Abstract
Previously, it was reported that the oxidative capacity and ability to grow on carbon sources such as pyruvate and glucose were severely diminished in the Rhizobium etli phaC::ΩSmr/Spr mutant CAR1, which is unable to synthesize poly-β-hydroxybutyric acid (PHB) (M. A. Cevallos, S. Encarnación, A. Leija, Y. Mora, and J. Mora, J. Bacteriol. 178:1646-1654, 1996). By random Tn5 mutagenesis of the phaC strain, we isolated the mutants VEM57 and VEM58, both of which contained single Tn5 insertions and had recovered the ability to grow on pyruvate or glucose. Nucleotide sequencing of the region surrounding the Tn5 insertions showed that they had interrupted an open reading frame designated aniA based on its high deduced amino acid sequence identity to the aniA gene product of Sinorhizobium meliloti. R. etli aniA was located adjacent to and divergently transcribed from genes encoding the PHB biosynthetic enzymes β-ketothiolase (PhaA) and acetoacetyl coenzyme A reductase (PhaB). An aniA::Tn5 mutant (VEM5854) was constructed and found to synthesize only 40% of the wild type level of PHB. Both VEM58 and VEM5854 produced significantly more extracellular polysaccharide than the wild type. Organic acid excretion and levels of intracellular reduced nucleotides were lowered to wild-type levels in VEM58 and VEM5854, in contrast to those of strain CAR1, which were significantly elevated. Proteome analysis of VEM58 showed a drastic alteration of protein expression, including the absence of a protein identified as PhaB. We propose that the aniA gene product plays an important role in directing carbon flow in R. etli.
Polyhydroxyalkanoic acids (PHAs) are a class of biodegradable plastics synthesized by many bacteria and thought to function as intracellular reserves of carbon and energy (2). Poly-β-hydroxybutyric acid (PHB) is a type of PHA composed of polymerized 3-hydroxybutyrate units. The pathways for the biosynthesis of PHA have been studied in various bacteria, and the genes encoding the biosynthetic enzymes have also been investigated (40). The most common PHB-biosynthetic pathway consists of three enzymes, the first of which is a β-ketothiolase which condenses two molecules of acetyl coenzyme A (acetyl-CoA) to form acetoacetyl-CoA. The acetoacetyl-CoA is then reduced to d-(−)-3-hydroxybutyryl-CoA by an acetoacetyl-CoA reductase. Lastly, the d-(−)-3-hydroxybutyryl-CoA monomers are linked by PHB synthase. The genes encoding β-ketothiolase, acetoacetyl-CoA reductase, and PHB synthase are designated phaA, phaB, and phaC, respectively.
Rhizobium etli accumulates PHB both in symbiosis and in free life (7, 14). PHB in rhizobia and other bacteria is thought to serve as a reserve of carbon and/or electrons to be utilized under suboptimal growth conditions (13, 23). An R. etli PHB-negative mutant (CAR1) with an insertionally inactivated PHB synthase structural gene (phaC) was described previously. Physiological studies showed that CAR1 was unable to synthesize PHB and excreted more organic acids than the wild-type strain CE3 (7). The NADH/NAD ratio in the mutant was higher than in the parent strain, which may be due to its inability to oxidize reducing power by synthesizing PHB. In rhizobia, as in other bacteria, excess reducing power can limit tricarboxylic acid (TCA) cycle function (13), and consistent with this, significantly less pyruvate dehydrogenase (PDH) activity was present in the PHB-negative mutant. In addition, the oxidative capacity of CAR1 was reduced and its ability to grow on glucose or pyruvate as the sole carbon source was severely diminished (7). These results and those of other studies on PHB synthase mutants of rhizobia (27, 42, 45, 46) show that the ability to synthesize PHB is an important component of the free-living and symbiotic metabolisms of these organisms. In the closely related bacterium Methylobacterium extorquens, the growth of a PHB synthase mutant on C1 and C2 compounds was impaired (19).
By transposon mutagenesis of the CAR1 phaC mutant, we obtained two double mutants which regained the ability to grow on pyruvate or glucose as the sole carbon source. The location of the Tn5 insertion in both mutants corresponds to an open reading frame (ORF) present in the PHB biosynthetic gene clusters of several proteobacteria (23). Because the R. etli ORF has the highest deduced amino acid sequence identity to the recently described AniA of S. meliloti (30), we have also designated it aniA. The aniA gene (named for anaerobically induced gene A) of S. meliloti strain 41 was shown to be expressed under microaerobic conditions, where its inactivation caused the overproduction of extracellular polymeric substances (30).
We present here a physiological and genetic characterization of aniA in R. etli and show that it exerts a profound effect on carbon metabolism, both in a phaC genetic background and in mutants in which only aniA has been inactivated. In contrast to S. meliloti AniA (30), R. etli AniA, described here, influences carbon flow and global protein expression during aerobic metabolism. We also report the cloning and sequencing of the R. etli phaAB operon and discuss the possible function of the product of aniA, located adjacent to phaAB, in the regulation of PHB, glycogen, and polysaccharide synthesis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. R. etli CE3 is a spontaneous Smr derivative of strain CFN42 (28) and is referred to here as a wild-type strain. Culture media and growth conditions for Rhizobium and Escherichia coli were as reported previously (13). When needed, antibiotics were used at the following concentrations (in micrograms per milliliter): carbenicillin, 100; tetracycline, 5; kanamycin, 30; streptomycin, 200; nalidixic acid, 20; spectinomycin, 100; and gentamicin, 30. To quantitate culture growth, total protein concentration of cells was determined by centrifuging duplicate 1-ml aliquots of cultures, resuspending the cell pellet in 5% (wt/vol) trichloroacetic acid, and centrifuging at 16,000 × g for 10 min. Supernatants were aspirated, and protein pellets were resuspended with 0.1 ml of 0.4 N NaOH. Protein concentrations were determined by the Lowry method (22) with bovine serum albumin as the standard.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| E. coli CMK | Host strain for plasmids, Smr Nalr | 4 |
| Laboratory stock | ||
| HB101 | F−recA13; Host for R. etli pLAFR1 genomic library | Laboratory stock |
| S17.1 | 294 recA; mobilizing strain for Tn5 | 36 |
| R. etli | ||
| CE3 | Smr derivative of wild-type strain CFN42 | 28 |
| CAR1 | phaC::ΩSmr/Spr derivative of CE3 | 7 |
| VEM57 | CE3 phaC::ΩSmr/SpraniA::Tn5 | This study |
| VEM58 | CE3 phaC::ΩSmr/SpraniA::Tn5 | This study |
| VEM5854 | CE3 double recombinant containing aniA::Tn5 insertion from VEM58 | This study |
| VEM5876 | VEM 58 containing plasmid pRCV-76 | This study |
| VEM5755 | CE3 double recombinant containing aniA::Tn5 insertion from VEM57 | This study |
| Plasmids | ||
| pLAFR1 | Broad-host-range cloning vector, Tcr | 16 |
| pRK2013 | Helper plasmid, Kmr | 10 |
| pBluescript | pBluescript II SK (+) cloning vector, Apr | Stratagene |
| pSUP5011 | pBR322 Tn5-mob, Apr Cmr Kmr | 36 |
| pRK415 | lacZ mob cloning vector, Tcr | 18 |
| pJQ200 | Positive selection gene replacement vector, sacB, Gmr | 31 |
| pCV12 | 12.5 kb EcoRI/EcoRI fragment containing the aniA::Tn5 from VEM58 cloned in pBluescript, Gmr Kmr | This study |
| pCV008 | pLAFR1 cosmid from a R. etli gene bank with a 20 kb EcoRI/EcoRI insert containing aniA, phaA phaB and orf1, Tcr | This study |
| pRCV-76 | 0.83-kb EcoRI/EcoRI fragment containing the aniA gene cloned in pRK415, Tcr | This study |
| pAD12 | pBluescript containing the 1.5-kb XhoI/EcoRI border fragment of aniA::Tn5 from pCV12, Apr | This study |
| pAD15 | pBluescript containing the 1.8-kb EcoRI/XhoI border fragment of aniA::Tn5 derived from pCV12, Apr | This study |
| pHP45 Ω-Km | Donor of Ω-Km interposon | 15 |
| pJCV1 | pJQ200 with 3.9 kb SalI insert containing phaC::ΩSp, Gmr Smr Spr | This study |
| pJCV2 | pJQ200 with 12.5 kb EcoRI insert containing the aniA::Tn5 insertion from VEM58, Gmr Kmr | This study |
| pJCV3 | pJQ200 with 12.5-kb EcoRI insert containing the aniA::Tn5 insertion from VEM57, Gmr Kmr | This study |
Cell extract preparation and enzyme and protein assays.
