Abstract
A β-1,3-xylanase gene (txyA) from a marine bacterium, Alcaligenes sp. strain XY-234, has been cloned and sequenced. txyA consists of a 1,410-bp open reading frame that encodes 469 amino acid residues with a calculated molecular mass of 52,256 Da. The domain structure of the β-1,3-xylanase (TxyA) consists of a signal peptide of 22 amino acid residues, followed by a catalytic domain which belongs to family 26 of the glycosyl hydrolases, a linker region with one array of DGG and six repeats of DNGG, and a novel carbohydrate-binding module (CBM) at the C terminus. The recombinant TxyA hydrolyzed β-1,3-xylan but not other polysaccharides such as β-1,4-xylan, carboxymethylcellulose, curdlan, glucomannan, or β-1,4-mannan. TxyA was capable of binding specifically to β-1,3-xylan. The analysis using truncated TxyA lacking either the N- or C-terminal region indicated that the region encoding the CBM was located between residues 376 and 469. Binding studies on the CBM revealed that the Kd and the maximum amount of protein bound to β-1,3-xylan were 4.2 μM and 18.2 μmol/g of β-1,3-xylan, respectively. Furthermore, comparison of the enzymatic properties between proteins with and without the CBM strongly indicated that the CBM of TxyA plays an important role in the hydrolysis of β-1,3-xylan.
β-1,3-Xylan, a β-1,3-linked d-xylose homopolymer, exists in the cell walls of red algae (Porphyra and Bangia spp.) and green algae (Caulerupa, Bryopsis, and Udotea spp.) (12, 14). β-1,3-Xylanase (1,3-β-d-xylan xylanohydrolase; EC 3.2.1.32) hydrolyzes β-1,3-xylosidic linkages to produce various xylooligosaccharides. This enzyme is very useful for structural analysis of the cell walls of these algae and also for preparing protoplasts (1) to perform cell fusion and gene manipulation.
Many glycoside hydrolases (GHs), particularly those involved in the hydrolysis of polysaccharides, frequently display a modular structure featuring a catalytic module (or unit) attached to one or several ancillary noncatalytic modules (NCMs), whose precise functions are often unknown (7). The NCMs of GHs can be identified in many cases by sequence comparisons (9). As their catalytic modules can be classified into several families based on amino acid sequence similarities, the NCMs also form a number of different families. A classification of such modules, independent and complementary to that of catalytic modules, is needed for a more meaningful description of GHs. For the enzymes acting on polysaccharides, such as cellulose, chitin, starch, β-1,3-glucan, and β-1,4-xylan, several NCMs have been shown to promote the attachment of the enzyme to the polysaccharide matrix, thereby facilitating the degradation of crystalline polysaccharides.
Alcaligenes sp. strain XY-234 is a marine bacterium that secretes an extracellular β-1,3-xylanase into the growth medium in the presence of β-1,3-xylan. We have previously reported the purification, properties, and N-terminal amino acid sequence of the enzyme from this organism (2). We have also cloned and sequenced a β-1,3-xylanase gene (txyA) from another bacterium, Vibrio sp. strain XY-214 (3), but have not yet characterized the unknown domain of TxyA at its C-terminal region.
To further elucidate the structure and the molecular architecture of β-1,3-xylanases, we have cloned and sequenced a new β-1,3-xylanase gene from Alcaligenes sp. strain XY-234. The present paper provides data that the txyA gene belongs to family 26 of the GHs and that the enzyme encoded by this gene can specifically bind to and degrade β-1,3-xylan. We have also identified and characterized a carbohydrate-binding module (CBM) in TxyA, which should be classified as a novel CBM.
MATERIALS AND METHODS
Materials.
Avicel PH-101 was obtained from FMC Corp., Philadelphia, Pa., and p-nitrophenyl-β-d-xyloside and p-nitrophenyl-α- and β-d-mannoside were from Sigma Chemical Co., St. Louis, Mo. Carboxymethylcellulose (CMC) and curdlan were purchased from Wako Pure Chemical Industries. β-1,4-Xylan was from Seikagaku Kogyo (Tokyo, Japan). β-1,4-Mannan and glucomannan were prepared as described previously (23). β-1,3-Xylan was prepared from a green alga, Caulerpa racemosa var. laetevirens, by the method of Iriki et al. (12). Glycol β-1,3-xylan was prepared by the method of Yamaura et al. (27).
