Abstract
Thirty-five phage-resistant mutants of Lactobacillus delbrueckii subsp. lactis ATCC 15808 were selected. Thirty-three of these mutants were assigned to the Bes group, while the remaining two were grouped under the Ads designation. Bes group mutants adsorbed phage LL-H but did not allow efficient phage development. Preliminary evidence suggests that these strains exhibit a mutation that changes the DNA specificity of a restriction-modification system. The Ads group mutants did not adsorb the small isometric-head phage LL-H. The results suggest that there are at least three different types of phage receptors in L. delbrueckii: two that are specific for small isometric-head phages and one that is specific for prolate-head phage JCL1032. Five LL-H host-range mutants which could overcome the adsorption block (a-type mutants) were selected and investigated by sequencing the genes g71 and g17, which encode minor and major tail proteins, respectively. Each of the a-type mutants carried a nucleotide change at the 3′ end of gene g71. No mutations were observed in gene g17. Comparison of the gene product of g71 of phage LL-H with its homolog in JCL1032 (ORF474) showed that these proteins had very similar C-terminal regions. No similarities were found at the N-terminal part of the proteins. We conclude that the C-terminal portion of the protein encoded by g71 of phage LL-H and its homolog in phage JCL1032 determines the adsorption specificities of these phages on L. delbrueckii.
Bacteriophages which infect and destroy lactic acid bacteria pose a common and constant threat to dairy fermentations, resulting in potentially serious economic losses. The first failures in Emmental cheese manufacturing caused by Lactobacillus delbrueckii subsp. lactis phages were reported in the 1950s in Finland (17), and since then L. delbrueckii phage infections have been considered a serious risk to the dairy industry. Detailed knowledge regarding both the molecular biology of these phages and the mechanisms of phage resistance in their host will facilitate the improvement of L. delbrueckii starter cultures.
Phage LL-H is a lytic phage of L. delbrueckii subsp. lactis that was isolated in a Finnish cheese plant in 1972. It is a typical small isometric-head and long noncontractile-tail phage, having a double-stranded DNA pac-type genome of 34,659 bp in size (1, 2, 19, 20, 28). The lytic phage LL-S, isolated in Sippola (Finland) in 1953, was physically characterized by Sarimo et al. (23) and is genetically similar to LL-H (11). In contrast, the prolate-head phage JCL1032 and phage LL-H exhibit only a limited genome homology which is restricted to three short DNA segments along the LL-H genome (3, 12, 13). One of these segments was preliminarily located in the region of g71, which encodes a minor structural component of phage LL-H, possibly a tail protein (9, 12, 19).
In spite of their obvious interest, phage resistance mechanisms operative in L. delbrueckii have remained largely uncharacterized so far. We have addressed this subject by isolating and studying LL-H-resistant mutants of L. delbrueckii subsp. lactis. A strain with a mutation which prevented the adsorption step was further used to isolate host-range mutants of the phage. Sequencing results indicate that the 3′ region of gene g71 determines the adsorption specificity of LL-H to its host. In addition, a g71 homolog in phage JCL1032 was identified (orf474). The C-terminal regions of Gp71 (the product of g71, previously designated Gp58 [19]) and ORF474 exhibited extensive similarities at the primary sequence level, strongly suggesting that binding of the morphologically distinct phages LL-H and JCL1032 to their common host is mediated through similar, albeit distinct, protein structures.
MATERIALS AND METHODS
Bacteria and phages.
L. delbrueckii subsp. lactis ATCC 15808 and phage LL-H-resistant mutants of this strain (Bes-1 [named for the block of early stages of infection]and Ads-5[named for adsorption block]) were maintained as frozen stocks at −25°C in MRS broth (8) containing 20% glycerol (vol/vol). Cultures were grown anaerobically at 37°C under static conditions, in liquid or on gelified MRS (with 1.5% agar). The same medium, supplemented with 10 mM CaCl2, was used for phage propagation (2). ATCC 15808 was used as the indicator strain for phages LL-H, LL-S, and JCL1032 unless stated otherwise. The designation LL-H(Bes-1) is used throughout the text to indicate LL-H lysates prepared in the Bes-1 strain. To obtain high LL-H titers, phages were initially precipitated with polyethylene glycol (2) and concentrated 10-fold in adsorption buffer (buffer A; 20 mM sodium phosphate [pH 5.8], 100 mM NaCl, 10 mM CaCl2), and the suspension was cleared by short centrifugation at low speed (3,000 × g; 5 min). Following a high-speed centrifugation step (25,000 × g; 45 min), pelleted phages were finally recovered in buffer A.
