (Fig. 2). Anaerobic reduction of Fe3+ is a respiratory process of the bacterium. The relationship between this novel cytochrome and anaerobic iron respiration was also significant, since transition from aerobic to anaerobic respiration markedly upregulated its expression (Fig. 4A). Likewise, high levels of expression were observed in the cells grown on S0/Fe3+ but not on Fe2+/O2, H2/O2, or H2/S0 (Fig. 6). Moreover, the new c-type cytochrome was reduced in the presence of the crude extract of the bacterium grown on anaerobic iron respiration, and the reduced protein was reoxidized by Fe3+ (Fig. 5). The cytochrome was able to serve as an electron acceptor for the crude extract and an electron donor for the reduction of Fe3+. This suggests that the this c-type cytochrome is functional for anaerobic respiration involving Fe3+ as an electron acceptor. There is growing evidence that cytochromes are involved in anaerobic metal reduction, serving as components in the respiratory chains of numerous bacteria (1, 6, 13, 23, 27-29, 31). Indeed, the c-type cytochrome described here would play an important part in anaerobic respiration as one of the electron transfer proteins in A. ferrooxidans.
A. ferrooxidans was able to grow with H2 as an electron donor and O2, S0, or Fe3+ as an electron acceptor (Fig. 1 and 3, Tables 1 and 2). The oxidation of H2 using each oxidant must therefore be coupled to the reduction of NAD(P) required for CO2 fixation. The entire electron transport pathways for these respirations remain unclear still, although hydrogenase was recently purified from the bacterium (19), suggesting that electrons from H2 would be first transferred to hydrogenase. However, the purified hydrogenase could not directly reduce NAD+ (19). Also, NAD+ could not be reduced by the reduced form of the purified cytochrome (data not shown). These results may mean that the regeneration of NADH is coupled to the electron transfer involving the flow from hydrogenase to Fe3+ via the reduced cytochrome through components in the inner membrane on H2/Fe3+ respiration. The existence of a reverse electron transfer to regenerate NADH in animal mitochondria has been reported. In addition, instances of reverse electron transfer in chemoautotrophic bacteria have also been reported (2, 3, 22). Recently, the uphill electron transfer from Fe2+ to NAD+ on aerobic respiration of A. ferrooxidans has been suggested (17). The uphill transfer may involve a putative cytochrome bc1 complex, according to the chemiosmotic mechanisms, possibly via Q-cycle mechanisms operating in reverse (17, 21). There is no evidence at present that the same mechanism of a reverse flow for NADH regeneration can be shared with aerobic and anaerobic respiration. However, a variety of autotrophic growth modes of the bacterium using H2, O2, S0, or Fe3+ suggest the existence of universal pathway for NADH regeneration by the uphill transfer connecting to each respiratory chain.
Rusticyanin is well known as one of the electron transfer components that is highly expressed when cells are grown on Fe2+ under aerobic conditions (8, 9). Surprisingly, immunostaining with an anti-rusticyanin antibody revealed the presence of rusticyanin in cells grown on H2/Fe3+ (data not shown). Although anaerobically grown cells did not early on exhibit oxidative activity in the presence of soluble Fe2+, the activity was recovered after aerobic incubation with Fe2+ for several weeks. The disappearance of the activity in the continued presence of rusticyanin was not surprising, since a large portion of the enzyme exists as an apo form, without the copper atom necessary for Fe2+-oxidizing activity (data not shown). It seems likely that there is no relationship between rusticyanin and iron-reducing activity and that the respiratory protein was constitutively expressed in this bacterium, depending upon respiration forms under anaerobic conditions. Nevertheless, the presence of Fe2+ may be important for induction of active form of rusticyanin, and O2 may play a similar role.
