Abstract
The biosynthesis of the 2′-(5"-phosphoribosyl)-3′-dephospho-coenzyme A (CoA) prosthetic group of citrate lyase (EC 4.1.3.6), a key enzyme of citrate fermentation, proceeds via the initial formation of the precursor 2′-(5"-triphosphoribosyl)-3′-dephospho-CoA and subsequent transfer to apo-citrate lyase with removal of pyrophosphate. In Escherichia coli, the two steps are catalyzed by CitG and CitX, respectively, and the corresponding genes are part of the citrate lyase gene cluster, citCDEFXG. In the homologous citCDEFG operon of Klebsiella pneumoniae, citX is missing. A search for K. pneumoniae citX led to the identification of a second genome region involved in citrate fermentation which comprised the citWX genes and the divergent citYZ genes. The citX gene was confirmed to encode holo-citrate lyase synthase, whereas citW was shown to encode a citrate carrier, the third one identified in this species. The citYZ genes were found to encode a two-component system consisting of the sensor kinase CitY and the response regulator CitZ. Remarkably, both proteins showed ≥40% sequence identity to the citrate-sensing CitA-CitB two-component system, which is essential for the induction of the citrate fermentation genes in K. pneumoniae. A citZ insertion mutant was able to grow anaerobically with citrate, indicating that CitZ is not essential for expression of citrate fermentation genes. CitX synthesis was induced to a basal level under anaerobic conditions, independent of citrate, CitB, and CitZ, and to maximal levels during anaerobic growth with citrate as the sole carbon source. Similar to the other citrate fermentation enzymes, CitX synthesis was apparently subject to catabolite repression.
Many species of enterobacteria, such as Klebsiella pneumoniae and Escherichia coli, are able to utilize citrate under anoxic, fermentative conditions. Whereas K. pneumoniae can grow with citrate as the sole carbon and energy source (for a review, see reference 6), E. coli is dependent on the presence of an oxidizable cosubstrate (18), due to the lack of oxaloacetate decarboxylase. The initial step in all known citrate fermentation pathways is the Mg2+-dependent cleavage of citrate to acetate and oxaloacetate, a reaction catalyzed by citrate lyase (2, 10, 33).
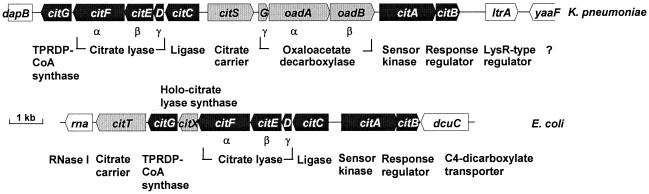
In K. pneumoniae, the structural genes for citrate lyase are part of the citCDEFG operon, which is located divergent to citS (Fig. 1). The proteins deduced from citC, citD, citE, and citF are citrate lyase ligase and the γ, β, and α subunits of citrate lyase, respectively (7). The citC operon is induced under anoxic conditions in the presence of citrate and Na+ ions. Its expression is strictly dependent on the citrate-sensing CitA-CitB two-component regulatory system (8, 16, 20) and is subject to catabolite repression, presumably by the cyclic AMP receptor protein (19).
FIG. 1.
Physical map of the citrate fermentation gene clusters in K. pneumoniae and E. coli. Genes in black are those present in both clusters, whereas genes in gray are present in only one. All other genes indicated presumably do not participate in citrate fermentation. TPRDP-CoA, 2′-(5"-triphosphoribosyl)-3′-dephospho-CoA.
In E. coli, a citCDEFXG gene cluster between 13.9 and 14.2 min (Fig. 1) that exhibited high similarity to the K. pneumoniae citrate lyase cluster (5) but differed by the presence of an additional gene, designated citX (25), was identified. Like in K. pneumoniae, expression of the E. coli citC operon is regulated by a two-component system named either CitA-CitB (25) or DpiB-DpiA (14), which shows high similarity to the corresponding proteins of K. pneumoniae.
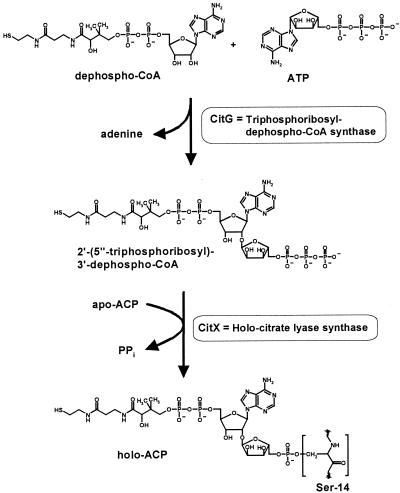
Citrate lyase contains the prosthetic group 2′-(5"-phosphoribosyl)-3′-dephospho-coenzyme A (CoA) that is attached via phosphodiester linkage to a serine residue of the γ subunit (4, 9, 22, 26, 31), which serves as an acyl carrier protein (ACP) (11). It was recently demonstrated (29, 30) that synthesis and attachment of the prosthetic group involve two reactions catalyzed by CitG and CitX (Fig. 2). In the first step, CitG catalyzes an unusual α-1,2-glycosidic linkage between ATP and dephospho-CoA, forming the prosthetic group precursor 2′-(5"-triphosphoribosyl)-3′-dephospho-CoA and adenine as products. CitG therefore functions as triphosphoribosyl-dephospho-CoA synthase (systematic name, ATP:dephospho-CoA 5′-triphosphoribosyl transferase). In the second step, the precursor is transferred to apo-ACP by CitX, resulting in the formation of holo-ACP and pyrophosphate. CitX thus functions as holo-citrate lyase synthase [systematic name, 2′-(5"-triphosphoribosyl)-3′-dephospho-CoA:apo-citrate lyase 2′-(5"-phosphoribosyl)-3′-dephospho-CoA transferase]. The mechanism for the biosynthesis of the 2′-(5"-phosphoribosyl)-3′-dephospho-CoA prosthetic group is not unique for citrate lyase but, as shown in a parallel work, follows a very similar route in the case of malonate decarboxylase (13).