Isolation of bacterial mutants.
LL-H-resistant mutants were isolated as described below from a total of 50 independent ATCC 15808 cultures grown from single colonies. Samples containing ≈3 × 108 late-log-phase bacteria were mixed with phage (6 × 109 PFU) and diluted 100-fold into MRS broth after an adsorption period of 10 min. Infected cells were incubated for 3 h at 37°C. Survivors were then recovered by centrifugation, reinfected with phage, and further incubated as described above. The whole procedure was repeated three to four times (until culture growth became visible) to decrease the likelihood of accumulation of mutant phages which could infect LL-H-resistant cells. After plating, individual colonies were picked and checked for phage resistance.
Phage adsorption and adsorption-competition tests.
Adsorption was usually determined by adding phages to late-log-phase bacteria in buffer A at a multiplicity of infection (MOI) of 0.1. Samples were taken at appropriate times, diluted 100-fold in cold buffer A, and centrifuged, after which the phage titer in the supernatant was determined. For the assessment of phage competition for cell receptors, the following procedure was used. Strain ATCC 15808 was grown to an optical density at 600 nm of 0.5 (≈5 × 108 CFU/ml) and cells were centrifuged and then resuspended in the same volume of buffer A. One-hundred microliters of cell suspension and 100 μl of a high-titer stock of phage LL-H (1.6 × 1011 PFU/ml) were mixed and adsorption was allowed to proceed for 10 min at 37°C. Under these conditions, only 50% of the phages adsorbed, resulting in an MOI of ≈130. After this first adsorption step, infected cells were pelleted by centrifugation and resuspended in 200 μl of buffer A. A second adsorption step was then started by adding phage LL-H(Bes-1) or phage LL-H-a21 at an input multiplicity of about 2 to LL-H-saturated cells. Adsorption was stopped and free phages were titrated as described above but, in this case, cultures of strain Bes-1 or Ads-5 were used as indicators for phages LL-H(Bes-1) and LL-H-a21, respectively, to avoid LL-H background (about 108 PFU/ml) remaining after the first adsorption. Control samples contained buffer A instead of phage LL-H.
Sequencing of phage genes.
The primers used for PCR amplification and sequencing of g17 and/or g71 from LL-H, LL-H mutants, and LL-S were designed from the published sequence of phage LL-H DNA (EMBL accession number L29568). A 2.2-kb DNA sequence containing g71 was amplified with primers p71F (5′-CGCGGCGAGGAAGGCGA-3′) and p71R (5′-TACTGCTAAGGTAGTCG-3′). Primers p17F (5′-GGGACTTTCACCTGCGA-3′) and p17R (5′-GCTGGCGATCCGGATAT-3′) were used for amplification of g17. PCR products were purified from an agarose gel, using the Qiaquick Gel Extraction kit (Qiagen, Hilden, Germany). Phage JCL1032 DNA was extracted from a polyethylene glycol-concentrated phage lysate by using a Qiagen Lambda Mini kit (Qiagen) and then it was digested with EcoRI and BamHI. A resulting 1.2-kb fragment was subsequently ligated to similarly digested plasmid pGEM3Z+. The standard primers SP6 and T7 and about 0.1 μg of ligation mixture were used for PCR amplification. The PCR product, purified as described above, was used for sequencing reactions. Phage JCL1032 DNA was used as template to extend the sequence to the flanking regions of the 1.2-kb EcoRI-BamHI fragment. Sequencing was carried out using an ABI Prism DNA sequencer (Perkin-Elmer). The redundancy of sequences was four- to eightfold. Database searches were performed using the FASTA program (22). For sequence alignment the program CLUSTAL W was used (27).
Nucleotide sequence accession number.
The g71 homolog for phage JCL1032 has been assigned accession number AJ294937 in the EMBL data library.
RESULTS
Isolation and characterization of two groups of phage LL-H-resistant mutants of L. delbrueckii subsp. lactis ATCC 15808.