Cells were harvested, washed, and disrupted by sonication as described by Encarnación et al. (14). Acetoacetyl-CoA reductase activity was determined under the reducing and oxidizing conditions described previously (32) but with 2-(N-morpholino)ethanesulfonic acid (MES) and Tris-HCl buffers, respectively, replacing phosphate buffer. The activity of β-ketothiolase was determined in the thiolysis direction as described by Maekawa et al. (26). Protein concentrations were determined as described above.
DNA manipulations.
R. etli genomic DNA was isolated by standard techniques (3). DNA manipulations including restriction enzyme digestions, gel electrophoresis, ligations, and transformation of E. coli were performed by standard methods (34). DNA hybridizations were carried out by the method of Southern (38) as described previously (11). DNA fragments to be sequenced were subcloned into pBluescript, and double-stranded DNA sequencing was carried out with a model 373A automatic DNA sequencer and a dye terminator cycle-sequencing FS Ready Reaction kit (Applied Biosystems, Foster City, Calif.). Nucleotide sequences were assembled with the GeneWorks package (release 2.3.1; IntelliGenetics, Inc.). Nucleotide and protein sequence homology searches were made by using the BLAST program (1) via the National Center for Biotechnology Information server. Multiple protein sequence alignments were made with Multalin program 1.5 (8) at the Network Protein Sequence Analysis server.
Transposon mutagenesis, cloning, analysis of transposon insertions, and construction and complementation of AniA mutant strains.
Transposon mutagenesis of R. etli CAR1, which is unable to grow on minimal medium containing pyruvate (MM-pyruvate), was carried out in a biparental mating with E. coli S171/pSUP5011 as described previously (11). The mating mixture was spread on MM-pyruvate containing tetracycline, resulting in the isolation of strains VEM57 and VEM58 (Table 1). The DNA fragments containing the Tn5 insertions from R. etli VEM57 and VEM58 were isolated by completely digesting genomic DNA with EcoRI, ligating the fragments into pJQ200 to give plasmids pJCV2 and pJCV3, respectively, and selecting transformants of E. coli CMK on Luria-Bertani medium containing gentamicin and kanamycin (Table 1). Plasmids pJCV2 and pJCV3 were separately mobilized by triparental mating into the R. etli wild type, and double recombinants were selected as described by Selbitschka et al. (35). The resulting aniA mutants were designated VEM5755 and VEM5854, respectively. The presence of a single insertion in each mutant was verified by Southern blotting. DNA flanking either end of the Tn5 insertion in VEM58 was subcloned in pBluescript as XhoI-EcoRI fragments to obtain plasmids pAD12 and pAD15 (Table 1) and used for DNA sequencing. Mobilization of the aniA phaAB genes cloned on pCV008 (Table 1) into R. etli VEM5854 was performed by a triparental mating with E. coli HB101/pCV008, HB101/pRK2013, and the recipient strain on YMA plates (44). Transconjugants were isolated on rich medium (peptone-yeast [PY]) plates containing kanamycin, and individual colonies were tested for the loss of their ability to grow on MM-pyruvate.
To clone the full-length aniA gene, we designed primers (upper primer, 5′-AGGAATTCTCGTTACGACCGCGATTCTC-3′; lower primer, 5′-AGGAATTCTGAATTGCTCAGGGGAGGCT-3′) to anneal to the upstream and downstream sequences, respectively, of the aniA gene. Both primers introduced EcoRI sites flanking aniA to facilitate subsequent cloning. Total DNA from R. etli CE3 was used as a PCR template, which resulted in the amplification of an 831-bp fragment. The PCR product was digested with EcoRI and ligated into pRK415 to create pRCV-76. This plasmid was introduced by triparental mating into VEM58 to create strain VEM5876 (Table 1).
Analytical procedures.
For the determination of nucleotides, culture samples were processed and nucleotide separations were performed by ion-exchange chromatography as described previously (7). Glycogen was isolated and quantitated as described by Cevallos et al. (7). PHB was assayed by the spectrophotometric method of Law and Slepecky (20), as described previously (14). For exopolysaccharide (EPS) determinations, cells from cultures grown in MM with succinate or pyruvate as the carbon source were pelleted by centrifugation (9,600 × g, 10 min), and EPS was precipitated from the supernatant with 2 volumes of 95% ethanol. Following storage at 4°C for 18 h, EPS was pelleted by centrifugation, redissolved in distilled water, and dialyzed against distilled water for 48 h. Total hexose in the dialyzed samples was determined by the anthrone method (9) with glucose as a standard. The concentrations of organic acids in cells and spent culture medium samples were determined as described previously (7).
Two-dimensional polyacrylamide gel electrophoresis and N-terminal protein sequencing.