FIG. 2.
Synthesis of the 2′-(5"-phosphoribosyl)-3′-dephospho-CoA prosthetic group of citrate lyase in E. coli and K. pneumoniae.
In E. coli the genes encoding CitX and CitG are part of the citrate lyase gene cluster. Therefore, expression of the plasmid-encoded citCDEFXG genes in E. coli led to the formation of a functional holo-citrate lyase (29). In contrast, expression of the K. pneumoniae cluster citCDEFG, which lacks citX, resulted in the synthesis of an inactive citrate lyase because only the apo- form of the ACP was formed (7). This deficiency could be complemented, however, by coexpression of the E. coli citX gene (29), which strongly indicated the existence of a citX homologous gene in K. pneumoniae. The fact that it is not part of the citCDEFG cluster might be incidental, but it could also reflect a transcriptional regulation different from that of the citC operon.
In this work, the K. pneumoniae citX gene was identified and its function was verified. Remarkably, citX clustered with three genes which presumably are also involved in citrate fermentation. One of these, citW, was shown to encode a citrate carrier, the third one to be identified, in addition to CitH (34, 35) and CitS (36, 37). The two other genes clustered with citX, named citY and citZ, encode a two-component regulatory system with high homology to the CitA-CitB system, which was previously shown to be essential for expression of the citC and citS operons (8).
MATERIALS AND METHODS
Bacterial strains and culture media.
E. coli and K. pneumoniae strains were routinely grown in Luria-Bertani (LB) medium at 37°C (27). E. coli DH5α (Bethesda Research Laboratories) was used for general cloning purposes. E. coli BL21(DE3) containing the T7 RNA polymerase gene under the control of a lacUV5 promoter (32) served as the host for overproduction of the CitX protein from pET expression plasmids. E. coli SM10λpir (21) served as the host during construction and mobilization of pKNG101-′citZ′.
For anaerobic growth, the K. pneumoniae cells were cultivated in a minimal medium which contained 100 mM potassium phosphate buffer (pH 7.0), 50 mM NaCl, 10 mM (NH4)2SO4, 1 mM MgSO4 and 0.4% trace element solution (180 mM CaCl2, 0.77 mM CoCl2, 0.51 MnCl2, 0.41 mM Na2MoO4, and 7.2 mM FeSO4). Carbon sources were added to a final concentration of 20 mM (potassium citrate or glucose) or 46 mM (glycerol). For analyzing the synthesis of CitX under anaerobic growth conditions, cells were cultivated in 16-ml tubes completely filled with medium and closed with screw caps containing a silicon septum. After 19 h of incubation at 37°C without agitation, when the cultures were in the stationary phase and citrate had been completely consumed, the cells were used for Western blot analysis of CitX. For comparing the growth properties of the citZ insertion mutant KS1 with those of the wild type and the citB deletion mutant KM4 (8), cells were grown at 37°C in serum bottles closed with rubber septa under nitrogen (150 kPa). Growth was monitored by measuring the optical density at 600 nm. The cells used for inoculation were routinely precultured aerobically on LB medium and washed in 100 mM potassium phosphate buffer (pH 7.0) prior to inoculation. For selective growth of K. pneumoniae and for testing the ability of E. coli recombinants to grow aerobically on citrate as the sole carbon and energy source, Simmons' citrate agar (Difco) supplemented with 12 μM thiamine was used. When required, ampicillin (100 μg ml−1) or streptomycin (50 μg ml−1) was added to the different media.
Amplification of a DNA fragment overlapping contigs 809 and 867.
In order to test whether contig 809 and contig 867 encompass adjacent regions on the genome, a PCR was carried out with oligonucleotides kp809-for2 (5′-TTCGCCGCCAGCAATGCCTC-3′) and kp867-rev (5′-CCGCACACGCTGATTCAGGG-3′). The reaction was performed with Taq DNA polymerase and chromosomal DNA of K. pneumoniae ATCC 13882 as the template in the presence of 8% (vol/vol) dimethyl sulfoxide (DMSO). The 1,172-bp PCR product was subjected to sequence analysis.
Plasmids.
For the amplification of K. pneumoniae genes by PCR, chromosomal DNA of strain ATCC 13882 was used as the template. The reactions were performed with Pfu DNA polymerase (Stratagene) according to the instructions of the supplier. The reaction mixture contained 4 to 6% (vol/vol) DMSO.
For the construction of pET124-kpcitX, the coding region of the K. pneumoniae citX gene was amplified by PCR using the primers kp-citX-for (5′-AAATTTCATATGTCAGTGGATACTCCCGCCCAG-3′) and kp-citX-rev (5′-TATTATCTCGAGTTAATCGCGGGCGAACCACGC-3′). With this procedure, the start codon became part of an NdeI restriction site while an XhoI site was introduced immediately after the stop codon. After digestion with NdeI and XhoI, the 543-bp PCR product was cloned into the vector pET124b (29) cut with the same enzymes to yield pET124-kpcitX. To construct pET124-kpcitXhis, the citX gene was amplified with the oligonucleotides kp-citX-for and kp-citXhis-rev (5′-TATTATCTCGAGATCGCGGGCGAACCACGCGTC-3′). Thereby, the citX stop codon was replaced with an XhoI restriction site. After digestion of the PCR product with NdeI and XhoI, the 540-bp DNA fragment was cloned into pET124b cut with the same enzymes. This led to a plasmid encoding CitX with a C-terminally attached His tag.