Thirty-five spontaneous LL-H-resistant mutants originating from L. delbrueckii strain ATCC 15808 were isolated from a total of 50 independently infected cultures, as described in Materials and Methods. Thirty-three mutant strains adsorbed LL-H but were poor indicators for the phage. Strain Bes-1 was chosen as representative of this group of mutants. The efficiency of plating (EOP) of LL-H in strain Bes-1 was about 3 × 10−4. However, after two cycles of propagation of LL-H in this strain, the resulting LL-H(Bes-1) phages formed plaques 3,000 times more efficiently in strain Bes-1 than in ATCC 15808. Conversely, the EOP of LL-H(Bes-1) in strain Bes-1 decreased by a similar factor if phages were previously grown in ATCC 15808 (data not shown). Similar shifts in plating efficiencies were observed for phages LL-S and JCL1032 in strains ATCC 15808 and Bes-1 (data not shown). No common L. delbrueckii indicator strain for phages LL-H and LL-H(Bes-1) was found in our collection.
Although both LL-H(Bes-1) and LL-H were able to adsorb equally well to strains ATCC 15808 and Bes-1 (Fig. 1 and data not shown), Northern hybridization studies indicated that there was no phage-specific transcription in strain Bes-1 when infected by LL-H or in ATCC 15808 when infected by LL-H(Bes-1) (data not shown). Overall, these results suggest that strains ATCC 15808 and Bes-1 display different restriction-modification (R-M) systems, leading to degradation of nonmodified phage DNA (see Discussion).
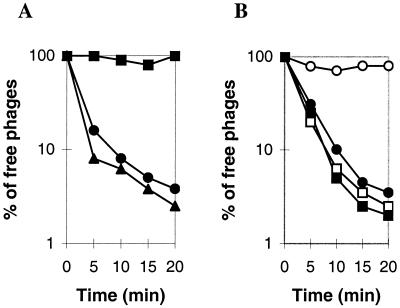
FIG. 1.
Adsorption of phage LL-H to L. delbrueckii subsp. lactis. (A) Adsorption of phage LL-H to ATCC 15808 (•) and its phage-resistant mutants ATCC 15808(Bes-1) (▴) and ATCC 15808(Ads-5) (▪). Late-log-phase bacteria were centrifuged and resuspended in buffer A at a concentration of 5 × 108 cells per ml. Cells at 37°C were infected with phage LL-H at an MOI of about 0.1. (B) Adsorption of phage LL-H(Bes-1) (○) and the mutant LL-H-a21 (□) to ATCC 15808 cells overloaded with phage LL-H. For controls, adsorption buffer was added instead of phage LL-H, and adsorption of phage LL-H(Bes-1) (•) and LL-H-a21 (▪) was measured. For details, see Materials and Methods.
The remaining two mutants among the 35 LL-H-resistant isolates did not adsorb phage LL-H, as shown for the representative of this group, strain Ads-5 (Fig. 1A). The EOP of phage LL-H in this strain was reduced to 10−6 to 10−7. Although the ads-5 mutation also blocked adsorption of LL-S, phage JCL1032 adsorbed normally to the corresponding strain (data not shown).
Phage LL-H host-range mutants.
Several independent mutants of phage LL-H capable of growing in strain Ads-5 were selected, and five of these, LL-H-a7, LL-H-a8, LL-H-a21, LL-H-a23, and LL-H-a24, were examined in greater detail. All these mutants adsorbed not only to Ads-5 but also to the wild-type strain (Fig. 1B and data not shown), suggesting that the receptor specificity of the LL-H host-range mutants had been changed. Phage adsorption-competition tests were carried out to investigate this possibility, using LL-H-a21 as the representative of host-range mutants. As shown in Fig. 1B, whereas LL-H-a21 could adsorb to ATCC 15808 after receptors were occupied by phage LL-H, LL-H(Bes-1) could not. Without previous saturation of receptors by LL-H, both phages adsorbed efficiently to ATCC 15808 cells (Fig. 1B). These results supported the notion that LL-H-a21 was able to use L. delbrueckii receptors other than those used by the wild-type phage.
Genetic control of adsorption specificity in phage LL-H.
Since no method for the transformation of ATCC 15808 is currently available, the location of phage mutations relies exclusively on direct sequencing of candidate genes. At least nine structural proteins have been identified in LL-H (19). Genes g17 (480 bp), encoding the major tail protein, and g71 (1,968 bp), coding for a putative minor tail component, have been checked in this study as likely targets of mutations affecting adsorption specificity.