Bacterial strains and plasmids.
Alcaligenes sp. strain XY-234 isolated from sea mud was grown as described previously (2) and used as the source of chromosomal DNA. Escherichia coli DH5α (Stratagene) and E. coli BL21(DE3) (Novagen) were the host strains for cloning experiments and for the production of recombinant proteins, respectively. pBluescriptII (Stratagene) was used as a cloning vector, and pET21a and pET22b (Novagen) were used as the expression vectors.
Recombinant DNA techniques.
Chromosomal DNA from Alcaligenes sp. strain XY-234 was isolated by the method of Saito et al. (18). Plasmid DNA was purified with the Wizard Plus miniprep DNA purification system (Promega). Restriction endonucleases were purchased from Takara (Kyoto, Japan) and Toyobo (Tokyo, Japan) and used in accordance with the manufacturer's specifications. DNA ligation was carried out by the Ligation Kit Ver.2 (Takara). DNA fragments were recovered after electrophoresis with a GeneClean II kit (Bio 101 Inc.).
To obtain the txyA gene from Alcaligenes sp. strain XY-234, Southern hybridization analysis was carried out for restriction enzyme digestion fragments of chromosomal DNA from strain XY-234 using the β-1,3-xylanase gene of Vibrio sp. strain XY-214 (3) labeled with AlkPhos Direct (Pharmacia, Uppsala, Sweden) as a probe. The 5-kb ClaI and 2-kb NheI digestion fragments that hybridized with the probe were ligated into the dephosphorylated ClaI and XbaI sites of pBluescriptII SK (−), respectively. These ligation mixtures were incubated at 4°C overnight and then transformed into competent E. coli DH5α by the method of Sambrook et al. (20).
TxyA activity and protein assays.
The enzyme solution (0.1 ml) was added to a mixture of 0.4 ml of 200 mM 2-morpholinoethanesulfonic acid (MES)-NaOH buffer (pH 7.0) and 0.5 ml of 1% β-1,3-xylan solution. After incubating at 37°C for 10 min, the reducing sugar generated was measured by the Somogyi-Nelson method (22) and expressed as xylose. One unit of enzyme activity was defined as the amount of enzyme that liberated 1 μmol of d-xylose per min under the above conditions.
Protein concentrations were measured by the method of Bradford (6) using bovine serum albumin as a standard.
Protein purification.
Native TxyA from Alcaligenes sp. strain XY-234 was purified as described previously (2). To express the recombinant enzymes, E. coli BL21(DE3) was grown at 37°C in 3 liters of Luria-Bertani (LB) medium (20) supplemented with 100 μg of ampicillin per ml. When the A600 reached 0.6, the culture was cooled at 25°C, and isopropyl-β-d-thiogalactopyranoside was added to a final concentration of 1 mM. Incubation was continued for another 3 h at 25°C, and the periplasmic fraction of the harvested cells was used as the crude enzyme. The crude enzyme solution derived from the recombinant pET21a was purified by a combination of Q Sepharose FF and Ether-Toyopearl 650S column chromatographies. His-tagged proteins from the recombinant pET22b were purified by HiTrap chelating (Pharmacia).
SDS-PAGE, zymogram, and Western blot analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed on a 12.5% polyacrylamide gel by the method of Laemmli (13). After electrophoresis, the gel was stained with Coomassie brilliant blue R. A low-molecular-weight SDS calibration kit (Pharmacia) was used as the standard. Zymogram was carried out by the method of Beguin (5). Western blot (immunoblot) analysis was performed by using a polyclonal anti-TxyA antiserum raised against β-1,3-xylanase (TxyA) of Vibrio sp. strain XY-214.
TLC analysis.