The method for labeling and sample preparation was as reported previously by Van Bogelen and Neidhardt (43). Electrophoresis for analytical gels was performed as described in the 2D Investigator instruction manual (Millipore Corp., Milford, Mass.) using 1-mm-thick gels. In the first dimension, pH 4 to 8 ampholines were used, and in the second dimension, the acrylamide concentration was 11.5%. After electrophoresis, the gels were fixed in 25% isopropanol-10% acetic acid-1% trifluoroacetic for 2 h and then immersed in a 20% solution of 2,5-diphenyloxazole in dimethyl sulfoxide for 2 h. The gels were washed well with water, placed on a sheet of Whatman no. 1 filter paper, and dried in a vacuum gel drier at 80°C. For autoradiographic detection of proteins, a sheet of Kodak Biomax MR film was exposed to the dried gel for 4 days at room temperature. The films were developed and analyzed with an image analysis system and PDQuest software (Protein Databases, Inc., Huntington Station, N.Y.).
Preparative two-dimensional gels were prepared as described for the analytical gels except that gels were 3 mm thick in the first dimension. Sample loads were increased to approximately 1 mg of total protein. The proteins were electroblotted to Immobilon-P membranes by using a Millipore Milliblot graphite type II electroblotter system according to the manufacturer's instructions and located by staining with Coomassie brilliant blue R-250. Stained proteins were excised and sequenced with a Porton LF 3000 protein sequencer (Beckman Instruments Inc., Fullerton, Calif.). Protein homology searches were made with the PeptideSearch, AACompident, or Fasta3 program available at the EMBL, Expasy, and EBI web servers, respectively.
Nodulation, nitrogen fixation assays, and nitrogen content in plants and seeds.
The symbiotic phenotypes of the R. etli strains in combination with Phaseolus vulgaris L. cv. Negro Jamapa were analyzed at 10 to 13, 17 to 19, 24 to 27, 31 to 33, 38 to 39, and 45 to 50 days postinoculation (dpi), as described previously (7). Nodule occupancy was determined at all time points by plating nodule homogenates on PY with or without the antibiotics (Table 1) required to differentiate each strain. The total nitrogen contents of dry samples of seeds and plants were determined with a nitrogen analyzer by chemiluminescence as described previously (7). The values obtained were analyzed by an on-line one-way ANOVA program for independent samples, and the differences were subjected to the Tukey honestly significant difference test (http.//faculty.vassar.edu/lowry/anova/u.html). Significance is given at a P value of <0.01 unless otherwise specified. Three plant experiments were performed; however, only two were monitored to harvest of seed.
Nucleotide sequence accession number.
The nucleotide sequence described here was deposited in GenBank under accession number AF342934.
RESULTS
Transposon mutagenesis of the phaC mutant and isolation of double mutants whose growth on pyruvate was restored.
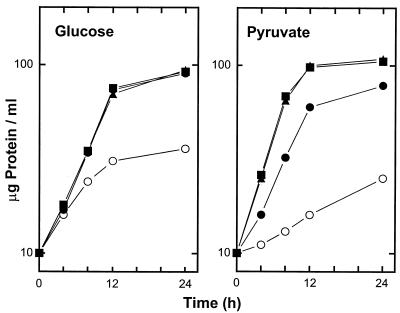
The phaC mutant CAR1 does not synthesize PHB, grows very poorly on MM with pyruvate or glucose, and has a glucose-oxidizing capacity that is only 30% of that of the parent strain (7). As an approach to analyzing the pleiotropic phenotype of the phaC mutant, we carried out random Tn5 mutagenesis of strain CAR1 and selected mutants whose growth on pyruvate was restored. Two double mutants able to grow on pyruvate retaining the phaC::ΩSmr/Spr insert and also containing a single Tn5 insertion, as evidenced by Southern blotting, were obtained. The double mutants, designated VEM57 and VEM58, were unable to accumulate PHB but grew as well as or better than the wild-type strain in MM containing pyruvate, glucose, ribose, glutamine, and mannitol as sole carbon sources (Fig. 1 and results not shown).
FIG. 1.
Growth of R. etli CE3 (wild type) (•), CAR1 (phaC) (○), VEM58 (phaC aniA) (▴), and VEM57 (phaC aniA) (▪) on MM with glucose or pyruvate as the carbon source. See Materials and Methods for details.
Genetic characterization of Tn5 insertions and surrounding DNA in the double mutants.
Genomic DNAs from mutants VEM58 and VEM57 were digested with EcoRI and, after electrophoresis and transfer to a membrane, were hybridized with a probe prepared from transposon Tn5. The hybridizing bands from both mutants were approximately 12.5 kb, and the similarity in restriction enzyme digestion patterns derived from each suggested that both insertions were in the same locus (results not shown). The DNA fragments containing the Tn5 insertions from both mutants were sequenced. The Tn5 insertions in VEM57 and VEM58 were found to be inserted following nucleotides 318 and 126, respectively, of a 570-bp ORF which we have designated aniA. The aniA::Tn5 insertions from VEM57 and VEM58 were mobilized into a wild-type R. etli genetic background, and the resulting aniA::Tn5 mutants were designated VEM5755 and VEM5854, respectively. Southern blotting confirmed that each mutant contained only a single Tn5 insertion. Since VEM5755 and VEM5854 had the same phenotype (results not shown), only VEM5854 was characterized in detail.
Sequence analysis of the R. etli aniA phaAB gene cluster.
The nucleotide sequence obtained from the Tn5-containing region cloned from VEM58 contained four potential ORFs. The AniA coding sequence encodes a gene product of 190 amino acids with a calculated molecular mass of 21,832 Da and a theoretical isoelectric point of 6.32. The aniA gene contains a putative ATG start codon preceded by a ribosomal binding site sequence (GAGA) six nucleotides upstream. R. etli AniA shares its highest sequence identity (78%) with AniA of S. meliloti strain 41 (42) and the aniA-like ORFs from Agrobacterium tumefaciens (66% identity; GenBank accession AE008190) and Mesorhizobium loti (56% identity) (17). When indigenous plasmids from the aniA mutant VEM58 were separated on Eckhardt gels and hybridized with Tn5, no hybridization was observed. As a positive control, plasmids isolated from an R. etli mutant with a Tn5 insertion in the b plasmid (A. Garcia-de los Santos, personal communication) were run in the same gel and gave a clear hybridization signal (results not shown). These results suggested that aniA is localized on the R. etli chromosome.