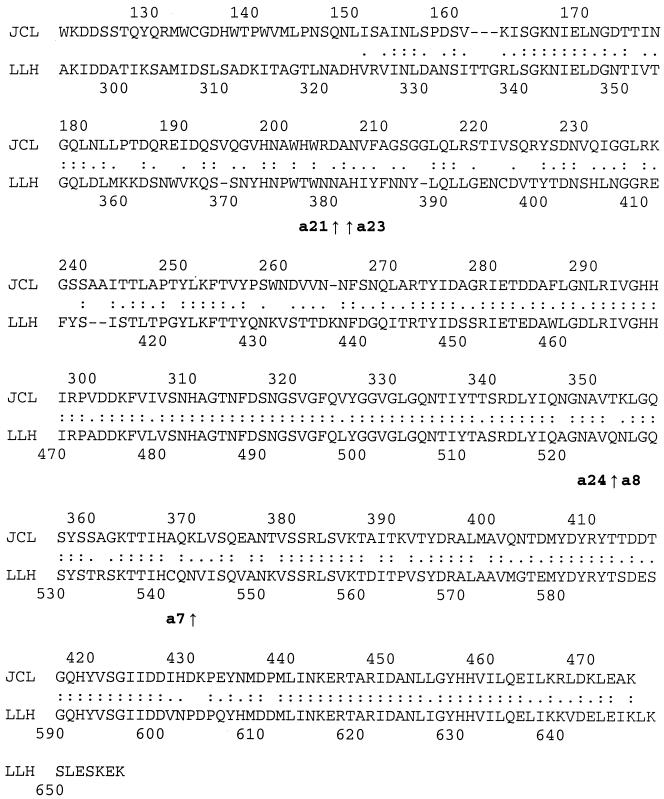
We carried out sequencing of g17 and g71 in the wild-type LL-H (control), the five LL-H-a mutants, and phage LL-H(Bes-1). In no tested phage were mutations observed in g17, and LL-H(Bes-1) g71 had the same sequence as the LL-H g71 gene. In contrast, all five mutants of the LL-H-a type had a mutation in g71 (identical in types a8 and a24). Interestingly, these mutations all resulted in amino acid replacements in the C-terminal half of the g71 product (Table 1 and Fig. 2).
TABLE 1.
Differences in g71 and its product found in LL-S and LL-H host-range mutants
| Phage | Nucleotide positiona | Base replacement | Amino acid positionb | Amino acid change |
|---|---|---|---|---|
| LL-H-a21 | 1140 | T → A | 380 | Asn → Lys |
| LL-H-a23 | 1141 | G → T | 381 | Ala → Ser |
| LL-H-a24 | 1575 | G → T | 525 | Gln → His |
| LL-H-a8 | 1575 | G → T | 525 | Gln → His |
| LL-H-a7 | 1629 | C → A | 543 | Asn → Lys |
| LL-S | 560 | C → T | 187 | Ala → Val |
| LL-S | 1309 | A → G | 437 | Lys → Glu |
| LL-S | 1958 | C → A | 653 | Ser → c |
The position in g71 where mutations occurred in host-range derivatives or where different nucleotides are found in LL-S is indicated.
Position in the Gp71 sequence where amino acid substitutions are found, relative to the LL-H product.
Missing, due to replacement of the serine codon with a stop codon.
FIG. 2.
Alignment of proteins encoded by LL-H g71 and JCL1032 orf474 created by the Clustal W program. Identical amino acids are marked by colons and similar amino acids are marked by dots. The positions where amino acids were substituted in the LL-H host-range mutants are indicated by small arrows (see also Table 1). The nonhomologous N-terminal parts of the proteins are not presented.
Homologs of g71 in phages LL-S and JCL1032.
Bacteriophages LL-H and LL-S are closely related and share the same host range. DNA heteroduplex analysis revealed three loops, each one covering approximately 0.9 kb, corresponding to less than 10% of their unit genome (11). The same primers as those used for sequencing LL-H g71 were successfully used for sequencing the LL-S homolog. The results showed that they differed in only three positions (Table 1). In two cases a nucleotide change resulted in amino acid substitutions. In the third case, a transversion from C to A resulted in the appearance of a TAA codon and hence in the truncation of the four C-terminal amino acids of the protein in LL-S.