Purified enzyme was incubated with 1% polysaccharide solutions containing β-1,3-xylan, β-1,4-xylan, curdlan, CMC, β-1,4-mannan, or glucomannan at 37°C. The reaction products were separated on a thin-layer chromatography (TLC) plate (Merck, Darmstadt, Germany) with a solvent system consisting of n-butanol-acetic acid-water (10:5:1). For detection of the products, the plates were sprayed with the diphenylamine-aniline-phosphate reagent (4) and baked for 10 min at 100°C.
Determination of the CBM-β-1,3-xylan dissociation constant and the β-1,3-xylan-binding capacity.
For quantitative analysis, the reaction mixture for the β-1,3-xylan-binding assay contained 1 mg of β-1,3-xylan and appropriate amounts of protein (100 to 500 μg/ml [total protein]) in a final volume of 1 ml of 50 mM Tris-HCl buffer (pH 7.5). After the mixture was incubated at 4°C for 1 h with slow vertical rotation (10 rpm), β-1,3-xylan was removed by centrifugation, and the concentration of unbound protein ([P], micromolar) in the supernatant was measured by the Bradford method (6). The bound protein concentration ([PC], in micromoles per gram of β-1,3-xylan) was determined by subtracting [P] from the total protein concentration. All assays were carried out in triplicate. Adsorption parameters were obtained by using the equation of Sakoda and Hiromi (19), [PC] = [P] [PC]max/(Kd + [P]), where Kd (micromolar) and [PC]max (micromoles per gram of β-1,3-xylan) are the equilibrium dissociation constant and maximum amount of protein bound, respectively.
For qualitative analysis, the assay tubes contained 0.5 mg of β-1,3-xylan and 2 μg of protein in 0.5 ml of 50 mM Tris-HCl buffer (pH 7.5). After incubation at 4°C for 1 h with vertical rotation, the assay tubes were centrifuged to sediment the β-1,3-xylan. After removing the buffer, the pellets were washed three times with 1 ml of 50 mM Tris-HCl buffer (pH 7.5) containing 1 M NaCl. The pellets were then resuspended in 20 μl of SDS sample buffer and analyzed by SDS-PAGE.
Determination of binding to other polysaccharides.
Avicel (β-1,4-glucan), curdlan (β-1,3-glucan), β-1,4-mannan, and β-1,4-xylan were tested to determine whether they bind to CBM protein. The method used was the same as that used in determining binding to β-1,3-xylan.
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper have been submitted to GenBank under accession no. AB039953.
RESULTS AND DISCUSSION
Nucleotide sequence of txyA and amino acid sequence of TxyA.
The txyA gene consists of 1,410 nucleotides encoding a protein of 469 amino acids with a predicted molecular mass of 52,256 Da. The putative initiation codon ATG was preceded by a spacing of 7 bp by a bacterial ribosome-binding sequence, AGGAAG, which was homologous to the consensus Shine-Dalgarno sequence. A rho-independent transcription termination signal was present 75 bp downstream of the translation stop codon TAA (17). The N-terminal amino acid sequence of TxyA exhibited a typical signal peptide and consensus sequence (Ala-X-Ala) (26) in which the predicted cleavage site is located between position 22 (Ala) and position 23 (Leu). The first 13 N-terminal amino acids of native TxyA were found to be LDGKLVPNEGVLV (2), which is identical to the deduced amino acids from positions 23 to 35. Therefore, removal of the signal peptide yields a mature protein with 447 amino acids and a molecular mass of 50,011 Da.
The deduced amino acid sequence encoded by the txyA gene was compared with protein sequences in the GenBank and EMBL databases as well as those in the literature. Based on the amino acid sequence similarity, TxyA was predicted to consist of a signal sequence followed by a catalytic domain, a linker sequence, and an unknown domain at the C terminus. The whole amino acid sequence of TxyA revealed 84.6% identity and 92.3% similarity with TxyA of Vibrio sp. strain XY-214 (3).
