Abstract
The effects of low extracellular pH and intracellular accumulation of weak organic acids were compared with respect to fatty acid synthesis by whole cells of Mycobacterium tuberculosis and Mycobacterium smegmatis. The profile of fatty acids synthesized during exposure to benzoic, nicotinic, or pyrazinoic acids, as well as that observed during intracellular hydrolysis of the corresponding amides, was not a direct consequence of modulation of fatty acid synthesis by these compounds but reflected the response to inorganic acid stress. Analysis of fatty acid synthesis in crude mycobacterial cell extracts demonstrated that pyrazinoic acid failed to directly modulate the fatty acid synthase activity catalyzed by fatty acid synthase I (FAS-I). However, fatty acid synthesis was irreversibly inhibited by 5-chloro-pyrazinamide in a time-dependent fashion. Moreover, we demonstrate that pyrazinoic acid does not inhibit purified mycobacterial FAS-I, suggesting that this enzyme is not the immediate target of pyrazinamide.
Pyrazinamide (PZA) is a first-line drug in tuberculosis chemotherapy. Its inclusion in the treatment regimen was pivotal in enabling present short-course chemotherapy, primarily because the addition of PZA significantly reduces relapse rates in patients on 6-month multidrug regimens (10, 26). PZA, although not as active as rifampin or isoniazid, has been shown to reduce viable organ counts in Mycobacterium tuberculosis-infected mice (22, 23) and to slightly reduce bacterial counts in sputum from human tuberculosis patients during monotherapy for 2 weeks (16). However, PZA studies in mice are usually performed in conjunction with other drugs, since PZA monotherapy rapidly leads to drug resistance (9). The disparity between the poor early killing activity of PZA and the profound effect on relapse rates has given rise to many hypotheses to explain this so-called “sterilizing effect.” One widely held notion is that PZA is effective against organisms residing in acidified compartments in the lung that arise during the early inflammatory stages of infection (11, 26).
Support for a specific mode of action involving organisms located within acidic compartments comes in part from in vitro studies on the mode of action of PZA that have demonstrated that PZA is only effective at a low extracellular pH and at low cell densities (20, 24, 31). The relevance of this in vitro analysis is controversial, since the intraphagosomal pH of infected macrophages equilibrates at a significantly higher value than that required for in vitro drug susceptibility (5, 13, 27). Although some studies have reported a bacteriostatic (8, 30) or even a bactericidal effect in human macrophages (6), more recent studies have demonstrated that PZA was not effective against M. tuberculosis residing in resting or activated human monocyte-derived macrophages (14).
PZA is hydrolyzed to pyrazinoic acid (POA) by the pyrazinamidase enzyme encoded by pncA in M. tuberculosis (33), and PZA resistance often correlates with loss of pyrazinamidase activity (32, 35). Although saprophytic mycobacteria such as Mycobacterium smegmatis are proficient at PZA hydrolysis, the drug nonetheless lacks activity against organisms outside the tuberculosis complex (3, 12). The lack of activity of PZA against M. smegmatis has been associated with the active efflux of the amidolysis product, the weak organic acid POA (37, 38, 41). Therefore, intracellular accumulation of POA appears to be an essential feature of the toxicity of PZA.
Recently, the inhibitory effects of POA accumulation (and the related analogue, nicotinic acid) were ascribed to inhibition of the de novo synthesis of fatty acids by the fatty acid synthase I (FAS-I) enzyme of mycobacteria (43). This conclusion was based upon an analysis of mutants resistant to the PZA analog 5-chloro-pyrazinamide (5-Cl-PZA). However, the conclusion that FAS-I inhibition is the result of a specific interaction of POA with the target enzyme is difficult to reconcile with other reported data. It has been demonstrated previously that complementation of a pncA knockout mutant of M. tuberculosis with the broader spectrum PzaA amidase from M. smegmatis (called PzaA) conferred sensitivity to the aromatic amides, benzamide, nicotinamide, and PZA. Inhibition by three such structurally diverse compounds argues against a simple inhibition of the FAS-I enzyme by direct binding and suggests that the aromatic acids formed upon hydrolysis of these compounds exert, at least in part, broad inhibitory effects on this organism (2). It was suggested that the intracellular accumulation of aromatic acids, driven by a pH gradient at low extracellular pH, places stress on the pH homeostasis mechanisms of the organism in addition to the metabolic disturbances caused by anion accumulation (2, 42).
In an attempt to reconcile these discrepant views, we compared the effects of low extracellular pH with those of intracellular aromatic anion accumulation with respect to fatty acid synthesis by whole cells. The results presented herein show that the effects of these amides on fatty acid synthesis were indistinguishable from the effects of inorganic acid stress caused by the low extracellular pH required for the inhibitory activity of these drugs. We also show that POA did not inhibit purified mycobacterial FAS-I, demonstrating that FAS-I is not the target for PZA.
MATERIALS AND METHODS
In vivo incorporation of [1-14C]acetate into fatty and mycolic acids.