For the construction of a citW expression plasmid, the citW coding region was amplified by using the oligonucleotides kp-citW-for (5′-GATTCGAAGCTTCATATGAGCACCACAGACAATGCATTCTC-3′) and kp-citW-rev (5′-ATAATAGGATCCTCACGCCAGATAGTGGCTGAGGAAC-3′). In kp-citW-for, the ATG start codon of citW is part of an NdeI restriction site, which is preceded by a HindIII restriction site. Primer kp-citW-rev introduced a BamHI site immediately after the citW stop codon. After restriction with HindIII and BamHI, the 1.34-kb PCR product was cloned into the vector pUC19 (40) digested with the same enzymes, resulting in plasmid pUC19-citW.
Construction of a K. pneumoniae citZ insertion mutant.
In order to test whether citZ is essential for anaerobic growth on citrate, a K. pneumoniae mutant with an insertion in the chromosomally encoded citZ gene was constructed. For this purpose, a 367-bp internal fragment of citZ was amplified from K. pneumoniae ATCC 13882 chromosomal DNA by PCR in the presence of 6% (vol/vol) DMSO with the oligonucleotides kp-citZint2-for (5′-TATTATGGATCCCTATTTACCGGACGGCAAAGGCG-3′) and kp-citZint-rev (5′-CCCGGGACTAGTCGGAAAAACAGTGCGCTTCCCG-3′), which introduced BamHI and SpeI restriction sites, respectively. Following restriction of the PCR product with these two enzymes, the fragment was cloned into the suicide vector pKNG101 (15), resulting in plasmid pKNG101-′citZ′. This plasmid was transferred into the K. pneumoniae wild-type strain via conjugation using E. coli SM10λpir as the donor. Cointegrates were isolated on Simmons' citrate agar plates containing streptomycin, and the genomic structure of two selected clones was confirmed by amplifying and sequencing the two chromosomal crossover sites (Fig. 3C) with the primer pairs pKNG-for (5′-CAAATCAGCGACACTGAATACGG-3′)/kp-yabH-for (5′-TATTATGCGGCCGCTTTGGCCGTTTCGATCTGATTCG-3′) and pKNG-rev (5′-TCCCCTGGATTTCACTGATGAG-3′)/kp-citY-for (5′-AACATCGAAGTCGCCGATAACGC-3′). The corresponding citZ insertion mutants were designated KS1 and KS2.
FIG. 3.
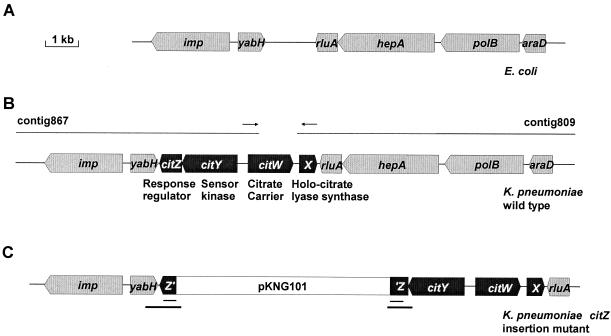
(A) Physical map of the E. coli genome region that contributed to the identification of the K. pneumoniae citX upstream region (see the text). (B) Physical map of the K. pneumoniae genome region containing the citX gene as deduced from the K. pneumoniae genome sequencing project (http://genome.wustl.edu/gsc/Projects/K.pneumoniae/) and the present study. The genes presumably involved in citrate fermentation in K. pneumoniae are in black, whereas the flanking genes, which are present in similar arrangement in K. pneumoniae and E. coli, are in grey. The arrows indicate the positions of the primers (kp867-rev and kp809-for2) used to amplify a DNA fragment that overlaps contig 809 and contig 867. (C) Physical map of the genomic citX region in the K. pneumoniae mutant KS1 obtained through the chromosomal integration of plasmid pKNG101-′citZ′. The duplicated part of citZ is underlined (thin lines). The DNA regions that were amplified by PCR and sequenced in order to confirm the genomic structure are indicated by thick lines.
DNA sequence analysis.
DNA sequence analysis was performed according to the dideoxynucleotide chain termination method (28) with a AmpliTaq DNA cycle sequencing kit (Applied Biosystems) and protocols and equipment for automated DNA sequencing (genetic analyzer 310; Applied Biosystems). Computer-assisted DNA and protein sequence analysis and alignments were performed with the Genetics Computer Group of the University of Wisconsin software package. Database searches were performed with the Basic Local Alignment Search Tool (BLAST) (1).
Biochemical methods.
The preparation of cell extracts as well as the determination of protein concentrations and citrate lyase activity were performed as described previously (29). In order to purify K. pneumoniae CitXHis, cell extracts of E. coli BL21(DE3) containing pET124-kpcitXhis were prepared with buffer containing 20 mM Tris, 500 mM NaCl, and 5 mM imidazole (TNI5; the number indicates the imidazole concentration in millimolar units). The pHs of all TNI buffers were adjusted to 7.9 by the addition of HCl. After passage through a 0.2-μm-pore-size filter, the cell extracts were loaded onto a column containing a 2-ml bed volume of His-bind resin (Novagen) equilibrated with TNI5. The column was washed with 15 ml each of TNI5, TNI15, TNI30, and TNI60 followed by 5 ml of TNI100. Subsequently, CitXHis was eluted with 10 ml of TNI200 in fractions of 1 ml, and the protein-containing fractions were pooled. For the preparation of polyclonal rabbit antibodies against CitXHis, aliquots of the purified protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (17). After Coomassie staining, the CitXHis-containing bands were excised and used for immunization (Eurogentec, Seraing, Belgium).