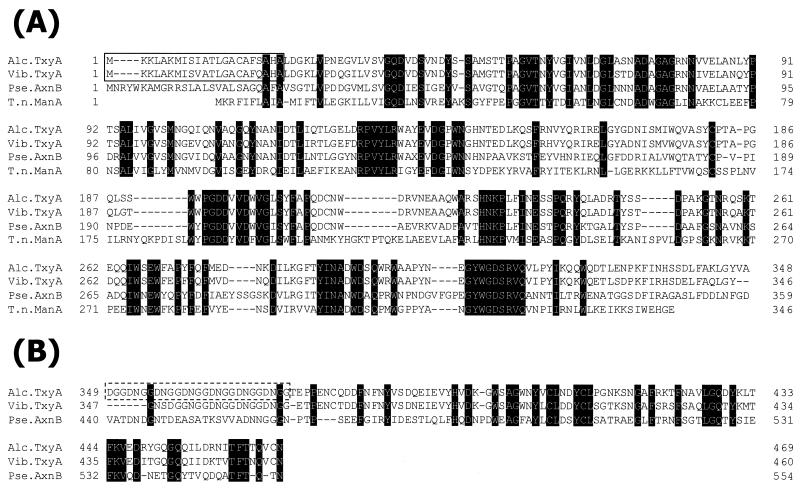
The N-terminal region (residues 23 to 348) of strain XY-234 TxyA contained a putative catalytic domain which was homologous to a member of the family 26 β-mannanases (11), i.e., Thermotoga neapolitana ManA (39.0% identity and 57.7% similarity among 322 amino acids; accession no. U58632) (Fig. 1A), Caldicellulosiruptor saccharolyticus ManA (24.7% identity and 45.2% similarity among 93 amino acids; accession no. U39812) (8), Clostridium thermocellum ManA (26.7% identity and 46.5% similarity among 101 amino acids; accession no. AB044406) (10) and Man26B (26.5% identity and 44.6% similarity among 83 amino acids; accession no. AJ242666), Dictyoglomus thermophilum ManA (25.8% identity and 44.9% similarity among 89 amino acids; accession no. AF013989), and Rhodothermus marinus ManA (31.1% identity and 48.9% similarity among 90 amino acids; accession no. X90947) (16). In particular, the putative catalytic domain of TxyA from strain XY-234 showed significant homology with β-1,3-xylanases such as Vibrio sp. strain XY-214 TxyA (88.8% identity and 96.3% similarity among 322 amino acids; accession no. AB029043) (3) and Pseudomonas sp. strain ND137 AxnB (57.2% identity and 71.4% similarity among 332 amino acids; accession no. AB063257) (Fig. 1A).
FIG. 1.
(A) Sequence alignment of the N-terminal regions of TxyA from Alcaligenes sp. strain XY-234 (Alc.TxyA), TxyA from Vibrio sp. strain XY-214 (Vib.TxyA), AxnB from Pseudomonas sp. strain ND137 (Pse.AxnB), and ManA from Thermotoga neapolitana (T.n.ManA). (B) Sequence alignment of the linker and CBM regions of TxyA from Alcaligenes sp. strain XY-234 with the putative linker and CBM regions of TxyA from Vibrio sp. strain XY-214 and AxnB from Pseudomonas sp. strain ND137. The conserved amino acid residues are highlighted. Gaps left to improve the alignment are indicated by dashes. The numbers refer to amino acid residues at the start of the respective lines; all sequences are numbered from Met-1 of the peptide. The signal peptides from amino acids 1 to 22 of Alcaligenes sp. strain XY-234 TxyA and Vibrio sp. strain XY-214 TxyA are boxed. The linker region containing six repeats of DNGG in Alcaligenes TxyA is boxed with a broken line.
As shown in Fig 1B, the C-terminal region (residues 376 to 469) of TxyA from strain XY-234 shows 77.4% identity and 87.1% similarity among 93 amino acids with Vibrio sp. strain XY-214 TxyA (3). It also shows 35.4% identity and 57.3% similarity among 82 amino acids with Pseudomonas sp. strain ND137 AxnB. The function of the C-terminal domain is still unknown. The glycine-rich region (residues 349 to 375), containing one array of DGG and six repeats of DNGG, is similar to some linker regions of β-1,4-xylanase XylD from Cellulomonas fimi (15) and β-1,4-xylanases STX-I and STX-II from Streptomyces thermoviolaceus OPC-520 (25). Interestingly, all these enzymes possess a CBM downstream of the linker region.