M. tuberculosis H37Rv pncA::hyg attB::pAIam (2) was grown under rotation at 37°C in Middlebrook 7H9 medium supplemented with 0.05% Tween 80 and albumin-NaCl-glucose (ADC) complex to an optical density at 650 nm (OD650) of 0.3. Cells were collected by centrifugation and resuspended to an OD650 of 0.040 in 15 ml of Middlebrook 7H9 medium adjusted to the relevant pH (see Results) with phosphoric acid and supplemented as described above with or without added amides, acids, or cerulenin (all from Sigma-Aldrich, Inc., St. Louis, Mo.). PZA, nicotinamide, benzamide, POA, nicotinic acid, and benzoic acid stocks (12 mg/ml for amides and 6 mg/ml for the acids) were made in Middlebrook 7H9 medium adjusted to pH 5.6 with 10 M NaOH where necessary. Cells were grown for 6 h in this medium before the addition of 80 μl of [1-14C]acetate (Na salt, 200 μCi/ml, 58 mCi/mmol; Amersham, Arlington Heights, Ill.). After a further 13 h of incubation, cells were harvested, washed twice in 10 ml of 1 mM EDTA, and resuspended in 1.5 ml of 14% tetrabutylammonium hydroxide. Cells were saponified and fatty acid methyl esters were prepared and analyzed by thin-layer chromatography (TLC) as described earlier (25, 34). M. tuberculosis H37Rv was similarly labeled, except that growth conditions and treatment times were similar to those described by Zimhony et al. (43). Thus, cells were grown in 7H12 medium to an OD650 of 0.4 followed by 10-fold dilution into this medium with a further 48 h of growth before 108 cells were harvested by centrifugation, resuspended in 15 ml of 7H12 medium, with or without added amides or cerulenin, and adjusted to the desired pH value with phosphoric acid, with 12 h of treatment under the relevant conditions before the addition of label for another 4 h. Fatty acids were prepared by the method described above as well as by saponification of soluble lipids prepared as described by Zimhony et al. (43). M. smegmatis mc2155 was labeled by treating cells at an OD650 of 0.05 for 75 min under the relevant conditions as described above, followed by the addition of 100 μl of [1-14C]acetate (Na salt, 200 μCi/ml, 58 mCi/mmol; Amersham) and incubation for a further 4 h before washing of cells and saponification as described above.
Cell-free assay for fatty acid synthase activity.
Cell extracts of M. smegmatis mc2155 and M. tuberculosis H37Rv were prepared and FAS-I activities were assayed by monitoring [2-14C]malonyl-coenzyme A (CoA) (American Radiolabeled Chemicals, Inc., St. Louis, Mo.) incorporation into fatty acid methyl esters as described by Slayden et al. (34), except that the cells were lysed in 10 mM potassium phosphate (pH 7)-1 mM EDTA-10 mM dithiothreitol (DTT).
Synthesis of 5-Cl-PZA.
The procedure employed was a modification of that described by Cynamon et al. (7). Three grams (23 mmol) of 5-hydroxy-POA (Lonza Inc.) was gently refluxed in 30 ml of phosphorus oxychloride (Sigma-Aldrich, Inc.) for 2 h under argon. The reaction was cooled on ice, followed by the addition of 30 ml of anhydrous tetrahydrofuran. This mixture was added dropwise to 150 ml of ammonium hydroxide on ice. The reaction mixture was stirred on ice for 30 min and then extracted three times with an equal volume of ethyl acetate. The organic extracts were pooled, washed with 100 ml of 4 M NaCl, dried over magnesium sulfate, and filtered, and the solvent was removed under rotary evaporation. The product was recrystallized from ethyl acetate and characterized by TLC by using a 5-Cl-PZA standard kindly provided by John Welch (State University of New York, Albany, N.Y.). The yield was 17%.
Purification and activity assays of FAS-I.
FAS-I was purified from M. smegmatis mc2155 cells as described by Kikuchi et al. (19), with the following modifications. Briefly, cell lysates were prepared from 4-liter cells grown in Luria-Bertani broth to an OD650 of 1.0. Cell pellets were washed with 200 ml of 4°C phosphate-buffered saline (PBS) and frozen at −20°C until use. Cells were disrupted by resuspension in 60 ml of 4°C 10 mM DTT buffer A (0.1 M potassium phosphate [pH 7.2], 1 mM DTT, 1 mM EDTA) and bead beating in batches with 10 g of glass beads (0.1 mm diameter) in a 15-ml ice-cooled bead beater (BioSpec Products, Inc., Bartlesville, Okla.) in 30-s intervals for a total of 4 min, with cooling for 2 min on ice between bead beatings. Unlysed cells were removed by centrifugation and the supernatant was clarified by the addition of streptomycin sulfate (0.3 mg/ml), followed by an initial centrifugation at 18,000 × g for 45 min, with subsequent ultracentrifugation at 105,000 × g for 90 min. The high-speed supernatant was partially fractionated by 20% ammonium sulfate precipitation. FAS-I activity was precipitated from the supernatant by using 40% ammonium sulfate, the precipitate was dialyzed overnight against 4 liters of buffer A, and 15 ml of the product was loaded on a Sephacryl S400HR column (Amersham). Protein was eluted at 0.8 ml/min with buffer A, and FAS-I-containing fractions were pooled and loaded on a 6-ml Resource Q column (Amersham). Anion exchange was performed at 2 ml/min by using a 0 to 1 M NaCl gradient in buffer A over 50 column volumes. Peak fractions (approximately 10 μg/ml) from the latter column were used for fatty acid synthesis assays as described by Slayden et al. (34). Stock solutions of acids (6 mg/ml) were prepared in 1 and 0.23 M potassium phosphate at a final pH of 7.2 or at the relevant pH of the assay as indicated in Results. The final concentration of potassium phosphate in all enzyme assays was adjusted to 0.1 M. FAS-I was preincubated with the amides, acids, or cerulenin additives for 30 min at room temperature before the addition of substrates. To investigate the effects of 5-Cl-PZA on FAS-I activity, purified enzyme (4 μg in 300 μl) was incubated for 30 min at 37°C in the presence of different concentrations of 5-Cl-PZA (0.03 to 0.4 mg/ml). The treated enzyme was subsequently diluted sevenfold into buffer A and concentrated to 200 μl on a 100-kDa exclusion limit Centricon concentrator (Amicon, Inc., Beverly, Mass.), followed by two washes with 2 ml of buffer A before analysis of fatty acid synthase activity. Time-dependent inhibition of FAS-I was monitored by preincubation of the purified enzyme (1.3 μg) with 0.03 mg of 5-Cl-PZA/ml before fivefold dilution into the enzyme assay.