REFERENCES
- 1.Akfar, E., and Y. Fukumori. 1999. Purification and characterization of triheme cytochrome C7 from the metal-reducing bacterium Geobacter metallireducens. FEMS Microbiol. Rev. 175:205-210. [DOI] [PubMed] [Google Scholar]
- 2.Aleem, M. I. H., H. Lee, and D. J. D. Nicholas. 1963. ATP dependent reduction of NAD+ by ferrocytochrome c in chemoautotrophic bacteria. Nature 200:759-761. [DOI] [PubMed] [Google Scholar]
- 3.Aleem, M. I. H. 1966. Generation of reducing power in chemosynthesis. II. Energy linked reduction of pyridine nucleotides in the chemoautotroph Nitrosomonas europaea. Biochem. Biophys. Acta 113:216-224. [DOI] [PubMed] [Google Scholar]
- 4.Appia-Ayme, C., A. Bengrine, C. Cavazza, M. T. Giudici-Orticoni, M. Bruschi, M. Chippaux, and V. Bonnefoy. 1998. Characterization and expression of the co-transcribed cyc1 and cyc2 genes encoding the cytochrome c4 (c552) and a high-molecular-mass cytochrome c from Thiobacillus ferrooxidans ATCC 33020. FEMS Microbiol. Lett. 167:171-177. [DOI] [PubMed] [Google Scholar]
- 5.Appia-Ayme, C., N. Guiliani, J. Ratouchniak, and V. Bonnefoy. 1998. Characterization of an operon encoding two c-type cytochromes, an aa3-type cytochrome oxidase, and rusticyanin in Thiobacillus ferrooxidans ATCC 33020. Appl. Environ. Microbiol. 65:4781-4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beliaev, A. S., and D. A. Saffarini. 1998. Shewanella putrefaciens mtrB encodes an outer membrane protein required for Fe(III) and Mn(IV) reduction. J. Bacteriol. 180:6292-6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bengrine, A., N. Guiliani, C. Appia-Ayme, E. Jedlicki, D. S. Holmes, M. Chippaux, and V. Bonnefoy. 1998. Sequence and expression of the rusticyanin structural gene from Thiobacillus ferrooxidans ATCC 33020 strain. Biochim. Biophys. Acta 1443:99-112. [DOI] [PubMed] [Google Scholar]
- 8.Blake, R. C., II, and D. B. Johnson. 2000. Phylogenetic and biochemical diversity among acidophilic bacteria that respire on iron, p. 53-78. In D. R. Lovly (ed.), Environmental microbe-metal interactions. American Society for Microbiology, Washington, D.C.
- 9.Blake, R. C., II, E. A. Shute, M. M. Greenwood, G. H. Spencer, and W. J. Ingledew. 1993. Enzymes of aerobic respiration on iron. FEMS Microbiol. Rev. 11:9-18. [DOI] [PubMed] [Google Scholar]
- 10.Brock, T. D., and J. Gustafson. 1976. Ferric iron reduction by sulfur-and iron-oxidizing bacteria. Appl. Environ. Microbiol. 32:567-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavazza, C., M. T. Giudici-Orticoni, W. Nitschke, C. Appia, V. Bonnefoy, and M. Bruschi. 1996. Characterisation of a soluble cytochrome c4 isolated from Thiobacillus ferrooxidans. Eur. J. Biochem. 242:308-314. [DOI] [PubMed] [Google Scholar]
- 12.Das, A., A. K. Mishra, and P. Roy. 1992. Anaerobic growth on elemental sulfur using dissimilar iron reduction by autotrophic Thiobacillus ferrooxidans. FEMS Microbiol. Lett. 97:167-172. [Google Scholar]
- 13.Dobbin, P. S., J. N. Butt, A. K. Powell, G. A. Reid, and D. J. Richardson. 1999. Characterization of a flavocytochrome that is induced during the anaerobic respiration of Fe3+ by Shewanella frigidimarina NCIMB400. Biochem. J. 342:439-448. [PMC free article] [PubMed] [Google Scholar]