JCL1032 is a prolate-head, possibly temperate bacteriophage. A region corresponding to a 1.2-kb EcoRI-BamHI fragment of JCL1032 DNA has previously been shown to hybridize with LL-H DNA probes covering the g71 region (12, 19). This 1.2-kb EcoRI-BamHI fragment of JCL1032 DNA, together with its flanking regions, was therefore sequenced. Analysis of the sequenced region (≈1.8 kb) revealed one open reading frame encoding a protein of 474 amino acids (ORF474). As shown in Fig. 2, a C-terminal region of about 310 amino acids is highly conserved in ORF474 and Gp71.
DISCUSSION
Four distinct types of natural phage resistance are recognized in lactic acid bacteria. They include those mechanisms which are directed towards inhibition of phage adsorption and/or DNA penetration and those which hinder any further intracellular phage development: R-M and abortive infection (10). We have approached the subject of phage resistance—for the first time in L. delbrueckii—by isolating and characterizing spontaneous mutants of strain ATCC 15808 that are resistant to bacteriophage LL-H. Two different phenotypic groups, represented by strains Bes-1 and Ads-5, were identified in this study.
Bes mutants: evidence for restriction and modification.
The efficiencies of plating of three phages (LL-H, LL-S, and JCL1032) in strain Bes-1 were reduced to about 3 × 10−4, a typical value for nonmodified phages plated in a restrictive strain (4). On the other hand, phages propagated in this mutant plated poorly in ATCC 15808, an indication that both the wild-type and the mutant exhibited R-M activities, albeit of different specificities. Evidence for restriction and modification in L. delbrueckii CNRZ 326 (ATCC 15808) was recently presented (5, 6), but its activity remains genetically and biochemically unexplored. To explain our data, two possibilities may be considered. In one hypothesis, the bes-1 mutation blocks the ATCC 15808-expressed R-M system and switches on a new system that was previously silent. The second possibility, which we favor, is that the mutation alters the DNA specificity of the R-M system present in ATCC 15808. According to this view, strain ATCC 15808 is probably endowed with a type I R-M system, because among the three classical type I, II, and III R-M systems only type I systems are known to change their sequence specificities after a single mutation (7). Type I systems appear to be widespread in lactococci (24).
Ads mutants: evidence for different types of phage receptors in L. delbrueckii.
Experiments conducted with the Ads-5 strain revealed that its mutation prevented adsorption of the small isometric-head phages LL-H and LL-S but not the binding of the unrelated prolate-head phage JCL1032. Ads-5 is, thus, a typical phage-specific receptor mutant. However, we also found that a host-range mutant of LL-H, able to adsorb and plate on Ads-5 (LL-H-a21), could still adsorb efficiently to ATCC 15808 cells, even when host receptors were saturated with bound LL-H. We therefore conclude that there are at least two types of surface structures which may be used as receptors for these small isometric-head phages. Although adsorption competition between LL-H-a21 and JCL1032 has not been tested, recent results indicate that the prolate-head phage binds to yet another type of receptor structure, different from those used by LL-H or LL-H-a21. In fact, using the same procedure as described here for selection of LL-H-resistant mutants, a strain resistant to LL-H-a21 was isolated from an infected culture of the Ads-5 strain. We observed that while LL-H-a21 was unable to adsorb to this double mutant, binding of JCL1032 was still not affected (unpublished results). Different types of receptors for small isometric-head and prolate-head phages have also been discovered in lactococci (18).
Phage adsorption proteins.