RESULTS
Fatty acid biosynthesis during extracellular acid stress and intracellular amide hydrolysis.
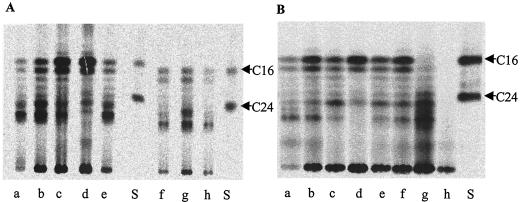
To investigate the effects of low extracellular pH on fatty acid biosynthesis in comparison to intracellular organic acid accumulation, the M. tuberculosis H37Rv pncA::hyg attB::pAIam strain was metabolically labeled with [14C]acetate under a variety of conditions. This strain shows enhanced susceptibility to PZA and nicotinamide as well as sensitivity to benzamide due to expression of the M. smegmatis PzaA amidase (2). Analysis of the methyl esters of saponified fatty acids prepared from intrinsically labeled cells (Fig. 1) demonstrated the previously described bimodal distribution of acyl chains generated by FAS-I (1, 19). The precise composition of fatty acids produced by the FAS-I complex was pH sensitive, so that upon exposure of the organism to low extracellular pH, a shift in distribution of newly synthesized acyl chains could be observed favoring increased production of longer chains (Fig. 1A, lanes a to d). This effect was also seen during exposure to PZA, nicotinamide, benzamide, POA, and nicotinic and benzoic acids (Fig. 1A, lanes e to h, and results not shown); however, these profiles were similar to those formed at or below the corresponding pH value in the absence of the drug (lanes a and b).
FIG. 1.
TLC showing the effect of acid stress and aromatic amide or acid exposure on biosynthesis of fatty acids by mycobacteria. Similar numbers of cells were labeled for each assay as described in Materials and Methods, and a quarter of each sample was analyzed by TLC. (A) Fatty acid profile from M. tuberculosis H37Rv pncA::hyg attB::pAIam formed after the following treatments: lane a, pH 5.0; lane b, pH 5.6; lane c, pH 6.0; lane d, pH 6.8; lane e, 1.2 mg of PZA/ml at pH 5.6; lane f, 1.2 mg of nicotinamide/ml at pH 5.6; lanes g and h, 0.024 and 0.12 mg/ml benzamide at pH 5.6, respectively. (B) Fatty acid profile from M. tuberculosis H37Rv formed during exposure to the following: lane a, pH 4.8; lane b, pH 5.6; lane c, pH 6.0; lane d, pH 7; lane e, 1 mg of PZA/ml at pH 5.8; lane f, 1.2 mg of PZA/ml at pH 5.6; lane g, 200 μg of 5-Cl-PZA/ml at pH 5.8; lane h, 10 μg of cerulenin/ml at pH 7. Lanes S, C16 and C24 fatty acid methyl ester standards.
To investigate the possibility that these observations might be specific to the amidase replacement recombinant strain, H37Rv pncA::hyg attB::pAIam, metabolic labeling of fatty acids was carried out by using wild-type M. tuberculosis H37Rv. This experiment was performed both in Middlebrook 7H9-based medium as used in the above experiment and in 7H12 medium (which lacks glycerol and glucose additives), under precisely the same conditions as had previously been reported to result in >90% inhibition of fatty acid biosynthesis by M. tuberculosis treated with PZA and POA (43). This analysis revealed that use of the wild-type H37Rv strain showed comparable effects to those observed with the H37Rv pncA::hyg attB::pAIam recombinant strain in terms of the fatty acid synthesis profiles observed during acid stress and intracellular aromatic acid accumulation in both types of medium (Fig. 1B and results not shown), despite minor strain differences in the fatty acid profiles observed by TLC. In these assays, only short-chain products of FAS-I near 16 carbons in length were inhibited under inorganic acid stress conditions, while longer chain products of about 26 carbons were overproduced.