Western blotting.
Proteins separated by SDS-PAGE were electroblotted onto a polyvinylidene difluoride membrane. For the immunological detection of CitX, the blots were incubated with rabbit antiserum (1:500 dilution) raised against the purified protein from K. pneumoniae. Bound immunoglobulins were probed with alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (Bio-Rad Laboratories) and visualized with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate.
Nucleotide sequence accession number.
The 2.16-kb DNA region of K. pneumoniae ATCC 13882 comprising citX and citW sequenced here was deposited in GenBank with the accession number AF411142.
RESULTS AND DISCUSSION
Identification and characterization of the K. pneumoniae DNA region containing the citX gene.
The observation that the E. coli citX gene can complement the K. pneumoniae citCDEFG genes in the formation of an active holo-citrate lyase (29) strongly indicated the presence of a citX homologous gene on the K. pneumoniae chromosome. In order to identify it, a TBlastN search (1) was performed with the E. coli CitX protein sequence against the DNA sequences available from the K. pneumoniae strain MGH78578 genome sequencing project at the Washington University School of Medicine (St. Louis, Mo.; http://genome.wustl.edu/gsc/Projects/K.pneumoniae/). This led to the identification of contig 809, which encoded a protein harboring a sequence motif conserved within the CitX family (29). However, several attempts to clone the putative citX gene from chromosomal DNA by PCR failed, because of faults in the available DNA sequence which were detected later on.
Since the putative citX gene was located at the 5′ end of contig 809, only downstream genes could be identified, i.e., rluA, hepA, polB, and araD (Fig. 3B). Interestingly, these genes are present in the same order on the E. coli chromosome at 1.3 min (5), but upstream of rluA the genes yabH and imp rather than citX are found (Fig. 3A). This observation raised the possibility that the upstream region of citX in K. pneumoniae might be located on a contig harboring yabH and imp. BLAST searches revealed the corresponding genes to be located on contig 867 of the K. pneumoniae database. Downstream of yabH, two genes (designated citY and citZ) which encoded a two-component regulatory system with significant similarity to the CitA-CitB system of K. pneumoniae (8) were identified on contig 867. Upstream of citY and in divergent orientation, the 5′ part of another gene, named citW, was identified. The deduced partial gene product showed significant similarity to the citrate transporter CitS from K. pneumoniae.
The identification of three genes downstream of yabH which are related to citrate fermentation genes supported the idea that contig 867 is located adjacent to contig 809. In order to test this assumption, a PCR with chromosomal DNA of K. pneumoniae was performed with one oligonucleotide (kp867-rev) priming within contig 867 and one (kp809-for2) priming within contig 809 (Fig. 3B). Indeed, a 1,172-bp DNA fragment was amplified, and sequence analysis confirmed that the two contigs encompass adjacent regions on the genome and led to the map presented in Fig. 3B. A 2.16-kb DNA region of K. pneumoniae ATCC 13882 comprising citX and citW was completely sequenced on both strands. In addition, selected regions of citY and citZ were sequenced and resulted in the removal of a frameshift present in the citY coding region of contig 867.
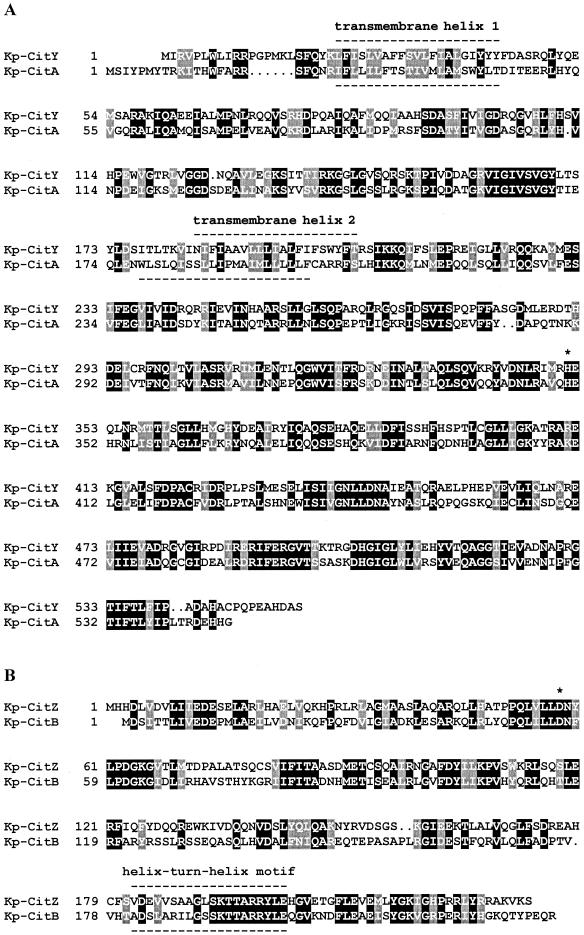
According to the corrected sequence, the protein deduced from citY consisted of 555 amino acids with a calculated molecular mass of 62.5 kDa and exhibited 44% sequence identity to the sensor kinase CitA from K. pneumoniae (Fig. 4A). Hydropathy analysis indicated that CitY had a similar topology as CitA with two transmembrane helices in the N-terminal part enclosing a periplasmic domain that extended from position 44 to 183. The corresponding domain of CitA was recently shown to function as a highly specific citrate receptor (16). The histidine residue which presumably is phosphorylated by the histidine autokinase CitY was located at position 351. In the C-terminal part of CitY, the conserved sequence motifs of the ATP binding site were found, i.e., the so-called N, G1, F, and G2 boxes.