CBMs are discrete protein modules found in a large number of carbohydrolases and a few nonhydrolytic proteins such as cellulosomal scaffoldin proteins (21). In rare instances, independent putative CBMs have also been described (24). To date, about 200 different CBMs have been identified and classified into 29 families according to their similarities in aminoacid sequence (http://afmb.cnrs-mrs.fr/∼pedro/CAZY/index.html).
Biochemical properties of rTxyA.
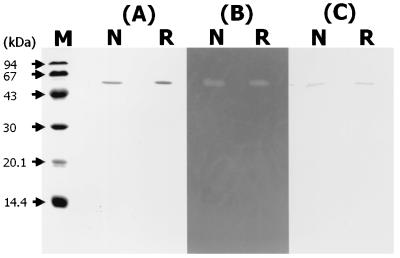
The native TxyA and recombinant TxyA (rTxyA) were purified by successive column choromatographies as described in Materials and Methods. The yields of the purified TxyA and rTxyA were 0.11 mg/liter and 15.7 mg/liter of culture medium, respectively. As shown in Fig. 2A and B, both the native and recombinant enzymes moved as a single protein band in SDS-PAGE (59,000 Da) and exhibited β-1,3-xylanase activities in a zymogram. The molecular mass of the purified rTxyA was found to be very close to that of the native enzyme. By Western blot analysis, the native and recombinant enzymes showed cross-reactivity with anti-TxyA antibodies prepared against β-1,3-xylanase (TxyA) of Vibrio sp. strain XY-214 (Fig. 2C). Thus, both enzymes were proved to be immunologically similar to TxyA of strain XY-214. The rTxyA of strain XY-234 exhibited an optimum pH of 7.0 and was stable in the pH range of 5.0 to 11 at 4°C for 24 h. The optimum temperature for rTxyA activity at pH 7.0 was found to be 40°C, and the activity was stable at 40°C for 10 min.
FIG. 2.
Analysis of purified native TxyA and rTxyA by SDS-PAGE (A), zymogram (B), and Western blotting (immunoblotting) (C). Lane M, size standards (low-molecular-weight SDS calibration kit; Pharmacia) (phosphorylase b [94 kDa], albumin [67 kDa], ovalbumin [43 kDa], carbonic anhydrase [30 kDa], trypsin inhibitor [20.1 kDa], and α-lactalbumin [14.4 kDa]); lanes N, native TxyA; lanes R, rTxyA.
The activity pattern of rTxyA was determined with several polysaccharides. The reaction solution containing 9.4 μg of purified rTxyA and 1% polysaccharide was incubated for 24 h at 37°C. TLC analysis revealed that rTxyA hydrolyzed β-1,3-xylan to produce a series of xylooligosaccharides, with xylobiose and xylotriose as the main products. The enzyme did not cleave CMC, curdlan, glucomannan, β-1,4-mannan, or β-1,4-xylan. It also did not hydrolyze p-nitrophenyl-β-d-xyloside or p-nitrophenyl-α- and β-d-mannoside (data not shown).
Identification of CBM.
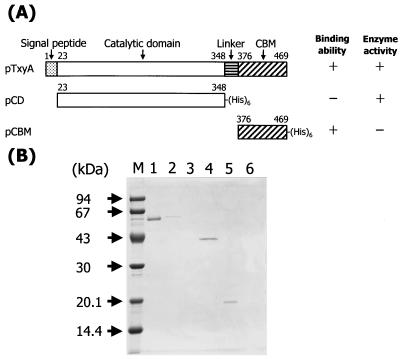
To clarify whether the unknown domain at the C-terminal region of TxyA is a CBM, we constructed two deletion mutants: pCD, containing the N-terminal region from Leu23 to Ala348, and pCBM, containing the C-terminal region from Thr376 to Gln469, based on the amino acid sequence. By using these truncated proteins and the original rTxyA, we assayed β-1,3-xylanase activity and β-1,3-xylan-binding ability according to the procedures described in Materials and Methods. As shown in Fig. 3A, rTxyA exhibited both catalytic activity with and ability to bind β-1,3-xylan, while the protein expressed by pCD (CD) had catalytic activity but no binding ability and the protein expressed by pCBM (CBM) had binding ability without catalytic activity. Moreover, β-1,3-xylan-bound protein could not be set free by washing with 1 M NaCl. SDS-PAGE analysis indicating that both rTxyA and CBM could bind to β-1,3-xylan, but this was not the case for CD (Fig. 3B). Therefore, it was evident that the unknown domain in TxyA is a CBM and that TxyA consists of two domains, the catalytic domain at the N-terminal region (residues 23 to 348) and the CBM at the C-terminal region (residues 376 to 469).