Metabolic labeling of the fatty acids of M. smegmatis, a PZA-resistant mycobacterium, also indicated that growth in an acidic environment induced a shift in the profile of fatty acid biosynthesis similar to that reported for M. tuberculosis but less pronounced (data not shown). M. smegmatis also predictably failed to respond to PZA treatment with an alteration in fatty acid synthesis at the whole cell level. These differences can be ascribed to the relative resistance of this organism to low pH (29) as well as to its insensitivity to PZA as a result of POA efflux (41).
Fatty acid synthesis in vitro is not inhibited by POA.
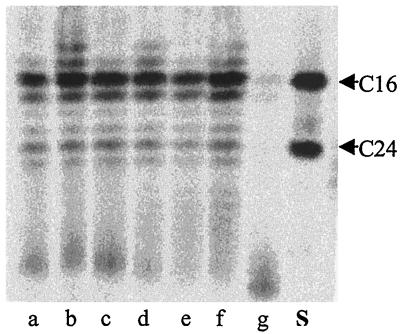
The observation that PZA and POA did not inhibit biosynthesis of fatty acids in whole cells of M. tuberculosis led us to examine whether fatty acid synthesis could be inhibited by POA or other aromatic acids and amides in whole cell lysates, where efflux of the potential inhibitor would not be a complicating issue. Incorporation of [14C]malonyl-CoA into fatty acid methyl esters was unaffected by POA concentrations of up to 3 mg of POA/ml in both M. tuberculosis and M. smegmatis whole cell lysates (Fig. 2, lanes d to f, and results not shown). Similarly, fatty acid synthesis was not inhibited by nicotinic and benzoic acids (results not shown). The relevant pH value for FAS-I activity assays is pH 7, since it has previously been demonstrated that M. tuberculosis is able to maintain a constant intracellular pH of 7 at POA concentrations of up to 0.50 mg/ml (41). However, the fatty acid synthase activity was also assayed at pH 6, which corresponds to the extracellular pH at which more than 90% inhibition of FAS-I activity could be obtained in M. tuberculosis cells in the presence of PZA (43). Even at this pH, POA did not inhibit fatty acid synthesis (Fig. 2, lanes a to c). Both cell lysates, however, were fully susceptible to cerulenin, a known inhibitor of the FAS-I enzyme (Fig. 2, lane g, and results not shown).
FIG. 2.
TLC showing the effect of POA and pH on fatty acid synthesis by M. tuberculosis whole cell lysates. Fatty acid synthase activity was assayed in 0.1 M potassium phosphate under the following conditions: lane a, pH 6; lane b, 0.6 mg of POA/ml at pH 6; lane c, 2.6 mg of POA/ml at pH 6; lane d, pH 7; lane e, 0.6 mg of POA/ml at pH 7; lane f, 2.6 mg of POA/ml at pH 7; lane g, 100 μg of cerulenin/ml at pH 7. Lane S, C16 and C24 fatty acid methyl ester standards.
Purified mycobacterial FAS-I is not inhibited by POA.
Since the cell lysate experiment potentially contained active components from the FAS-II system or from polyketide synthases whose activity might obstruct our ability to monitor FAS-I inhibition directly, we purified the FAS-I enzyme from M. smegmatis to homogeneity by using an adaptation of the published procedure (see Materials and Methods) (Fig. 3A). The M. smegmatis enzyme was chosen for practical reasons, as well as because it had been reported that POA inhibited both the M. smegmatis and the M. tuberculosis enzymes (43).
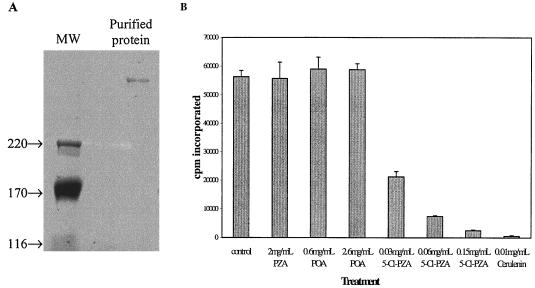
FIG. 3.
Effect of PZA, POA, and 5-Cl-PZA on purified M. smegmatis FAS-I. (A) Purification of FAS-I from M. smegmatis. Protein was fractionated by SDS-PAGE in a 4% gel, and bands were visualized by colloidal blue staining (Invitrogen). Lane 1, molecular mass markers (sizes shown in kDa); lane 2, Resource Q fraction that was used for enzyme assays. (B) FAS-I activity in the presence of PZA, POA, or 5-Cl-PZA. Fatty acid synthase activity was determined by monitoring the incorporation of [2-14C]malonyl-CoA into petroleum ether-extractable material after saponification and acidification of the assay mix.
Fatty acid synthase assays were performed by monitoring the incorporation of [14C]malonyl-CoA into petroleum ether-extractable material following saponification and methyl esterification. Analysis of fatty acid synthase activity in the presence of different amides and acids revealed that it was not affected by POA concentrations of up to 2.6 mg/ml (Fig. 3B). Similarly, no inhibition could be obtained with PZA (up to 2 mg/ml) or with benzoic or nicotinic acids (results not shown). However, 5-Cl-PZA was able to inhibit the incorporation of [14C]malonyl-CoA into fatty acids, giving 96% inhibition at 0.15 mg/ml. Addition of cerulenin, a known inhibitor of the β-ketoacyl synthase domain in type I synthases (28), resulted in more than 99% inhibition at 0.010 mg/ml. All assays were performed at pH 7.2, the optimal pH for FAS-I (19).