FIG. 4.
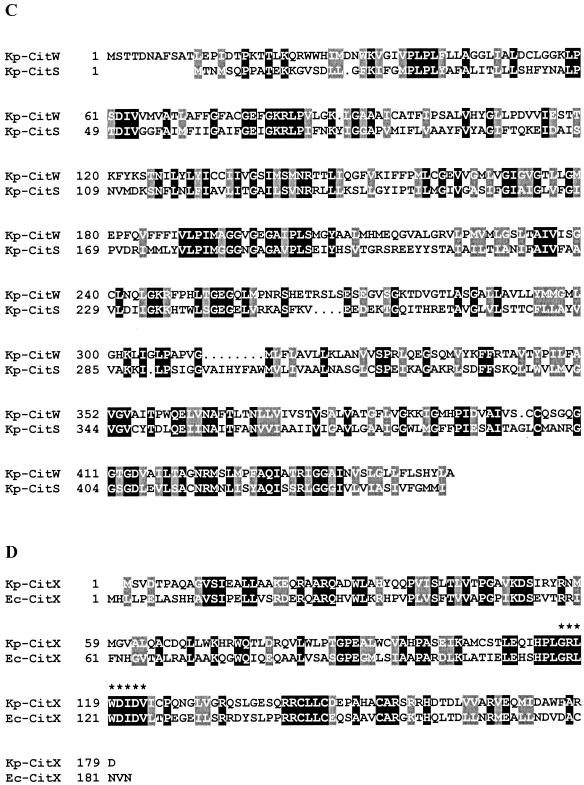
Sequence alignments of the K. pneumoniae (Kp) sensor kinases CitY and CitA (A), the K. pneumoniae response regulators CitZ and CitB (B), the K. pneumoniae secondary carriers CitW and CitS (C), and the CitX proteins from K. pneumoniae and E. coli (Ec) (D). The phosphorylation sites of the sensor kinases (His-350 of CitA and His-351 of CitY) and the response regulators (Asp-56 of CitB and Asp-58 of CitZ) and the signature sequence previously reported to be conserved in members of the CitX family (29) are indicated by asterisks.
The protein deduced from citZ consisted of 232 amino acids (26.2 kDa) and exhibited 40% sequence identity to the response regulator CitB from K. pneumoniae (Fig. 4B). The conserved aspartate residue which presumably is phosphorylated by CitY was located at position 58. In the C-terminal part, a putative DNA-binding helix-turn-helix motif extending from position 182 to 201 was detected. Remarkably, the sequence of the second helix was completely identical in CitZ and CitB, indicating similar DNA target sequences.
The protein deduced from citW was composed of 454 amino acids (48.2 kDa) and showed 29% identity to the Na+-dependent citrate carrier CitS of K. pneumoniae (Fig. 4C). As in the case of CitS, hydropathy analysis predicted 12 putative transmembrane helices for CitW; however, in the case of CitS, evidence for a membrane topology with 11 transmembrane helices was provided (38), which might also apply to CitW.
The protein deduced from citX consisted of 179 amino acids (20.1 kDa) and displayed 38% identity to CitX from E. coli (Fig. 4D). The sequence G/A-R-L-X-D-L/I-D-V, previously proposed as a signature sequence for proteins with citrate lyase apo-ACP nucleotidyltransferase activity (29), was completely conserved in the K. pneumoniae CitX protein.
Identification of the citX gene product as holo-citrate lyase synthase.
On the basis of the new sequence information, it was possible to amplify the putative citX gene from chromosomal DNA of K. pneumoniae ATCC 13882. Subsequently, the PCR product was cloned into the expression vector pET124b, resulting in plasmid pET124-kpcitX. In order to confirm that the cloned gene actually encodes a protein with holo-citrate lyase synthase activity, its ability to complement the K. pneumoniae citCDEFG genes with respect to the formation of an active holo-citrate lyase was tested. To this end, E. coli BL21(DE3) harboring plasmid pT7-CL (7) was transformed with the compatible plasmid pET124-kpcitX, and expression of the target genes was induced by IPTG (isopropyl-β-d-thiogalactopyranoside). Extracts prepared from these cells contained citrate lyase activity of up to 8.3 μmol min−1 (mg of protein)−1, whereas extracts from cells containing the vector pET124b instead of pET124-kpcitX possessed an activity of <0.01 μmol min−1 (mg of protein)−1 (29). This result provided conclusive evidence that the cloned K. pneumoniae citX gene indeed encoded holo-citrate lyase synthase. The specific activity of 8.3 μmol min−1 (mg of protein)−1 was almost identical to that obtained previously (29) with the E. coli CitX protein (8.2 μmol min−1 [mg of protein]−1) instead of K. pneumoniae CitX. This indicates that under the conditions employed, E. coli CitX could fully replace K. pneumoniae CitX in the transfer of phosphoribosyl-dephospho-CoA from triphosphoribosyl-dephospho-CoA to K. pneumoniae apo-ACP.
Identification of the citW gene product as a citrate transporter.