Besides LL-H-a21, four other LL-H mutants which could adsorb to and propagate in strain Ads-5 were selected. Each of these mutants had a single mutation in g71, defining altogether four residues in the corresponding product which determine LL-H binding properties. The genetic evidence provided in this study thus credits a recent suggestion from a comparative genome analysis (9) for the assignment of g71 as the gene encoding the receptor-binding protein of phage LL-H. The four amino acid substitutions affecting the adsorption specificity of phage LL-H were located in the C-terminal half of Gp71. Interestingly, we have established that most residues in this region are also conserved in a putative protein of phage JCL1032, encoded by orf474. The high degree of sequence conservation in the C-terminal regions of these proteins strongly argues for their common function as host-interacting protein domains. The use of different receptors by JCL1032, LL-H, and LL-H-a21 (see above) additionally indicates that receptor specificity is dictated by crucial but still-elusive motifs embedded in the otherwise highly conserved structure. Although additional biochemical and genetic data are required to confirm these conclusions, it is worth noting that our observations fit well into the general pattern that emerges from studies by other groups that have focused mainly on phages infecting gram-negative hosts. For instance, it has been shown that only the C-terminal domain of the tail fiber protein of the phages lambda (16, 29, 30) and T7 (25) interacts with Escherichia coli receptors. Again, phages of the T4 family (T4, TuIa, TuIb) also recognize their cellular receptors by means of the C-terminal region of a protein that is located at the distal part of their long tail fiber (15, 21, 26). Evidence for horizontal transfer of tail fiber genes among unrelated E. coli bacteriophages is well documented, and a fast evolution of these proteins as a result of intense host-range selection was previously suggested (14). The homology between JCL1032 ORF474 and LL-H Gp71 may be taken as additional evidence both for the occurrence of horizontal transfer of tail adsorption protein genes and for application of the same evolutionary principles to both gram-negative and gram-positive phage systems.
Acknowledgments
This study was supported by the grants from the Academy of Finland (SA 46921) and from the EC Biotech II program (BIO4-CT98-0406).
REFERENCES
- 1.Alatossava, T., T. Juvonen, and R. L. Huhtinen. 1983. Effect of cadmium on the infection of Lactobacillus lactis by bacteriophage LL-H. J. Gen. Virol. 64:1623-1627. [DOI] [PubMed] [Google Scholar]
- 2.Alatossava, T., and M. J. Pyhtilä. 1980. Characterization of a new Lactobacillus lactis bacteriophage. IRCS Med. Sci. 8:297-298. [Google Scholar]
- 3.Alatossava, T., P. Forsman, M. Mikkonen, L. Räisänen, and A. Vasala. 1998. Molecular genetics and evolution of Lactobacillus phage LL-H and its related phages. Recent Res. Dev. Agric. Biol. Chem. 2:345-359. [Google Scholar]
- 4.Arber, W., and S. Linn. 1969. DNA modification and restriction. Annu. Rev. Biochem. 38:467-500. [DOI] [PubMed] [Google Scholar]
- 5.Auad, L., M. A. Azcarate Peril, A. P. de Ruiz Holgado, and R. R. Raya. 1998. Evidence of restriction/modification system in Lactobacillus delbrueckii subsp. lactis CNRZ 326. Curr. Microbiol. 36:271-273. [DOI] [PubMed] [Google Scholar]
- 6.Azcarate Peril, M. A., L. Auad, and R. R. Raya. 2000. Deoxyribonuclease activities in Lactobacillus delbrueckii. Microbiol. Res. 155:101-106. [DOI] [PubMed] [Google Scholar]
- 7.Bickle, T. A., and D. H. Kruger. 1993. Biology of DNA restriction. Microbiol. Rev. 75:407-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Man, J. C., M. Rogosa, and M. E. Sharpe. 1960. A medium for cultivation of lactobacilli. J. Appl. Bacteriol. 23:130-135. [Google Scholar]
- 9.Desire, F., R. D. Pridmore, and H. Brüssow. 2000. Comparative genomics of the late gene cluster from Lactobacillus phages. Virology 275:294-305. [DOI] [PubMed] [Google Scholar]
- 10.Forde, A., and G. F. Fitzgerald. 1999. Bacteriophage defence systems in lactic acid bacteria. Antonie Leeuwenhoek 76:89-113. [PubMed] [Google Scholar]