FIG. 3.
(A) Schematic representation of the domain structure of TxyA. Binding ability was measured by a β-1,3-xylan-binding assay, and enzyme activity toward β-1,3-xylan was detected by the release of reducing sugar equivalent (12). + and − indicate binding ability or β-1,3-xylanase activity for β-1,3-xylan. The numbers refer to amino acid residues of TxyA. (B) SDS-PAGE analysis of β-1,3-xylan binding by the recombinant proteins. Lane M, molecular mass markers; lanes 1 and 2, rTxyA; lanes 3 and 4, the purified protein expressed by pCD; lanes 5 and 6, the purified protein expressed by pCBM. Each protein was mixed and incubated with β-1,3-xylan. After centrifugation, the concentrated supernatants (lanes 2, 4, and 6) and the precipitates (lanes 1, 3, and 5) containing β-1,3-xylan-bound proteins were mixed with SDS sample buffer and analyzed by SDS-12.5% PAGE.
To compare the function of the proteins with and without the CBM, we determined the enzymatic properties of rTxyA and CD. The kinetic parameters obtained from Lineweaver-Burke plots showed a straight line within experimental error (Table 1). The Km value of CD for β-1,3-xylan was 2.41-fold higher than that of rTxyA, while the difference in Km value between CD and rTxyA for glycol β-1,3-xylan was small. The overall catalytic efficiency (kcat/Km) of rTxyA for β-1,3-xylan was 2.14-fold higher than that of CD. These results indicate that the CBM of TxyA contributes to the rate of depolymerization through increased binding to the polymeric substrate.
TABLE 1.
Kinetic parameters of rTxyA and CD with β-1,3-xylan and glycol β-1,3-xylana
| Enzyme | β-1,3-Xylan
|
Glycol β-1,3-xylan
|
||||
|---|---|---|---|---|---|---|
| Km (mg/ml) | kcat (s−1) | kcat/Km | Km (mg/ml) | kcat (s−1) | kcat/Km | |
| rTxyA | 4.86 | 14.12 | 2.91 | 0.47 | 3.96 | 8.52 |
| CD | 11.72 | 15.98 | 1.36 | 0.73 | 4.32 | 5.88 |
Data are the mean values of three independent experiments. The assay was carried out with 1.0 nmol of rTxyA and 1.3 nmol of CD.
TxyA from Vibrio sp. strain XY-214 was also found to have β-1,3-xylan-binding ability (unpublished data). The CBM that has been defined here for TxyA from the Alcaligenes sp. likely has an analog in the TxyA from the Vibrio sp. Therefore, the CBMs of both the Alcaligenes and Vibrio enzymes should be classified into a new CBM family.
Characterization of CBM.
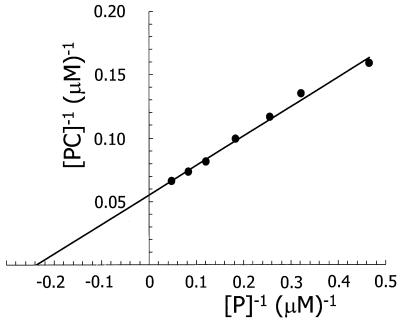
Figure 4 shows a typical double reciprocal plot for the binding of pure CBM to β-1,3-xylan. Within experimental error, the plots were linear, yielding a Kd of about 4.2 μM and [PC] max of 18.2 μmol of CBM bound per g of β-1,3-xylan. The latter value corresponds to approximately 221 mg of CBM protein per g of β-1,3-xylan. The linearity of the plot suggests that there is only one type of CBM-β-1,3-xylan interaction and that the CBM of TxyA had a high affinity for β-1,3-xylan.