The fact that CitW showed 29% sequence identity to CitS suggested that this protein might also function as a citrate carrier. In order to test this assumption, the citW gene was amplified by PCR from chromosomal DNA and ligated as a 1.34-kb BamHI/HindIII fragment into pUC19, allowing transcription of citW from the vector-encoded lac promoter. The ligation mixture was transformed into E. coli DH5α and plated on Simmons' citrate agar containing ampicillin. After 24 h at 30°C, several citrate-positive colonies could be identified by a local color change of the bromthymol blue-containing agar from green to blue, caused by alkalinization due to citrate consumption. Cells unable to utilize citrate, such as E. coli DH5α containing the vector pUC19, formed very small colonies, presumably by using residual carbon sources present in the agar, and bromthymol blue remained green. Restriction analysis of plasmid DNA isolated from four Cit+ clones revealed that all contained pUC19 with the BamHI/HindIII insert carrying citW. One of the pUC19-citW plasmids was transformed again into E. coli DH5α, which was plated on Simmons' citrate agar. In this case, all transformants were able to utilize citrate, confirming that citW was responsible for the Cit+ phenotype and therefore must encode a citrate transporter.
CitW represents the third secondary carrier for citrate in K. pneumoniae besides CitH and CitS. CitH is a Na+-independent transporter which was proposed to catalyze the uptake of HCit2− (the protonated species of citrate, or the divalent citrate species) in symport with protons (34, 35). It is assumed that CitH is responsible for citrate uptake under oxic growth conditions. CitS is a Na+-dependent citrate carrier (36, 37), and studies with the purified, reconstituted carrier showed that it catalyzes the electroneutral transport of HCit2− using ΔpNa and ΔpH as driving forces (23, 24). The citS gene is part of the CitA-CitB regulon and thus expressed only under anoxic conditions in the presence of citrate and Na+ (8). The fact that citW is located in front of the citX gene required for the synthesis of holo-citrate lyase suggested that CitW could also be involved in citrate fermentation and posed the question on its specific function. Reverse transcription-PCR experiments revealed that citW mRNA was indeed present in K. pneumoniae cells grown anaerobically on citrate, and transport studies provided good evidence that CitW functions as a citrate-acetate antiporter (C. N. Kästner, K. Schneider, P. Dimroth, and K. M. Pos, unpublished data). Since acetate is the major end product of citrate fermentation in K. pneumoniae (6), such a function makes sense physiologically. Nevertheless, the reason for the probable coexistence of CitS and CitW in citrate-fermenting K. pneumoniae cells is not yet clear.
Anaerobic growth on citrate of a citZ insertion mutant.
Sequence comparisons clearly assigned CitY-CitZ to the CitA-CitB family of two-component systems (16). The common function of this family, which includes, e.g., DcuS-DcuR from E. coli (12, 41) and CitS-CitT from Bacillus subtilis (39), is to sense the presence of tri- or dicarboxylates in the environment and to induce the synthesis of secondary transporters for the corresponding compounds (16). To analyze the role of the CitY-CitZ system in citrate fermentation, a citZ insertion mutant was constructed. For that purpose, an internal 374-bp fragment of the citZ coding region was cloned into the suicide vector pKNG101 and transferred via conjugation into the K. pneumoniae wild-type strain. Exconjugants carrying a chromosomally integrated plasmid were selected on Simmons' citrate agar containing streptomycin. The expected gene arrangement (Fig. 3C) obtained after integration was confirmed by PCR amplification of the crossover sites and DNA sequence analysis of the corresponding PCR products.
One of the resulting citZ mutants, designated strain KS1, was used for growth studies. When cultivated anaerobically on citrate minimal medium, the citZ mutant was able to grow at the same rate and to the same density as the wild type, whereas the citB deletion mutant KM4 failed to grow, as reported previously (8). This indicated that in contrast to CitA-CitB the CitY-CitZ two-component system is not essential for the expression of one or several citrate fermentation genes.
Regulation of CitX synthesis.
In all bacteria containing a citX gene—except K. pneumoniae—it is clustered with the citrate lyase structural genes, either as a separate gene between citF and citG, as in E. coli (25), or fused with citG, as in Leuconostoc mesenteroides (3). This raised the question of whether the separate location of citX in K. pneumoniae is incidental or reflects a transcriptional regulation that differs from that of the citCDEFG genes. In order to analyze the synthesis of CitX under different growth conditions and in different strains, the protein was overproduced in E. coli, purified by means of a C-terminally attached hexahistidine tag, and used for the production of polyclonal rabbit antibodies. The specificity of the antiserum was confirmed by Western blots with whole-cell lysates of E. coli BL21(DE3) harboring the K. pneumoniae citX expression plasmid pET124-citX. Before induction of citX expression, a weak band of about 20 kDa was detected by the antiserum, which became much stronger after induction. In whole-cell lysates of E. coli harboring only the vector pET124, this 20-kDa band was not detectable. These results confirmed that the antiserum reacts with the K. pneumoniae CitX protein.
CitX synthesis was examined in the K. pneumoniae wild-type strain, in the citZ insertion mutant KS1, and in the citB deletion mutant KM4 (Fig. 5). The protein migrating below CitX during SDS-PAGE that was detected with the CitX antiserum in all samples was also observed with the preimmune serum. It served as an internal control to show that equal amounts of protein were loaded onto the different lanes.
FIG. 5.
Western blot analysis of K. pneumoniae whole-cell extracts with polyclonal rabbit antiserum against K. pneumoniae CitX. The wild type was grown aerobically on LB medium (lane 1) or anaerobically on glucose (lane 2), citrate (lane 3), or citrate plus glycerol (lane 4). The citZ insertion mutant KS1 was grown anaerobically on glucose (lane 5) or citrate (lane 6). The citB deletion mutant KM4 was grown anaerobically on glucose (lane 7) or citrate plus glycerol (lane 8). The band below CitX was also detected with the preimmune serum and served to confirm that equal amounts of protein were present in each lane.