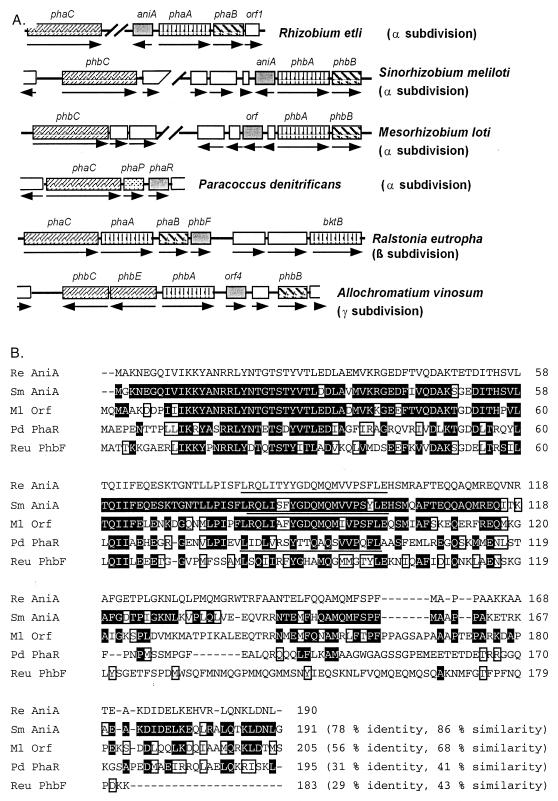
The genetic organization surrounding several aniA-like genes reported in the literature is shown in Fig. 2A. Genes encoding AniA-like proteins have so far been found only in the proteobacterial branch of the eubacteria. The specific location of aniA homologs within PHB biosynthesis gene clusters is variable, but in the α proteobacteria R. etli, S. meliloti, M. loti (Fig. 2A), Caulobacter crescentus (The Institute for Genomic Research, C. crescentus_12574), A. tumefaciens (GenBank accession numbers AE008190 and AE008191), and Methylobacterium extorquens (19) they are located next to or near phaA and transcribed divergently from it (Fig. 2A and results not shown). AniA homologs in members of the β and γ subdivisions (represented in Fig. 2A by Ralstonia eutropha and Allochromatium vinosum, respectively) of the proteobacteria are located next to phaB or phbA and are transcribed in the same direction.
FIG. 2.
(A) Schematic representation of several proteobacterial gene clusters containing aniA homologs (gray boxes). Open boxes represent various potential ORFs whose predicted products do not appear to be involved in PHB synthesis. Boxes representing ORFs encoding enzymes involved in PHB synthesis are represented as follows: PHB synthase, fine cross-hatching; β-ketothiolase, vertical bars; acetoacetyl-CoA reductase, bold cross-hatching. The transcriptional orientation of the genes is indicated by arrows. Sources of the sequences are as follows: R. etli aniA, this report; S. meliloti strain 41 aniA, reference 30; P. denitrificans phaR, references 24 and 25; R. eutropha phbF, reference 37; M. loti, reference 17; A. vinosum orf4, reference 21. (B) Alignment of the predicted amino acid sequences of AniA-like proteins produced with Multalin. Deduced amino acid residues identical to those in R. etli AniA are highlighted on a black background, and conservative amino acid substitutions (AST, ILMV, KR, NQ, ED, and FWY) are boxed. Potential helix-turn-helix motifs described in Results for R. etli, S. meliloti, and P. denitrificans are underlined. Sequences were obtained from the sources used for panel A and are abbreviated as follows: Re, R. etli; Sm, S. meliloti; Ml, M. loti; Pd, P. denitrificans; Reu, R. eutropha.
The presence of potential helix-turn-helix motifs occurring in AniA from S. meliloti (30), PhaR from Paracoccus denitrificans (24) and StdC from Comamonas testosteroni (5) have been hypothesized to play a role in DNA binding. Prediction of the secondary structure of the R. etli AniA sequence at the BioMolecular Engineering Research Center server (http://bmerc-www.bu.edu) also showed the presence of a potential helix-turn-helix motif in the region from amino acids 79 to 99 (Fig. 2B).
Upstream and transcribed divergently from R. etli aniA are two genes putatively encoding the PHB biosynthetic enzymes β-ketothiolase (PhaA) and acetoacetyl-CoA reductase (PhaB) (Fig. 2A). The highest deduced amino acid sequence identity (73%) of R. etli phaA was to the deduced sequence of S. meliloti PhaA (42). R. etli phaB encodes a putative protein with high similarity to a variety of acetoacetyl-CoA reductases encoded in PHB biosynthesis gene clusters, with its highest deduced amino acid sequence identity (83%) being to the corresponding deduced protein sequence from Zoogloea ramigera (29). It should be noted that some Z. ramigera strains have been misidentified and actually belong to the Rhizobiaceae (33). The deduced protein product of R. etli orf1 (Fig. 2A), initiating very close to the termination codon of phaB, did not have any significant homology to known proteins.
Cloning and complementation analysis of the aniA gene from R. etli.
Using the aniA::Tn5-containing fragment from pAD15 as a hybridization probe against an R. etli genomic DNA bank, we obtained a clone containing a wild-type copy of aniA on a cosmid designated pCV008 (Table 1). For complementation studies, plasmid pCV008 (which contains aniA, phaA, and phaB) or pRCV-76 (which contains only aniA) was conjugated into R. etli CE3, VEM58, and VEM5854. As expected, the presence of either pCV008 or pRCV-76 in the double mutant VEM58 (phaC::ΩSmr/Spr aniA::Tn5) prevented its growth on MM-pyruvate (results not shown). When plasmid pCV008 was introduced into VEM5854 (aniA::Tn5), PHB accumulation was restored to a level similar to that of wild-type strain CE3 (results not shown). When R. etli CE3 containing pCV008 was grown in MM-succinate, it accumulated fourfold more PHB and produced 44% less EPS than R. etli CE3. Similarly, wild-type strain CE3 complemented with pRCV-76 accumulated twofold more PHB and 39% less EPS. The growth rates of the wild type and the complemented strains were similar.
Growth characteristics of the phaC aniA single and double mutants and accumulation of storage polymers.
The growth curves presented in Fig. 1 show that the phaC aniA double mutants VEM57 and VEM58 had regained the ability to grow with pyruvate or glucose as a carbon source, and in fact the growth rates of these mutants were significantly higher than those of wild-type strain CE3 or the phaC mutant CAR1 in MM-pyruvate.
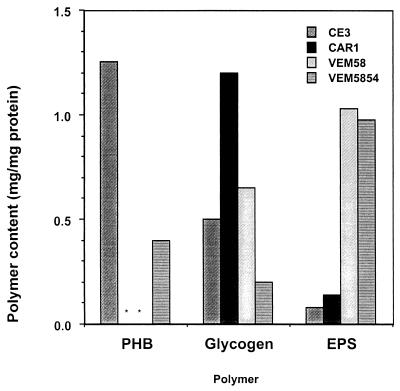
The ability of strains VEM58 and VEM5854 to accumulate glycogen, PHB, and EPS was compared to that of the parental strain and the single mutant CAR1. As expected, PHB accumulation was not detected in CAR1 and VEM58 owing to the phaC mutation. The aniA mutant VEM5854 accumulated only 40% as much PHB as wild-type strain CE3 (Fig. 3).
FIG. 3.
PHB, glycogen, and EPS accumulation in R. etli CE3, CAR1, VEM58, and VEM5854 cultured on MM-pyruvate as described in Materials and Methods. Symbols in the key from top to bottom correspond to the bars in the graph from left to right. ∗, not detected.