5-Cl-PZA is an irreversible FAS-I inhibitor.
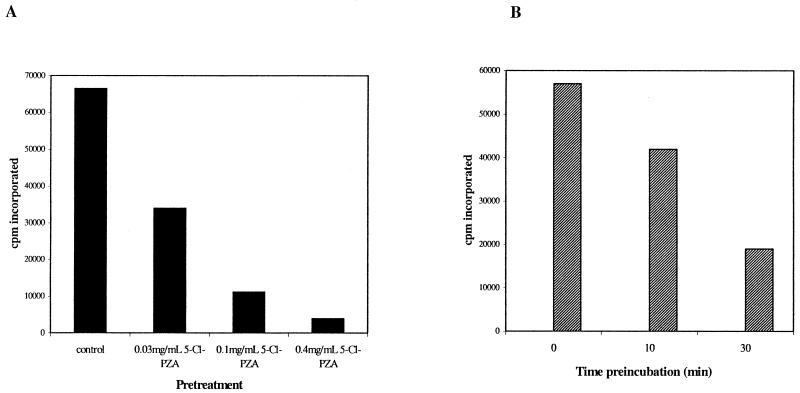
The chloro substituent at the 5 position of the pyrazine ring in the active PZA analog 5-Cl-PZA may activate this position for attack by a suitable nucleophile at an enzyme-active site. To check for irreversible inhibition of the FAS-I enzyme by 5-Cl-PZA, purified enzyme was incubated with different concentrations of 5-Cl-PZA prior to removal of the drug by dialysis. Under these conditions, FAS-I activity was still 50% inhibited by preincubation with 0.03 mg of 5-Cl-PZA/ml (Fig. 4A), a concentration that caused 60% inhibition of [14C]malonyl-CoA incorporation when added directly to the FAS-I assay mix. Furthermore, a time-dependent increase in inhibition of FAS-I was observed by preincubation of the purified enzyme with 0.03 mg of 5-Cl-PZA/ml prior to fivefold dilution into the assay mix (26% and 67% increases at 10 and 30 min of preincubation, respectively) (Fig. 4B). Taken together, these results suggest that 5-Cl-PZA covalently modifies FAS-I and that inhibition of FAS-I activity by 5-Cl-PZA can be ascribed to a unique chemical property of this drug that is not shared by PZA.
FIG. 4.
5-Cl-PZA is not a competitive inhibitor of FAS-I. (A) Purified FAS-I enzyme was incubated for 30 min at 37°C in the presence of different concentrations of 5-Cl-PZA. The treated enzyme was subsequently diluted sevenfold into buffer A (see text) and concentrated on a 100-kDa Centricon concentrator, followed by two washes in a 10-fold excess of buffer A before analysis of fatty acid synthase activity. (B) Purified enzyme was preincubated with 0.030 mg of 5-Cl-PZA/ml for different lengths of time before fivefold dilution into the enzyme assay mix.
DISCUSSION
The low bactericidal activity on M. tuberculosis by PZA and nicotinamide, both in vitro and in vivo, argues against the suggestion that POA and nicotinic acid, the known active metabolites of these amides, target the essential enzyme FAS-I (43). Inhibition by PZA in vitro is dependent on inoculum size (24, 31, 36) as well as on low pH (20, 24, 31), with significant bactericidal activity only observed at pH values (pH 4.8 to 5.0) that fail to support growth of the organism (15). This contrasts with the bactericidal activity of isoniazid (39, 40), which is a drug that inhibits the essential FAS-II complex.
Moreover, the inhibition of FAS-I by compounds such as nicotinic acid would be a surprising evolutionary development. Mycobacteria appear to have lost the pncB gene in the Preiss-Handler recycling pathway for pyridine nucleotides (4, 17) but have retained the pncA gene, encoding the nicotinamidase enzyme, which would result in the previously reported nicotinic acid accumulation in these organisms (17). Thus, there has been no evolutionary pressure to inactivate the pncA gene, despite the fact that pncA inactivation confers no obvious growth disadvantage as far as in vitro phenotype or ability to infect its human host is concerned (2, 32, 35), supporting the argument that nicotinic acid and its analogues cannot be very potent inhibitors of the essential FAS-I enzyme.
The failure to obtain POA-resistant mutants of M. tuberculosis (32) could suggest that the drug targets an essential enzyme in which any mutations that abolish drug binding are lethal (43) or that the drug has pleiotropic effects on cellular metabolism which cannot be alleviated by a simple mutation. In addition, the observed growth inhibition caused by intracellular accumulation of POA and nicotinic and benzoic acids, as a result of hydrolysis of these compounds in an M. tuberculosis recombinant expressing a broad-spectrum amidase from M. smegmatis (2), makes it unlikely that POA acts by binding and inhibiting a specific macromolecular target.