- 14.Drobner, E., H. Huber, and K. O. Stetter. 1990. Thiobacillus ferrooxidans, a facultative hydrogen oxidizer. Appl. Environ. Microbiol. 56:2922-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eddowes, M. J., and H. A. O. Hill. 1979. Electrochemistry of horse heart cytochrome c. J. Am. Chem. Soc. 101:4461-4464. [Google Scholar]
- 16.Elbehti, A., and D. Lemesle-Meunier. 1996. Identification of membrane-bound c-type cytochromes in an acidophilic ferrous ion oxidizing bacterium Thiobacillus ferrooxidans. FEMS Microbiol. Lett. 136:51-56. [DOI] [PubMed] [Google Scholar]
- 17.Elbehti, A., G. Brasseur, and D. Lemesle-Meunier. 2000. First evidence of an uphill electron transfer through bc1 and NADH-Q oxidoreductase complex of the acidophilic obligate chemolithotrophic ferrous ion-oxidizing bacterium. J. Bacteriol. 182:3602-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer, F., W. Zillig, K. O. Stetter, and G. Schreiber. 1983. Chemolithoautotrophic metabolism of anaerobic extremely thermophilic archaebacteria. Nature 301:511-513. [DOI] [PubMed] [Google Scholar]
- 19.Fischer, J., A. Quentmeier, S. Kostka, R. Kraft, and C. G. Friedrich. 1996. Purification and characterization of the hydrogenase from Thiobacillus ferrooxidans. Arch. Microbiol. 165:289-296. [DOI] [PubMed] [Google Scholar]
- 20.Giudici-Orticoni, M. T., G. Leroy, W. Nitschke, and M. Bruschi. 2000. Characterization of a new dihemic c4-type cytochrome isolated from Thiobacillus ferrooxidans. Biochemistry 39:7205-7211. [DOI] [PubMed] [Google Scholar]
- 21.Ingledew, W. J. 1982. Thiobacillus ferrooxidans. The bioenergetics of an acidophilic chemolithotroph. Biochem. Biophys. Acta 683:89-117. [DOI] [PubMed] [Google Scholar]
- 22.Kiesow, L. 1964. On the assimilation of energy from inorganic sources in autotrophic forms of life. Proc. Natl. Acad. Sci. USA 52:980-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knight, V., and R. Blakemore. 1998. Reduction of diverse electron acceptors by Aeromonas hydrophila. Arch. Microbiol. 169:239-248. [DOI] [PubMed] [Google Scholar]
- 24.Liu, S. V., J. Zhou, C. Zhang, D. R. Cole, M. Gajdarziska-Jostifovska, and T. J. Phelps. 1997. Thermophilic Fe(III)-reducing bacteria from the deep subsurface: the evolutionary implications. Science 22:1106-1109. [Google Scholar]
- 25.Lovley, D. R. 1991. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol. Rev. 55:259-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma, K., R. N. Schicho, R. M. Kelly, and M. W. Adams. 1993. Hydrogenase of the hyperthermophile Pyrococcus furiosus is an elemental sulfur reductase or sulfhydrogenase: evidence for a sulfur-reducing hydrogenase ancestor. Proc. Natl. Acad. Sci. USA 90:5341-5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myers, C. R., and J. M. Myers. 1992. Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. J. Bacteriol. 174:3429-3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myers, C. R., and J. M. Myers. 1997. Outer membrane cytochromes of Shewanella putrefaciens MR-1: spectral analysis, and purification of the 83-kDa c-type cytochrome. Biochim. Biophys. Acta 1326:307-318. [DOI] [PubMed] [Google Scholar]
- 29.Myers, J. M., and C. R. Myers. 1998. Isolation and sequence of omcA, a gene encoding a decaheme outer membrane cytochrome c of Shewanella putrefaciens MR-1, and detection of omcA homologs in other strains of S. putrefaciens. Biochim. Biophys. Acta 1373:237-251. [DOI] [PubMed] [Google Scholar]