Previously, we reported that high levels of glycogen were accumulated in R. etli CAR1 when this strain was serially subcultured in MM-succinate (7). After 24 h of growth in MM-pyruvate, CAR1 accumulated 2.5-fold more glycogen than the wild-type strain CE3. The double mutant VEM58 (phaC aniA) produced slightly more glycogen than CE3, while VEM5854 (aniA) accumulated about half as much glycogen as the parent strain (Fig. 3). The levels of EPS produced by CE3 were very low, and EPS production by CAR1 was only slightly (1.6-fold) higher. In contrast, VEM58 and VEM5854 each produced 12 to 13 times as much EPS as the wild-type strain (Fig. 3).
Intracellular nucleotide content and the excretion of organic acids by the phaC and phaC aniA mutants.
We reported previously that R. etli CAR1 contained high levels of intracellular NADH and NADPH and that this may have resulted in the inhibition of PDH, causing the lower level of PDH activity found in R. etli CAR1. We hypothesized that the high levels of reduced nucleotides resulted from the inability of CAR1 to sequester reducing power in PHB (7). We confirmed that PDH activity in mutant CAR1 was reduced relative to the wild-type activity during growth in MM-pyruvate and that the activity of pyruvate carboxylase was also significantly reduced. We found that these activities were restored to wild-type levels in the double mutant VEM58 grown under the same conditions (12). These enzyme deficiencies explain the very limited ability of CAR1 to grow with pyruvate or glucose as a carbon source.
Significantly, reduced nucleotides were diminished in double mutant VEM58 to a level similar to, or lower than, that of wild-type strain CE3 (Table 2). The high excretion of organic acids observed in strain CAR1 was also drastically reduced in the double mutant (Table 3).
TABLE 2.
Reduced-nucleotide content of R. etli wild-type strain CE3 and mutant strains grown in MM with glucose or pyruvate as a carbon source
| Strain | Reduced-nucleotide content with carbon source at timea
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Pyruvate
|
Glucose
|
|||||||
| NADH
|
NADPH
|
NADH
|
NADPH
|
|||||
| 10 h | 24 h | 10 h | 24 h | 10 h | 24 h | 10 h | 24 h | |
| CE3 | 0.11 | 0.36 | 0.32 | 0.54 | ND | 0.30 | ND | 0.11 |
| CAR1 | 1.00 | 0.53 | 0.56 | 0.61 | ND | 1.67 | ND | 0.51 |
| VEM58 | 0.11 | 0.39 | 0.41 | 0.38 | ND | 0.05 | ND | 0.11 |
| VEM5854 | 0.12 | 0.13 | 0.17 | 0.32 | ND | 0.05 | ND | 0.03 |
Reduced-nucleotide concentrations are expressed as nanomoles per milligram of protein and are the means of duplicate cultures. ND, not determined.
TABLE 3.
Excretion of organic acids by R. etli wild-type strain CE3 and mutant strains grown on MM-pyruvate
| Strain | Organic acid content at time of growtha
|
|||||
|---|---|---|---|---|---|---|
| 2-Oxoglutarate
|
Succinate
|
Lactate
|
||||
| 12 h | 24 h | 12 h | 24 h | 12 h | 24 h | |
| CE3 | 14.2 | 0.84 | 0.06 | ND | 0.36 | ND |
| CAR1 | 37.3 | 14.7 | 0.23 | 0.28 | 0.96 | ND |
| VEM58 | 8.8 | 2.85 | ND | 0.15 | 0.19 | ND |
| VEM5854 | 13.0 | 0.24 | ND | 0.18 | 0.12 | ND |
The organic acid content of spent medium was determined as described in Materials and Methods and is expressed in micromoles per milligram of protein. ND, not determined.
Proteome analysis of the phaC aniA single and double mutants.
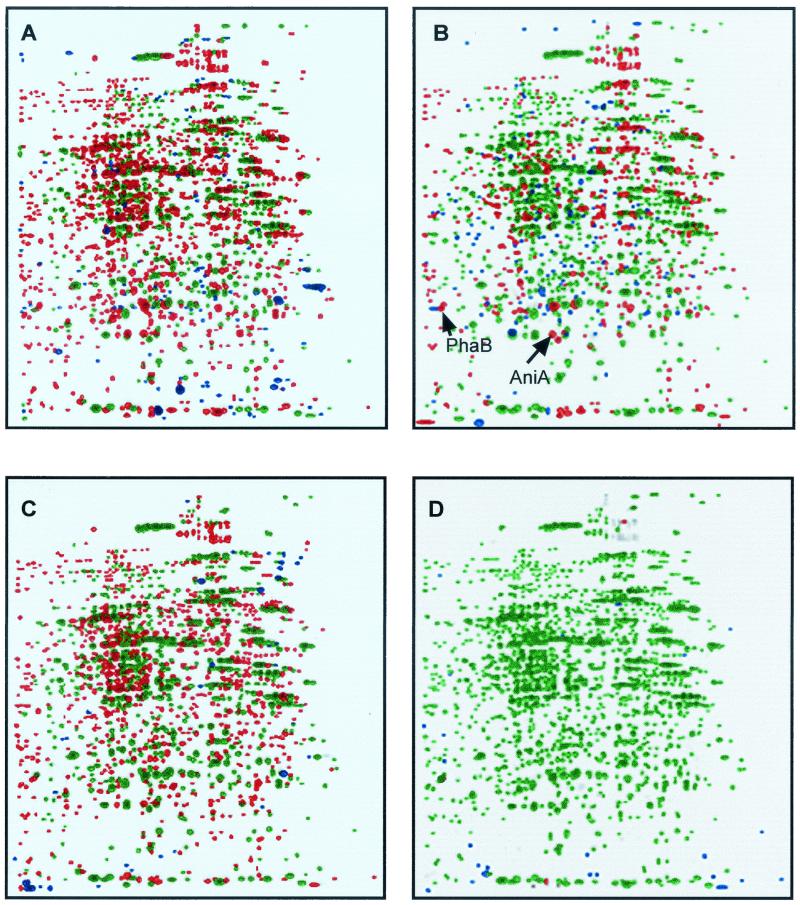
Up to 1,400 proteins were resolved by two-dimensional polyacrylamide gel electrophoresis in extracts prepared from cells of R. etli CE3 grown for 6 h in MM-pyruvate. In the phaC mutant CAR1, only 598 proteins were resolved. Of these proteins, 443 were also present in strain CE3, and the remaining 155 were unique to the mutant (Fig. 4A). In VEM58 (phaC aniA), 1,340 proteins were resolved, of which 738 were also present in strain CE3 and 602 were unique to VEM58 (Fig. 4B). The aniA mutant VEM5854 produced 850 proteins, 605 of which were also present in strain CE3 and 245 of which were unique (Fig. 4C). One of the most striking differences in the protein patterns of the different strains was that 795 proteins present in CE3 were absent from VEM5854.