These considerations prompted us to investigate the effects of an acidic environment on fatty acid biosynthesis in comparison to the effects of intracellular aromatic anion accumulation produced by hydrolysis of the corresponding amides. For this purpose we used an M. tuberculosis pncA mutant heterologously expressing the M. smegmatis pzaA gene that had previously been shown to confer hypersensitivity to benzamide, nicotinamide, and PZA (2). All three aromatic amides resulted in a profile of newly synthesized fatty acids that was similar to that generated by the relevant low pH control, which reflects biosynthesis in response to extracellular acid stress. However, under these conditions, use of 5-Cl-PZA did result in inhibition of fatty acid synthesis, which is consistent with the observation that all reported 5-Cl-PZA-resistant M. smegmatis transformants expressed FAS-I at higher levels (43). It is also consistent with previous studies of amidase-deficient PZA-resistant mutants that nonetheless retain sensitivity to 5-Cl-PZA, a result used in that study to conclude that these two agents (PZA and 5-Cl-PZA) had different mechanisms of action (7). In the study reported herein, PZA treatment did result in modest inhibition of fatty acid synthesis at a pH of 5.6, although a similar degree of inhibition was obtained during growth at lower extracellular pH values (≤pH 5). This study was further extended by analyzing the profile of fatty acids synthesized in the wild-type M. tuberculosis H37Rv strain in response to low external pH, in the presence or absence of PZA and nicotinamide. This analysis corroborated the observation that PZA and nicotinamide are not specific inhibitors of fatty acid synthesis in whole cells and confirmed that the conclusions drawn from studies using H37Rv pncA::hyg attB::pAIam were not due to a peculiarity of this recombinant strain.
The observation that acid stress resulted in an overall increase in length of the fatty acid acyl chains may be a consequence of allosteric regulation of FAS-I under certain stress conditions or of an effect on intracellular levels of specific mycobacterial polysaccharides that show high-affinity binding to acyl-CoA esters of fatty acids (1, 18, 21). Altered concentrations or destabilization of these glycolipid complexes might result in preferential elongation of C16 acyl chain primers to C26-C28 products. The mechanism of this regulation is not a direct effect of altered intracellular pH, since M. tuberculosis and M. smegmatis are capable of intracellular pH homeostasis under these conditions (29, 41).
The evidence for the lack of inhibition of fatty acid biosynthesis by PZA and the aromatic amides benzamide and nicotinamide in vivo was corroborated by in vitro enzyme assays on mycobacterial whole cell lysates, which showed that fatty acid profiles were unaltered in response to these amides and their respective acid metabolites at concentrations greater than 20-fold above their MICs. These studies were performed both at the relevant intracellular pH of 7, as demonstrated by the capacity of M. tuberculosis to maintain a neutral pH during PZA and POA exposure (41), and at pH 6. Zhang and coworkers (41) demonstrated that the requirement for an acidic environment during PZA susceptibility studies could be ascribed to the resultant accumulation of POA driven by the transmembrane pH gradient created by the intracellular pH homeostasis mechanisms which maintained a neutral intracellular pH.
To confirm that FAS-I activity was not affected by POA or nicotinic acid, the enzyme was purified from M. smegmatis, an organism whose FAS-I activity was reportedly inhibited by these compounds in whole cells (43). The lack of inhibition of [14C]malonyl-CoA incorporation into fatty acids by nicotinic acid and POA at concentrations as high as 2.6 mg/ml in enzyme assays using purified FAS-I provided further evidence that FAS-I is not the target for PZA. However, our results unequivocally confirmed the observation that FAS-I is inhibited by 5-Cl-PZA (43).
The disparity between the lack of inhibition of FAS-I by POA and the apparent inhibition obtained with 5-Cl-PZA could be linked to the electronic effect of the chlorine atom at the 5 position of the pyrazine ring, which could render this ring carbon susceptible to attack by a suitable nucleophile. Indeed, it was found that 5-Cl-PZA was an irreversible inhibitor of FAS-I, as evidenced by the inability to relieve inhibition by dialysis after incubation with the purified enzyme as well as by time-dependent inhibition of FAS-I by the molecule before dilution of the protein into enzyme assays.
In conclusion, our results fail to support the notion that FAS-I is the target for PZA. The most consistent interpretation of the data obtained to date is that accumulation of aromatic anions such as benzoate, pyrazinoate, and nicotinate may exert nonspecific inhibitory effects on cellular metabolism. The continuing effort to understand the molecular mechanism of PZA action remains one of the major unsolved dilemmas of tuberculosis chemotherapy.
Acknowledgments
We thank Ric Slayden for help with FAS-I assays and John Welch for the kind gift of 5-Cl-PZA during the initial stages of this work. Lonza Inc. is gratefully acknowledged for the generous donation of 5-hydroxypyrazinoic acid.
This work was initiated with grants from the South African Medical Research Council, the South African Institute for Medical Research, and the Tuberculosis Lead Program of the Department of Arts, Culture, Science, and Technology.