FIG. 4.
Double-reciprocal plot of CBM binding to β-1,3-xylan. The assay conditions are described in Materials and Methods.
The ability of CBM to bind several polysaccharides, such as Avicel (β-1,4-glucan), curdlan (β-1,3-glucan), β-1,4-mannan, β-1,3-xylan, and β-1,4-xylan, was investigated. The CBM did not show measurable binding to these polysaccharides except for β-1,3-xylan. To determine whether soluble carbohydrates competed with β-1,3-xylan for the CBM protein, d-xylose, β-1,3-xylooligosaccharides, and glycol β-1,3-xylan were included in some assays at four times the weight per volume of β-1,3-xylan (1 mg of β-1,3-xylan and 4 mg of soluble carbohydrate per ml of assay mix). No appreciable differences in Kd or [PC]max were observed, indicating that these soluble carbohydrates had little or no effect on the binding of CBM to β-1,3-xylan.
By expression of the individual domains and activity measurements of the protein products, we have demonstrated that the unknown domain at the C terminus represents a novel CBM capable of specifically recognizing β-1,3-xylosyl linkages. The validity of the assay is supported by the observation that [PC]max increases linearly with the amount of β-1,3-xylan used, while Kd is independent of the amount of β-1,3-xylan. Since xylose and β-1,3-xylooligosaccharides had no effect on the binding between CBM and β-1,3-xylan, the CBM recognition site is not governed simply by the β-1,3-xylooligosaccharide chain. A specific three-dimensional arrangement of β-1,3-linked xylose chains must be needed for this interaction. Also, as a result of the comparison of the enzymatic properties between rTxyA and the protein expressed by pCD, removal of the CBM from TxyA caused a significant increase in the Km of TxyA toward β-1,3-xylan. These results indicate that this CBM plays an important role in the hydrolysis of insoluble substrates such as β-1,3-xylan.
Acknowledgments
We thank Roy H. Doi, Section of Molecular and Cellular Biology, University of California, Davis, for useful discussions and suggestions.
Y.-T. Li was supported by National Institutes of Health grant NS 09626.
REFERENCES
- 1.Araki, T., M. Hayakawa, Y. Tamaru, K. Yoshimatu, and T. Morishita. 1994. Isolation and regeneration of haploid protoplasts from Bangia atropurpurea (Rhodophyta) with marine bacterial enzymes. J. Phycol. 30:1040-1046. [Google Scholar]
- 2.Araki, T., N. Inoue, and T. Morishita. 1998. Purification and characterization of β-1,3-xylanase from a marine bacterium, Alcaligenes sp. XY-234. J. Gen. Appl. Microbiol. 44:269-274. [DOI] [PubMed] [Google Scholar]
- 3.Araki, T., S. Hashikawa, and T. Morishita. 2000. Cloning, sequencing, and expression in Escherichia coli of the new gene encoding β-1,3-xylanase from a marine bacterium, Vibrio sp. strain XY-214. Appl. Environ. Microbiol. 66:1741-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey, R. W., and E. J. Bourne. 1960. Colour reagents given by sugars and diphenylamineaniline spray reagents on paper chromatograms. J. Chromatogr. 4:206-213. [Google Scholar]
- 5.Beguin, P. 1983. Detection of cellulase activity in polyacrylamide gels using Congo red-stained agar replicas. Anal. Biochem. 131:333-336. [DOI] [PubMed] [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Coutinho, P. M., and B. Henrissat. 1999. The molecular structure of cellulases and other carbohydrate-active enzymes: an integrated database approach, p. 15-23. In K. Ohmiya, K. Hayashi, K. Sakka, Y. Kobayashi, S. Karita, and T. Kimura (ed.), Genetics, biochemistry and ecology of cellulose degradation. Uni Publishers, Tokyo, Japan.