FIG. 4.
Proteome maps of R. etli wild-type CE3 and mutant strains grown on MM-pyruvate displayed by using pDQuest software. In all comparisons, R. etli CE3 proteins are in red; blue spots indicate proteins from strains CAR1 (A), VEM58 (phaC aniA) (B), VEM5854 (aniA) (C), and VEM5854(pCV008) (D). Green spots indicate proteins present in both of the strains being compared.
During the routine N-terminal sequencing of R. etli CE3 proteins isolated from two-dimensional polyacrylamide electrophoresis gels, we identified N-terminal sequences identical to those of the deduced N-terminal sequences of AniA and PhaB (acetoacetyl-CoA reductase). The molecular masses of AniA and PhaB determined from the gels were 20 and 25 kDa, respectively (Fig. 4B), similar to the 21,832- and 25,578-Da molecular masses deduced from their nucleotide sequences. Figure 4B shows that AniA and PhaB are absent from the proteome of mutant VEM58 (phaC aniA). Likewise, neither AniA nor PhaB was detected in the proteome of aniA mutant VEM5854 (Fig. 4C). To see if the absence of the PhaB protein resulted in a corresponding loss of enzyme activity in these strains, we assayed acetoacetyl-CoA reductase. When measured in both the oxidizing and reducing directions with NAD(P) and NAD(P)H, respectively, as cofactors, no significant differences in acetoacetyl-CoA reductase activities were apparent between the different strains, and all of the strains had similar β-ketothiolase activities (results not shown).
There was a pronounced reduction in PHB accumulation by mutant VEM5854, which produced only 40% as much polymer as the wild-type strain (Fig. 3). PHB production (results not shown) and a proteome protein pattern closely resembling that of wild-type strain CE3 (Fig. 4) were restored when VEM5854 was complemented by plasmid pCV008, which carries aniA and phaAB (Table 1), or with plasmid pRCV-76, which carries only aniA (results not shown).
Symbiotic phenotype of the phaC and aniA single and double mutants in bean plants.
At all time points assayed in three experiments with bean plants, the symbiotic phenotypes of CE3, CAR1, VEM58, and VEM5854 strains were statistically indistinguishable by the criteria of number of nodules formed and nodule and plant dry weight (results not shown). As reported previously (7), the phaC mutant CAR1 displayed significantly more nitrogenase activity than the wild-type strain at 24 to 27, 32 to 33, 38 to 39, and 45 dpi. At all time points, VEM58 (phaC aniA) and CE3 gave nitrogenase values that were not significantly different, except that VEM58 gave lower activity at 10 to 13 dpi (P < 0.05). VEM5854 and CE3 nitrogenase values were not significantly different at all times measured. VEM58 displayed a significantly lower activity than CAR1 at 17 to 19, 24 to 27, and 31 to 33 dpi. On the other hand, VEM5854 exhibited significantly less activity than CAR1 at 24 to 27, 31 to 33, and 38 to 39 dpi; lastly, VEM58 and VEM5854 nitrogenase values were not significantly different at all times measured, except at 24 to 27 dpi (VEM58 had less nitrogenase than VEM5854). There is a correspondence of these data with the nitrogen content of plants (measured as milligrams of nitrogen per milligram of plant dry weight). The results indicated that CE3 contained significantly less nitrogen than CAR1 at all times measured. CE3 had a significantly lower nitrogen content than VEM58 up to 24 to 27 dpi. In comparisons of CE3 versus VEM5854 and CAR1 versus VEM58, the nitrogen content was not significantly different. However, VEM5854 contained significantly less nitrogen than CAR1 at 10 to 13 to 38 to 39 dpi. Finally, VEM5854 contained less nitrogen than VEM58 up to 32 to 33 dpi. The yields of seeds (in grams per plant) at harvest time were 106% in phaC mutant CAR1, 75% in VEM58, and 81% in VEM5854 relative to CE3. Nitrogen content in seeds (in milligrams of nitrogen in seeds per plant) were 132% in CAR1, 87% in VEM58, and 80% in VEM5854 relative to CE3.
DISCUSSION
To investigate the metabolic or regulatory factors which determine the pleiotropic phenotype of an R. etli phaC mutant, we performed a second mutagenesis on CAR1 and isolated the phenotypic suppressor mutants VEM57 and VEM58. The gene inactivated in both double mutants was designated aniA, based on the nomenclature used to designate a homologous gene in S. meliloti (30). Literature and gene database searches showed that aniA homologs occur in many, but not all, bacteria which synthesize PHB (results not shown). The product of S. meliloti aniA is involved in regulating carbon flow during growth under low oxygen conditions (30). Although the R. etli AniA characterized here clearly exerts its influence on carbon trafficking and global protein expression under aerobic growth conditions, we use the designation AniA to describe the R. etli gene product to avoid further confusion in the nomenclature of AniA-like genes, which have been designated by a variety of names in different bacteria (23).
AniA exerts a profound effect on metabolism in R. etli. The inactivation of aniA in an R. etli wild-type background causes a pronounced decrease in PHB accumulation, a manyfold increase in EPS biosynthesis and a drastic alteration of global protein expression, including the disappearance of PhaB. It is important to note that the drastic decrease in protein expression in the phaC mutant could result, at least in part, from a general reduction in protein expression caused by its very poor growth in MM-pyruvate. However, the finding that the aniA mutant VEM5854 also has a drastically altered pattern of protein expression, despite its growing like the wild type on MM-pyruvate, indicates a significant, growth-independent effect of AniA on global protein expression.
The introduction of plasmids containing aniA (pCV008 or pRCV-76) into wild-type strain CE3 caused an increase in PHB production and a decrease in EPS accumulation. Strain CE3 with plasmid pRCV-76, which contains only aniA, has a less pronounced increase in PHB accumulation than CE3 containing pCV008, which contains phaAB in addition to aniA. The increased accumulation of PHB in CE3/pCV008 may result in part from the presence of phaAB on this plasmid. The inactivation of aniA in a phaC mutant background restored a normal growth phenotype to the double mutant.
The potential helix-turn-helix motif in AniA suggests the possibility that it binds to DNA and functions, perhaps as part of a regulatory cascade, to control carbon trafficking in the cell. The product of the aniA homolog PhaR in P. denitrificans has been shown to bind to DNA in the phaC-phaP intergenic region of that organism. The in vivo expression of phaP, which encodes a PHB granule-associated phasin, was repressed by PhaR (25). The relevance of these results to the mechanism by which AniA of R. etli modulates carbon flow remains to be elucidated, although it is clear that R. etli does not contain a phaP homolog in proximity to aniA. The results of proteome analysis in R. etli suggest that the synthesis of PhaB (acetoacetyl-CoA reductase) may be directly positively regulated by the aniA product, although it will be necessary to demonstrate that AniA binds to DNA and functions as a transcriptional regulator of phaB or other genes. The failure to detect a decrease in acetoacetyl-CoA reductase activity in mutants VEM58 and VEM5854 may be because some bacteria contain more than one acetoacetyl-CoA reductase which participates in PHB synthesis (23, 37). In addition to the gene annotated as encoding acetoacetyl-CoA reductase in each of the genomes of S. meliloti (6) and M. loti (17), each species contains additional ORFs with significant homology to acetoacetyl-CoA reductases.