REFERENCES
- 1.Bloch, K. 1977. Control mechanisms for fatty acid synthesis in Mycobacterium smegmatis. Adv. Enzymol. Relat. Areas Mol. Biol. 45:1-84. [DOI] [PubMed] [Google Scholar]
- 2.Boshoff, H. I., and V. Mizrahi. 2000. Expression of Mycobacterium smegmatis pyrazinamidase in Mycobacterium tuberculosis confers hypersensitivity to pyrazinamide and related amides. J. Bacteriol. 182:5479-5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boshoff, H. I., and V. Mizrahi. 1998. Purification, gene cloning, targeted knockout, overexpression, and biochemical characterization of the major pyrazinamidase from Mycobacterium smegmatis. J. Bacteriol. 180:5809-5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, B. G. Barrell, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 5.Crowle, A. J., R. Dahl, E. Ross, and M. H. May. 1991. Evidence that vesicles containing living, virulent Mycobacterium tuberculosis or Mycobacterium avium in cultured human macrophages are not acidic. Infect. Immun. 59:1823-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crowle, A. J., J. A. Sbarbaro, and M. H. May. 1986. Inhibition by pyrazinamide of tubercle bacilli within cultured human macrophages. Am. Rev. Respir. Dis. 134:1052-1055. [DOI] [PubMed] [Google Scholar]
- 7.Cynamon, M. H., R. J. Speirs, and J. T. Welch. 1998. In vitro antimycobacterial activity of 5-chloropyrazinamide. Antimicrob. Agents Chemother. 42:462-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhillon, J., and D. A. Mitchison. 1989. Activity and penetration of antituberculosis drugs in mouse peritoneal macrophages infected with Mycobacterium microti OV254. Antimicrob. Agents Chemother. 33:1255-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickinson, J. M., and D. A. Mitchison. 1991. Efficacy of intermittent pyrazinamide in experimental murine tuberculosis. Tubercle 72:110-114. [DOI] [PubMed] [Google Scholar]
- 10.Grosset, J. 1980. Bacteriologic basis of short-course chemotherapy for tuberculosis. Clin. Chest Med. 1:231-241. [PubMed] [Google Scholar]
- 11.Grosset, J. 1990. New experimental regimens for preventive therapy of tuberculosis. Bull. Int. Union Tuberc. Lung Dis. 66:15-16. [PubMed] [Google Scholar]
- 12.Guo, M., Z. Sun, and Y. Zhang. 2000. Mycobacterium smegmatis has two pyrazinamidase enzymes, PncA and PzaA. J. Bacteriol. 182:3881-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hackam, D. J., O. D. Rotstein, W. J. Zhang, N. Demaurex, M. Woodside, O. Tsai, and S. Grinstein. 1997. Regulation of phagosomal acidification. Differential targeting of Na+/H+ exchangers, Na+/K+-ATPases, and vacuolar-type H+-ATPases. J. Biol. Chem. 272:29810-29820. [DOI] [PubMed] [Google Scholar]
- 14.Heifets, L., M. Higgins, and B. Simon. 2000. Pyrazinamide is not active against Mycobacterium tuberculosis residing in cultured human monocyte-derived macrophages. Int. J. Tuberc. Lung Dis. 4:491-495. [PubMed] [Google Scholar]
- 15.Heifets, L., and P. Lindholm-Levy. 1992. Pyrazinamide sterilizing activity in vitro against semidormant Mycobacterium tuberculosis bacterial populations. Am. Rev. Respir. Dis. 145:1223-1225. [DOI] [PubMed] [Google Scholar]
- 16.Jindani, A., V. R. Aber, E. A. Edwards, and D. A. Mitchison. 1980. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am. Rev. Respir. Dis. 121:939-949. [DOI] [PubMed] [Google Scholar]
- 17.Kasarov, L. B., and A. G. Moat. 1972. Metabolism of nicotinamide adenine dinucleotide in human and bovine strains of Mycobacterium tuberculosis. J. Bacteriol. 110:600-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiho, T., and C. E. Ballou. 1988. Thermodynamic parameters and shape of the mycobacterial polymethylpolysaccharide-fatty acid complex. Biochemistry 27:5824-5828. [DOI] [PubMed] [Google Scholar]
- 19.Kikuchi, S., D. L. Rainwater, and P. E. Kolattukudy. 1992. Purification and characterization of an unusually large fatty acid synthase from Mycobacterium tuberculosis var. bovis BCG. Arch. Biochem. Biophys. 295:318-326. [DOI] [PubMed] [Google Scholar]
- 20.Mackaness, G. B. 1956. The intracellular activation of pyrazinamide and nicotinamide. Am. Rev. Tuberc. 74:718-728. [DOI] [PubMed] [Google Scholar]
- 21.Maggio, J. E. 1980. Structure of a mycobacterial polysaccharide-fatty acyl-CoA complex: nuclear magnetic resonance studies. Proc. Natl. Acad. Sci. USA 77:2582-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malone, L., A. Schurr, H. Lindh, D. McKenzie, J. S. Kiser, and J. H. Williams. 1952. The effect of pyrazinamide (Aldinamide) on experimental tuberculosis in mice. Am. Rev. Respir. Dis. 65:511-518. [PubMed] [Google Scholar]