In the case of the wild type, only a very faint band corresponding to CitX was observed in cells grown aerobically in LB medium, but a significant CitX level was observed in cells grown anaerobically in glucose minimal medium. This indicated that anoxic conditions alone are sufficient to induce a low basal level of citX expression, whereas in the case of the genes for citrate lyase and oxaloacetate decarboxylase both anoxic conditions and the presence of citrate are required for significant expression (8). The maximal CitX content was present in cells grown anaerobically with citrate as the sole carbon and energy source, showing that besides the oxygen (or redox) status, citrate represents a second important signal for citX expression. In cells grown anaerobically with citrate plus glycerol, the CitX level was about half-maximal, indicating that citX expression is subject to catabolite repression, similar to the other citrate fermentation genes (19).
In cells of the citZ insertion mutant KS1 grown anaerobically on glucose (Fig. 5, lane 5) or citrate (Fig. 5, lane 6), the CitX content was comparable to that of the wild type, indicating that the response regulator CitZ is required neither for anaerobiosis-induced citX expression nor for citrate-induced citX expression. This result was in accordance with the growth phenotype of strain KS1.
In cells of the citB deletion mutant KM4 grown anaerobically on glucose (Fig. 5, lane 7) the CitX content was comparable to that of the wild type grown under the respective conditions. In contrast, the CitX level in KM4 cells grown anaerobically on citrate plus glycerol appeared to be lower than in the wild type cultivated under these conditions. This suggested that the CitA-CitB two-component system is not required for anaerobiosis-induced citX expression but might be responsible for citrate-inducible citX expression. The latter assumption is supported by the presence of putative CitB binding sites in the 282-bp citW-citY intergenic region. It contains two identical 18-bp sequence motifs, each representing a 9-bp repeat (5′-TAAAAACCATAAAAACCA-3′). The CitB-binding sites in the citC-citS intergenic region as determined by DNase I footprints contain one perfect copy of this 9-bp repeat and two copies differing in only a single position (20).
In summary, the finding that the K. pneumoniae citX gene is clustered with genes for a novel citrate carrier and a CitA-CitB-homologous two-component system indicates that citrate fermentation and its regulation is more complex than previously assumed. Although the present study indicates that the CitY-CitZ system is not essential for citrate fermentation, a role of this two-component system in fine-tuning the expression of citrate fermentation genes in the natural environment is an attractive possibility to be addressed in further studies.
Acknowledgments
This work was financially supported by Boehringer Mannheim GmbH (now Roche Diagnostics GmbH, Penzberg, Germany) and by the Commission for Technology and Innovation (Bern, Switzerland).
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Antranikian, G., and F. Giffhorn. 1987. Citrate metabolism in anaerobic bacteria. FEMS Microbiol. Rev. 46:175-198. [Google Scholar]
- 3.Bekal, S., J. V. Beeumen, B. Samyn, D. Garmyn, S. Henini, C. Diviès, and H. Prévost. 1998. Purification of Leuconostoc mesenteroides citrate lyase and cloning and characterization of the citCDEF gene cluster. J. Bacteriol. 180:647-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyreuther, K., H. Böhmer, and P. Dimroth. 1978. Amino-acid sequence of citrate-lyase acyl-carrier protein from Klebsiella aerogenes. Eur. J. Biochem. 87:101-110. [DOI] [PubMed] [Google Scholar]
- 5.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 6.Bott, M. 1997. Anaerobic citrate metabolism and its regulation in enterobacteria. Arch. Microbiol. 167:78-88. [PubMed] [Google Scholar]
- 7.Bott, M., and P. Dimroth. 1994. Klebsiella pneumoniae genes for citrate lyase and citrate lyase ligase: localization, sequencing, and expression. Mol. Microbiol. 14:347-356. [DOI] [PubMed] [Google Scholar]
- 8.Bott, M., M. Meyer, and P. Dimroth. 1995. Regulation of anaerobic citrate metabolism in Klebsiella pneumoniae. Mol. Microbiol. 18:533-546. [DOI] [PubMed] [Google Scholar]
- 9.Dimroth, P. 1976. The prosthetic group of citrate lyase acyl carrier protein. Eur. J. Biochem. 64:269-281. [DOI] [PubMed] [Google Scholar]
- 10.Dimroth, P. 1988. The role of vitamins and their carrier proteins in citrate fermentation, p. 191-204. In H. Kleinkauf, H. von Döhren, and L. Jaenicke (ed.), The roots of modern biochemistry. Walter de Gruyter and Co., Berlin, Germany.