The S. meliloti AniA mutant displays a very low level of nitrogen-fixing activity on alfalfa (30). Consistent with previous results (7), seed yield and seed nitrogen content were higher in the R. etli phaC mutant than in the wild type. In contrast, the aniA mutation, alone or in combination with the phaC lesion, reduced seed yield and nitrogen content to levels below those of the wild type. This may be explained by the derivation of reductive power in these mutants for polysaccharide synthesis and accumulation.
Our physiological analysis suggests that high levels of reducing power in the phaC mutant might be responsible for preventing its growth on pyruvate or glucose, since the inactivation of aniA in this mutant drastically reduced nucleotide levels and reversed the growth defects. This may in turn result because the double mutant synthesizes very high levels of EPS, which may have the role of sequestering reducing power normally fulfilled by PHB synthesis (30), thus allowing the mutant to better utilize the TCA cycle for growth on pyruvate or glucose.
The production of PHB and other storage compounds is an important component of rhizobial metabolism under both free-living and symbiotic conditions (7, 30, 39, 41, 47). During symbiosis, the microaerobic flux of carbon into storage compounds could be regulated to favor energy production and NADH consumption, thus avoiding a drastic redox inhibition of TCA cycle enzymes (13). For this reason, we are pursuing further studies to clarify the role of the aniA product in regulating storage polymer synthesis and carbon metabolism in general. Clearly, the fact that AniA modulates the pattern of protein synthesis and flux of carbon and energy is extremely important in metabolism, as demonstrated by the fact that cells lacking AniA failed to produce 795 proteins, including the phaB gene product.
Acknowledgments
We thank Magdalena Hernández and Gabriela Guerrero for help in image analysis, Sandra Contreras for technical assistance in chromatographic determinations, and Ignacio Alvear, Antonia Jaimes, and José Luis Zitlalpopoca for help in plant experiments.
This work was supported by grant IN227598 from DGAPA-UNAM.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, A. J., and E. A. Dawes. 1990. Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol. Rev. 54:450-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. Kingston, D. Moore, J. Smith, R. Seidman, and K. Struhl. 1994. Current protocols in molecular biology. Wiley Interscience, New York, N.Y.
- 4.Bien, M., H. Steffen, and D. Schulte-Frohlinde. 1988. Repair of plasmid pBR322 damaged by γ-irradiation or by restriction endonucleases using different recombination proficient E. coli strains. Mutat. Res. 194:193-205. [DOI] [PubMed] [Google Scholar]
- 5.Cabrera, J. E., G. Panzetta-Dutari, J. L. Pruneda, and S. Genti-Raimondi. 1997. A new Comamonas testosteroni steroid-inducible gene: cloning and sequence analysis. J. Steroid Biochem. Mol. Biol. 63:91-98. [DOI] [PubMed] [Google Scholar]
- 6.Capela, D., F. Barloy-Hubler, J. Gouzy, G. Bothe, F. Ampe, J. Batut, P. Boistard, A. Becker, M. Boutry, E. Cadieu, S. Dréano, S. Gloux, T. Godrie, A. Goffeau, D. Kanh, E. Kiss, V. Lelaure, D. Masuy, T. Pohl, D. Poretelle, A. Pühler, B. Plurnelle, U. Ramsperger, C. Renard, P. Thébault, M. Vandenbol, S. Weidner, and F. Galibert. 2001. Analysis of the chromosome sequence of the legume symbiont Sinorhizobium meliloti strain 1021. Proc. Natl. Acad. Sci. USA 98:9877-9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cevallos, M. A., S. Encarnación, A. Leija, Y. Mora, and J. Mora. 1996. Genetic and physiological characterization of a Rhizobium etli mutant strain unable to synthesize poly β-hydroxybutyrate. J. Bacteriol. 178:1646-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corpet, F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dische, Z. 1962. Color reactions of carbohydrates, p. 478-481. In R. L. Whistler and M. L. Wolfram (ed.), Methods in carbohydrate chemistry, vol. I. Academic Press, New York, N.Y. [Google Scholar]
- 10.Ditta, G., S. Stanfield, D. Corbin, and D. R. Helinski. 1980. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 77:7347-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn, M. F., S. Encarnación, G. Araíza, M. C. Vargas, A. Dávalos, H. Peralta, Y. Mora, and J. Mora. 1996. Pyruvate carboxylase from Rhizobium etli: mutant characterization, nucleotide sequence, and physiological role. J. Bacteriol. 178:5960-5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn, M. F., G. Araíza, S. Encarnación, M. del Carmen Vargas, and J. Mora. 2002. Effect of aniA (carbon flux regulator) and phaC (poly-β-hydroxybutyrate synthase) mutations on pyruvate metabolism in Rhizobium etli. J. Bacteriol. 184:2296-2299. [DOI] [PMC free article] [PubMed]
- 13.Dunn, M. F. 1998. Tricarboxylic acid cycle and anaplerotic enzymes in rhizobia. FEMS Microbiol. Rev. 22:105-123. [DOI] [PubMed] [Google Scholar]
- 14.Encarnación, S., M. Dunn, K. Willms, and J. Mora. 1995. Fermentative and aerobic metabolism in Rhizobium etli. J. Bacteriol. 177:3058-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fellay, R., J. Frey, and H. Krisch. 1987. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vivo insertional mutagenesis of gram-negative bacteria. Gene 52:147-154. [DOI] [PubMed] [Google Scholar]
- 16.Friedman, A. M., S. R. Long, S. E. Brown, W. J. Buikema, and F. M. Ausubel. 1982. Construction of a broad host range cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene 18:289-296. [DOI] [PubMed] [Google Scholar]
- 17.Kaneko, T., Y. Nakamura, S. Sato, E. Asamizu, T. Kato, S. Sasamoto, A. Watanabe, K. Idesawa, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, C. Kiyokawa, M. Kohara, M. Matsumoto, A. Matsuno, Y. Mochizuki, S. Nakayama, N. Nakazaki, S. Shimpo, M. Sugimoto, C. Takeuchi, M. Yamada, and S. Tabata. 2000. Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res. 7:331-338. [DOI] [PubMed] [Google Scholar]