- 30.Nealson, K. H., and D. Saffarini. 1994. Iron and manganese in anaerobic respiration: environmental significance, physiology, and regulation. Annu. Rev. Microbiol. 48:311-343. [DOI] [PubMed] [Google Scholar]
- 31.Obuekwe, C. O., D. W. Westlake, and F. D. Cook. 1981. Effect of nitrate on reduction of ferric iron by a bacterium isolated from crude oil. Can. J. Microbiol. 27:692-697. [DOI] [PubMed] [Google Scholar]
- 32.Pronk, J. T., B. J. C. De, P. Bos, and J. G. Kuenen. 1992. Anaerobic growth of Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 58:2227-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pronk, J. T., K. Liem, P. Bos, and J. G. Kuenen. 1991. Energy transduction by anaerobic ferric iron respiration in Thiobacillus ferrooxidans. Appl. Environ. Microbiol. 57:2063-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quayle, J. R., and T. Ferenci. 1978. Evolutionary aspects of autotrophy. Microbiol. Rev. 42:251-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sato, A., Y. Fukumori, T. Yano, M. Kai, and T. Yamanaka. 1989. Thiobacillus ferrooxidans cytochrome c-552: purification and some of its molecular features. Biochim. Biophys. Acta 976:129-134. [DOI] [PubMed] [Google Scholar]
- 36.Segerer, A., K. O. Stetter, and F. Klink. 1985. Two contrary modes of chemolithotrophy in the same archaebacterium. Nature 313:787-789. [DOI] [PubMed] [Google Scholar]
- 37.Slobodkin, A. I., C. Jeanthon, S. l'Haridon, T. Nazina, M. Miroshnichenko, and E. Bonch-Osmolovskaya. 1999. Dissimilatory reduction of Fe(III) by thermophilic bacteria and archaea in deep subsurface petroleum reservoirs of western Siberia. Curr. Microbiol. 39:99-102. [DOI] [PubMed] [Google Scholar]
- 38.Stetter, K. O. 1996. Hyperthermopilic procaryotes. FEMS Microbiol. Rev. 18:149-158. [Google Scholar]
- 39.Sugio, T., W. Mizunashi, K. Inagaki, and T. Tano. 1987. Purification and some properties of sulfur:ferric ion oxidoreductase from Thiobacillus ferrooxidans. J. Bacteriol. 169:4916-4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamegai, H., M. Kai, Y. Fukumori, and T. Yamanaka. 1994. Two membrane-bound c-type cytochromes of Thiobacillus ferrooxidans: purification and properties. FEMS Microbiol. Lett. 119:147-153. [DOI] [PubMed] [Google Scholar]
- 41.Valkova-Valchanova, M. B., and S. H. Chan. 1994. Purification and characterization of two new c-type cytochromes involved in Fe2+ oxidation from Thiobacillus ferrooxidans. FEMS Microbiol. Lett. 121:61-69. [DOI] [PubMed] [Google Scholar]
- 42.Vargas, M., K. Kashefi, E. L. Blunt-Harris, and D. R. Lovley. 1998. Microbiological evidence for Fe(III) reduction on early Earth. Nature 395:65-67. [DOI] [PubMed] [Google Scholar]
- 43.Yamanaka, T., and Y. Fukumori. 1995. Molecular aspects of the electron transfer system which participates in the oxidation of ferrous ion by Thiobacillus ferrooxidans. FEMS Microbiol. Rev. 17:401-413. [DOI] [PubMed] [Google Scholar]
- 44.Zillig, W., S. Yeats, I. Holz, A. Bock, F. Gropp, M. Rettenberger, and S. Lutz. 1985. Plasmid-related anaerobic autotrophy of the novel archaebacterium Sulfolobus ambivalens. Nature 313:789-791. [DOI] [PubMed] [Google Scholar]