- 11.Dimroth, P., W. Dittmar, G. Walther, and H. Eggerer. 1973. The acyl-carrier protein of citrate lyase. Eur. J. Biochem. 37:305-315. [DOI] [PubMed] [Google Scholar]
- 12.Golby, P., S. Davies, D. J. Kelly, J. R. Guest, and S. C. Andrews. 1999. Identification and characterization of a two-component sensor-kinase and response-regulator system (DcuS-DcuR) controlling gene expression in response to C4-dicarboxylates in Escherichia coli. J. Bacteriol. 181:1238-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoenke, S., M. Wild, and P. Dimroth. 2000. Biosynthesis of triphosphoribosyl-dephospho-coenzyme A, the precursor of the prosthetic group of malonate decarboxylase. Biochemistry 39:13223-13232. [DOI] [PubMed] [Google Scholar]
- 14.Ingmer, H., C. A. Miller, and S. N. Cohen. 1998. Destabilized inheritance of pSC101 and other Escherichia coli plasmids by DpiA, a novel two-component system regulator. Mol. Microbiol. 29:49-59. [DOI] [PubMed] [Google Scholar]
- 15.Kaniga, K., I. Delor, and G. R. Cornelis. 1991. A wide-host-range suicide vector for improving reverse genetics in Gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109:137-141. [DOI] [PubMed] [Google Scholar]
- 16.Kaspar, S., R. Perozzo, S. Reinelt, M. Meyer, K. Pfister, L. Scapozza, and M. Bott. 1999. The periplasmic domain of the histidine autokinase CitA functions as a highly specific citrate receptor. Mol. Microbiol. 33:858-872. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 18.Lütgens, M., and G. Gottschalk. 1980. Why a co-substrate is required for anaerobic growth of Escherichia coli on citrate. J. Gen. Microbiol. 119:63-70. [DOI] [PubMed] [Google Scholar]
- 19.Meyer, M., P. Dimroth, and M. Bott. 2001. Catabolite repression of the citrate fermentation genes in Klebsiella pneumoniae: evidence for involvement of the cyclic AMP receptor protein. J. Bacteriol. 183:5248-5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer, M., P. Dimroth, and M. Bott. 1997. In vitro binding of the response regulator CitB and of its carboxy-terminal domain to A + T-rich DNA target sequences in the control region of the divergent citC and citS operons of Klebsiella pneumoniae. J. Mol. Biol. 269:719-731. [DOI] [PubMed] [Google Scholar]
- 21.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of the outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oppenheimer, N. J., M. Singh, C. C. Sweeley, S.-J. Sung, and P. A. Srere. 1979. The configuration and location of the ribosidic linkage in the prosthetic group of citrate lyase (Klebsiella aerogenes). J. Biol. Chem. 254:1000-1002. [PubMed] [Google Scholar]
- 23.Pos, K. M., M. Bott, and P. Dimroth. 1994. Purification of two active fusion proteins of the Na+-dependent citrate carrier of Klebsiella pneumoniae. FEBS Lett. 347:37-41. [DOI] [PubMed] [Google Scholar]
- 24.Pos, K. M., and P. Dimroth. 1996. Functional properties of the purified Na+-dependent citrate carrier of Klebsiella pneumoniae: evidence for asymmetric orientation of the carrier protein in proteoliposomes. Biochemistry 35:1018-1026. [DOI] [PubMed] [Google Scholar]
- 25.Pos, K. M., P. Dimroth, and M. Bott. 1998. The Escherichia coli citrate carrier CitT: a member of a novel eubacterial transporter family related to the 2-oxoglutarate/malate translocator from spinach chloroplasts. J. Bacteriol. 180:4160-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson, J. B., M. Singh, and P. A. Srere. 1976. Structure of the prosthetic group of Klebsiella aerogenes citrate (pro-3 S)-lyase. Proc. Natl. Acad. Sci. USA 73:1872-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 28.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider, K., P. Dimroth, and M. Bott. 2000. Biosynthesis of the prosthetic group of citrate lyase. Biochemistry 39:9438-9450. [DOI] [PubMed] [Google Scholar]
- 30.Schneider, K., P. Dimroth, and M. Bott. 2000. Identification of triphosphoribosyl-dephospho-CoA as precursor of the citrate lyase prosthetic group. FEBS Lett. 483:165-168. [DOI] [PubMed] [Google Scholar]
- 31.Singh, M., J. B. Robinson, and P. A. Srere. 1977. On the structure of the prosthetic group of citrate (pro-3 S)-lyase. J. Biol. Chem. 252:6061-6068. [PubMed] [Google Scholar]
- 32.Studier, F. W., and B. A. Moffatt. 1986. Use of T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189:113-130. [DOI] [PubMed] [Google Scholar]
- 33.Subramanian, S., and C. Sivaraman. 1984. Bacterial citrate lyase. J. Biosci. 6:379-401. [Google Scholar]
- 34.Van der Rest, M. E., E. Schwarz, D. Oesterhelt, and W. N. Konings. 1990. DNA sequence of a citrate carrier of Klebsiella pneumoniae. Eur. J. Biochem. 189:401-407. [DOI] [PubMed] [Google Scholar]
- 35.Van der Rest, M. E., T. Abee, D. Molenaar, and W. N. Konings. 1991. Mechanism and energetics of a citrate-transport system of Klebsiella pneumoniae. Eur. J. Biochem. 195:71-77. [DOI] [PubMed] [Google Scholar]
- 36.Van der Rest, M. E., R. M. Siewe, T. Abee, E. Schwarz, D. Oesterhelt, and W. N. Konings. 1992. Nucleotide sequence and functional properties of a sodium-dependent citrate transport system from Klebsiella pneumoniae. J. Biol. Chem. 267:8971-8976. [PubMed] [Google Scholar]
- 37.Van der Rest, M. E., D. Molenaar, and W. N. Konings. 1992. Mechanism of Na+-dependent citrate transport in Klebsiella pneumoniae. J. Bacteriol. 174:4893-4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Geest, M., and J. Lolkema. 2000. Membrane topology of the Na+/citrate transporter CitS of Klebsiella pneumoniae by insertion mutagenesis. Biochim. Biophys. Acta 1466:328-338. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto, H., M. Murata, and J. Sekiguchi. 2000. The CitST two-component system regulates the expression of the Mg-citrate transporter in Bacillus subtilis. Mol. Microbiol. 37:898-912. [DOI] [PubMed] [Google Scholar]
- 40.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequence of the M13mp18 and pUC19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 41.Zientz, E., J. Bongaerts, and G. Unden. 1998. Fumarate regulation of gene expression in Escherichia coli by the DcuSR (dcuSR genes) two-component regulatory system. J. Bacteriol. 180:5421-5425. [DOI] [PMC free article] [PubMed] [Google Scholar]