- 18.Keen, N. T., S. Tamaski, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 19.Korotkova, N., and M. E. Lidstrom. 2001. Connection between poly-β-hydroxybutyrate biosynthesis and growth on C1 and C2 compounds in the methylotroph Methylobacterium extorquens AM1. J. Bacteriol. 183:1038-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Law, J. H., and R. A. Slepecky. 1961. Assay of poly-β-hydroxybutyric acid. J. Bacteriol. 82:33-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liebergesell, M., and A. Steinbüchel. 1992. Cloning and nucleotide sequences of genes relevant for biosynthesis of poly(3-hydroxybutyric acid) in Chromatium vinosum strain D. Eur. J. Biochem. 209:135-150. [DOI] [PubMed] [Google Scholar]
- 22.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 23.Madison, L. L., and G. W. Huisman. 1999. Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol. Mol. Biol. Rev. 63:21-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maehara, A., S. Ueda, H. Nakano, and T. Yamane. 1999. Analyses of a polyhydroxyalkanoic acid granule-associated 16-kilodalton protein and its putative regulator in the pha locus of Paracoccus denitrificans. J. Bacteriol. 181:2914-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maehara, A., Y. Doi, T. Nishiyama, Y. Takagi, S. Ueda, H. Nakano, and T. Yamane. 2001. PhaR, a protein of unknown function conserved among short-chain-length polyhydroxyalkanoic acids producing bacteria, is a DNA-binding protein and represses Paracoccus denitrificans phaP expression in vitro. FEMS Microbiol. Lett. 200:9-15. [DOI] [PubMed] [Google Scholar]
- 26.Maekawa, B., N. Koyama, and Y. Doi. 1993. Purification and properties of 3-ketothiolase from Alcaligenes latus. Biotechnol. Lett. 15:691-696. [Google Scholar]
- 27.Mandon, K., N. Michel-Reydellet, S. Encarnacion, P. A. Kaminski, A. Leija, M. A. Cevallos, C. Elmerich, and J. Mora. 1998. Poly-β-hydroxybutyrate turnover in Azorhizobium caulinodans is required for growth and affects nifA expression. J. Bacteriol. 180:5070-5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noel, K. D., A. Sánchez, L. Fernández, J. Leemans, and M. A. Cevallos. 1984. Rhizobium phaseoli symbiotic mutants with transposon Tn5 insertions. J. Bacteriol. 158:148-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peoples, O. P., and A. J. Sinskey. 1989. Fine structural analysis of the Zoogloea ramigera phbA-phbB locus encoding β-ketothiolase and acetoacetyl-CoA reductase: nucleotide sequence of phbB. Mol. Microbiol. 3:349-357. [DOI] [PubMed] [Google Scholar]
- 30.Povolo, S., and S. Casella. 2000. A critical role for aniA in energy-carbon flux and symbiotic nitrogen fixation in Sinorhizobium meliloti. Arch. Microbiol. 174:42-49. [DOI] [PubMed] [Google Scholar]
- 31.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors that allow direct selection for gene replacement in gram-negative bacteria. Gene 127:15-21. [DOI] [PubMed] [Google Scholar]
- 32.Ritchie, G. A., P. J. Senior, and E. A. Dawes. 1971. The purification and characterization of acetoacetyl-coenzyme A reductase from Azotobacter beijerinckii. Biochem. J. 121:309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosselló-Mora, R. A., M. Wagner, R. Amann, and K.-H. Schleifer. 1995. The abundance of Zoogloea ramigera in sewage treatment plants. Appl. Environ. Microbiol. 61:702-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Selbitschka, W., S. Niemann, and A. Pühler. 1993. Construction of gene replacement vectors from gram-negative bacteria using a genetically modified SacRB gene as a positive selection marker. Appl. Microbiol. Biotechnol. 38:615-618. [Google Scholar]
- 36.Simon, R. 1984. High frequency mobilization of gram-negative bacterial replicons by the in vitro constructed Tn5-mob transposon. Mol. Gen. Genet. 196:413-420. [DOI] [PubMed] [Google Scholar]
- 37.Slater, S., K. L. Houmiel, M. Tran, T. A. Mitsky, N. B. Taylor, S. R. Padgette, and K. J. Gruys. 1998. Multiple β-ketothiolases mediate poly(β-hydroxyalkanoate) copolymer synthesis in Ralstonia eutropha. J. Bacteriol. 180:1979-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Southern, E. M. 1975. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J. Mol. Biol. 98:503-517. [DOI] [PubMed] [Google Scholar]
- 39.Stam, H., H. W. van Verseveld, W. de Vries, and A. H. Stouthamer. 1986. Utilization of poly-β-hydroxybutyrate in free-living cultures of Rhizobium ORS571. FEMS Microbiol. Lett. 35:215-220. [Google Scholar]
- 40.Steinbüchel, A., and S. Hein. 2001. Biochemical and molecular basis of microbial synthesis of polyhydroxyalkanoates in microorganisms. Adv. Biochem. Eng. Biotechnol. 71:81-123. [DOI] [PubMed] [Google Scholar]
- 41.Tavernier, P., J. C. Portais, J. E. Nava-Saucedo, J. Courtois, B. Courtois, and J. N. Barbotin. 1997. Exopolysaccharide and poly-β-hydroxybutyrate coproduction in two Rhizobium meliloti strains. Appl. Environ. Microbiol. 63:21-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tombolini, R., S. Povolo, A. Buson, A. Squartini, and M. P. Nuti. 1995. Poly-β-hydroxybutyrate (PHB) biosynthetic genes in Rhizobium meliloti 41. Microbiology 141:2553-2559. [DOI] [PubMed] [Google Scholar]
- 43.Van Bogelen, R. A., and F. C. Neidhardt. 1992. The gene-protein database of Escherichia coli: edition 5. Electrophoresis 13:1014-1054. [DOI] [PubMed] [Google Scholar]
- 44.Vincent, J. M. 1970. A manual for the practical study of root-nodule bacteria. Blackwell Scientific Publications, Oxford, United Kingdom.
- 45.Walshaw, D. L., A. Wilkinson, M. Mundy, M. Smith, and P. S. Poole. 1997. Regulation of the TCA cycle and the general amino acid permease by overflow metabolism in Rhizobium leguminosarum. Microbiology 143:2209-2221. [DOI] [PubMed] [Google Scholar]
- 46.Willis, L. B., and G. C. Walker. 1988. The phbC (poly-β-hydroxybutyrate synthase) gene of Rhizobium (Sinorhizobium) meliloti and characterization of phbC mutants. Can. J. Microbiol. 44:554-564. [DOI] [PubMed] [Google Scholar]
- 47.Zevenhuizen, L. P. T. M. 1981. Cellular glycogen, β-1,2-glucan, poly-β-hydroxybutyric acid and extracellular polysaccharides in fast-growing species of Rhizobium. Antonie Leeuwenhoek 47:481-497. [DOI] [PubMed] [Google Scholar]