- 23.McCune, R. M., R. Tompsett, and W. McDermott. 1956. The fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. II. The conversion of tuberculosis infection to the latent state by the administration of pyrazinamide and a companion drug. J. Exp. Med. 104:763-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDermott, W., and R. Tompsett. 1954. Activation of pyrazinamide and nicotinamide in acidic environments in vitro. Am. Rev. Tuberc. 70:748-754. [DOI] [PubMed] [Google Scholar]
- 25.Mdluli, K., R. A. Slayden, Y. Zhu, S. Ramaswamy, X. Pan, D. Mead, D. D. Crane, J. M. Musser, and C. E. Barry III. 1998. Inhibition of a Mycobacterium tuberculosis beta-ketoacyl ACP synthase by isoniazid. Science 280:1607-1610. [DOI] [PubMed] [Google Scholar]
- 26.Mitchison, D. A. 1985. The action of antituberculosis drugs in short-course chemotherapy. Tubercle 66:219-225. [DOI] [PubMed] [Google Scholar]
- 27.Oh, Y. K., and R. M. Straubinger. 1996. Intracellular fate of Mycobacterium avium: use of dual-label spectrofluorometry to investigate the influence of bacterial viability and opsonization on phagosomal pH and phagosome-lysosome interaction. Infect. Immun. 64:319-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omura, S. 1981. Cerulenin. Methods Enzymol. 72:520-532. [PubMed] [Google Scholar]
- 29.Rao, M., T. L. Streur, F. E. Aldwell, and G. M. Cook. 2001. Intracellular pH regulation by Mycobacterium smegmatis and Mycobacterium bovis BCG. Microbiology 147:1017-1024. [DOI] [PubMed] [Google Scholar]
- 30.Salfinger, M., A. J. Crowle, and L. B. Reller. 1990. Pyrazinamide and pyrazinoic acid activity against tubercle bacilli in cultured human macrophages and in the BACTEC system. J. Infect. Dis. 162: 201-207. [DOI] [PubMed] [Google Scholar]
- 31.Salfinger, M., and L. B. Heifets. 1988. Determination of pyrazinamide MICs for Mycobacterium tuberculosis at different pHs by the radiometric method. Antimicrob. Agents Chemother. 32:1002-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scorpio, A., P. Lindholm-Levy, L. Heifets, R. Gilman, S. Siddiqi, M. Cynamon, and Y. Zhang. 1997. Characterization of pncA mutations in pyrazinamide-resistant Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 41:540-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scorpio, A., and Y. Zhang. 1996. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat. Med. 2:662-667. [DOI] [PubMed] [Google Scholar]
- 34.Slayden, R. A., R. E. Lee, J. W. Armour, A. M. Cooper, I. M. Orme, P. J. Brennan, and G. S. Besra. 1996. Antimycobacterial action of thiolactomycin: an inhibitor of fatty acid and mycolic acid synthesis. Antimicrob. Agents Chemother. 40:2813-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sreevatsan, S., X. Pan, Y. Zhang, B. N. Kreiswirth, and J. M. Musser. 1997. Mutations associated with pyrazinamide resistance in pncA of Mycobacterium tuberculosis complex organisms. Antimicrob. Agents Chemother. 41:636-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stottmeier, K. D., R. E. Beam, and G. P. Kubica. 1967. Determination of drug susceptibility of mycobacteria to pyrazinamide in 7H10 agar. Am. Rev. Respir. Dis. 96:1072-1075. [DOI] [PubMed] [Google Scholar]
- 37.Sun, Z., A. Scorpio, and Y. Zhang. 1997. The pncA gene from naturally pyrazinamide-resistant Mycobacterium avium encodes pyrazinamidase and confers pyrazinamide susceptibility to resistant M. tuberculosis complex organisms. Microbiology 143:3367-3373. [DOI] [PubMed] [Google Scholar]
- 38.Sun, Z., and Y. Zhang. 1999. Reduced pyrazinamidase activity and the natural resistance of Mycobacterium kansasii to the antituberculosis drug pyrazinamide. Antimicrob. Agents Chemother. 43:537-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takayama, K., L. Wang, and R. S. Merkal. 1973. Scanning electron microscopy of the H37Ra strain of Mycobacterium tuberculosis exposed to isoniazid. Antimicrob. Agents Chemother. 4:62-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vilcheze, C., H. R. Morbidoni, T. R. Weisbrod, H. Iwamoto, M. Kuo, J. C. Sacchettini, and W. R. Jacobs, Jr. 2000. Inactivation of the inhA-encoded fatty acid synthase II (FASII) enoyl-acyl carrier protein reductase induces accumulation of the FASI end products and cell lysis of Mycobacterium smegmatis. J. Bacteriol. 182:4059-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang, Y., A. Scorpio, H. Nikaido, and Z. Sun. 1999. Role of acid pH and deficient efflux of pyrazinoic acid in unique susceptibility of Mycobacterium tuberculosis to pyrazinamide. J. Bacteriol. 181:2044-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang, Y., and A. Telenti. 2000. Genetics of drug resistance in Mycobacterium tuberculosis, p. 235-254. In G. F. Hatfull and W. R. Jacobs, Jr. (ed.), Molecular genetics of mycobacteria. ASM Press, Washington, D.C.
- 43.Zimhony, O., J. S. Cox, J. T. Welch, C. Vilcheze, and W. R. Jacobs, Jr. 2000. Pyrazinamide inhibits the eukaryotic-like fatty acid synthetase I (FASI) of Mycobacterium tuberculosis. Nat. Med. 6:1043-1047. [DOI] [PubMed] [Google Scholar]