- 11.Forsman, P., and T. Alatossava. 1991. Genetic variation of Lactobacillus delbrueckii subsp. lactis bacteriophages isolated from cheese processing plants in Finland. Appl. Environ. Microbiol. 57:1805-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forsman, P. 1993. Characterization of prolate-headed bacteriophage of Lactobacillus delbrueckii subsp. lactis and its DNA homology with isometric-headed phages. Arch. Virol. 132:321-330. [DOI] [PubMed] [Google Scholar]
- 13.Forsman, P., and T. Alatossava. 1994. Repeated sequences and its sites of genome rearrangements in bacteriophages of Lactobacillus delbrueckii subsp. lactis. Arch. Virol. 137:43-54. [DOI] [PubMed] [Google Scholar]
- 14.Haggård-Ljungquist, E. C., C. Halling, and R. Calendar. 1992. DNA sequences of the tail fiber genes of bacteriophage P2: evidence for horizontal transfer of tail fiber genes among unrelated bacteriophages. J. Bacteriol. 174:1462-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashemolhosseini, S., D. Montag, L. Kramer, and U. Henning. 1994. Determinants of the receptor specificity of coliphages of the T4 family. A chaperone alters the host range. J. Mol. Biol. 241:524-533. [DOI] [PubMed] [Google Scholar]
- 16.Hofnung, M., A. Jezierska, and C. Braun-Breton. 1976. lamB mutations in Escherichia coli K12: growth of lambda host range mutants and effect of nonsense suppressors. Mol. Gen. Genet. 145:207-213. [DOI] [PubMed] [Google Scholar]
- 17.Kiuru, V. J. Y., and E. Tybeck. 1955. Characteristics of bacteriophage active against lactic bacteria in Swiss cheese. Suom. Kem. B 28:57-62. [Google Scholar]
- 18.Kraus, J., and B. L. Geller. 1998. Membrane receptor for prolate phages is not required for infection of Lactococcus lactis by small or large isometric phages. J. Dairy Sci. 81:2329-2335. [Google Scholar]
- 19.Mikkonen, M., and T. Alatossava. 1994. Characterization of the genome region encoding structural proteins of Lactobacillus delbrueckii subsp. lactis bacteriophage LL-H. Gene 151:53-59. [DOI] [PubMed] [Google Scholar]
- 20.Mikkonen, M., L. Räisänen, and T. Alatossava. 1996. The early gene region completes the nucleotide sequence of Lactobacillus delbrueckii subsp. lactis phage LL-H. Gene 175:49-57. [DOI] [PubMed] [Google Scholar]
- 21.Montag, D., S. Hashemolhosseini, and U. Henning. 1990. Receptor-recognizing protein of T-even type bacteriophages. The receptor-recognizing area of proteins 37 of phages T4, TuIa and TuIb. J. Mol. Biol. 216:327-334. [DOI] [PubMed] [Google Scholar]
- 22.Pearson, W. R., and D. J. Lipman. 1988. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 85:2444-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarimo, S. S., M. Hartiala, and L. Aaltonen. 1976. Preparation and partial characterization of Lactobacillus lactis bacteriophage. Arch. Microbiol. 116:191-195. [DOI] [PubMed] [Google Scholar]
- 24.Schouler, C., M. Gautier, S. D. Ehrlich, and M. C. Chopin. 1998. Combinational variation of restriction modification specificities in Lactococcus lactis. Mol. Microbiol. 28:167-178. [DOI] [PubMed] [Google Scholar]
- 25.Steven, A. C., B. L. Trus, J. V. Maizel, M. Unser, D. A. D. Parry, J. S. Wall, J. F. Hainfeld, and F. M. Studier. 1988. Molecular substructure of a viral receptor-recognition protein. The gp17 tail-fiber of bacteriophage T7. J. Mol. Biol. 200:351-356. [DOI] [PubMed] [Google Scholar]
- 26.Tetart, F., C. Desplats, and H. M. Krisch. 1998. Genome plasticity in the distal tail fiber locus of the T-even bacteriophage: recombination between conserved motifs swaps adhesin specificity. J. Mol. Biol. 282:543-556. [DOI] [PubMed] [Google Scholar]
- 27.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trautwetter, A., P. Ritzenthaler, T. Alatossava, and M. Mata-Gilsinger. 1986. Physical and genetic characterization of the genome of Lactobacillus lactis bacteriophage LL-H. J. Virol. 59:551-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, J., M. Hofnung, and A. Charbit. 2000. The C-terminal portion of the tail fiber protein of bacteriophage lambda is responsible for binding to LamB, its receptor at the surface of Escherichia coli K-12. J. Bacteriol. 182:508-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Werts, C., V. Michel, M. Hofnung, and A. Charbit. 1994. Adsorption of bacteriophage lambda on LamB protein of Escherichia coli K-12: point mutations in gene J of lambda responsible for extended host range. J. Bacteriol. 176:941-947. [DOI] [PMC free article] [PubMed] [Google Scholar]