- 8.Gibbs, M. D., A. U. Elinder, R. A. Reeves, and P. L. Bergquist. 1996. Sequencing, cloning and expression of a β-1,4-mannanase gene, manA, from the extremely thermophilic anaerobic bacterium, Caldicellulosiruptor Rt8B.4. FEMS Microbiol. Lett. 141:37-43. [DOI] [PubMed] [Google Scholar]
- 9.Gilkes, N. R., B. Henrissat, D. G. Kilburn, R. C. Miller Jr., and R. A. Warren. 1991. Domains in microbial β-1,4-glycanases: sequence conservation, function, and enzyme families. Microbiol. Rev. 55:303-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halstead, J. R., P. E. Vercoe, H. J. Gilbert, K. Davidson, and G. P. Hazlewood. 1999. A family 26 mannanase produced by Clostridium thermocellum as a component of the cellulosome contains a domain which is conserved in mannanases from anaerobic fungi. Microbiology 145:3101-3108. [DOI] [PubMed] [Google Scholar]
- 11.Henrissat, B., and A. Bairoch. 1993. New families in the classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 293:781-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iriki, Y., T. Suzuki, K. Nisizawa, and T. Miwa. 1960. Xylan of siphonaceous green algae. Nature 187:82-83. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 14.McDowell, R. H. 1967. Chemistry and enzymology of marine algal polysaccharides, p. 88-96, 134-137. Academic Press, London, United Kingdom.
- 15.Millward-Sadler, S. J., D. M. Poole, B. Henrissat, G. P. Hazlewood, J. H. Clarke, and H. J. Gilbert. 1994. Evidence for a general role for high-affinity noncatalytic cellulose binding domains in microbial plant cell wall hydrolases. Mol. Microbiol. 11:375-382. [DOI] [PubMed] [Google Scholar]
- 16.Politz, O., M. Krah, K. K. Thomsen, and R. Borriss. 2000. A highly thermostable endo-(1,4)-β-mannanase from the marine bacterium Rhodothermus marinus. Appl. Microbiol. Biotechnol. 53:715-721. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg, M., and D. Court. 1979. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu. Rev. Genet. 13:319-353. [DOI] [PubMed] [Google Scholar]
- 18.Saito, H., and K. Miura. 1963. Preparation of transforming deoxyribonucleic acid by phenol-treatment. Biochim. Biophys. Acta 72:619-629. [PubMed] [Google Scholar]
- 19.Sakoda, M., and K. Hiromi. 1976. Determination of the best-fit values of kinetic parameters of the Michaelis-Menten equation by the method of least squares with the Taylor expansion. J. Biochem. (Tokyo) 80:547-555. [DOI] [PubMed] [Google Scholar]
- 20.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 21.Shoham, Y., R. Lamed, and E. A. Bayer. 1999. The cellulosome concept as an efficient microbial strategy for the degradation of insoluble polysaccharides. Trends Microbiol. 7:275-281. [DOI] [PubMed] [Google Scholar]
- 22.Somogyi, M. 1952. Notes on sugar determination. J. Biol. Chem. 195:19-23. [PubMed] [Google Scholar]
- 23.Tamaru, Y., T. Araki, H. Amagoi, H. Mori, and T. Morishita. 1995. Purification and characterization of an extracellular β-1,4-mannanase from a marine bacterium, Vibrio sp. strain MA-138. Appl. Environ. Microbiol. 61:4454-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomme, P., R. A. J. Warren, R. C. Miller, D. G. Kilburn, and N. R. Gilkes. 1995. Cellulose binding domains: classification and properties, p. 142-161. In J. N. Saddler and M. H. Penner (ed.), Enzymatic degradation of insoluble carbohydrates. American Chemical Society, Washington, D.C.
- 25.Tsujibo, H., T. Ohtsuki, T. Iio, I. Yamazaki, K. Miyamoto, M. Sugiyama, and Y. Inamori. 1997. Cloning and sequence analysis of genes encoding xylanases and acetyl xylan esterase from Streptomyces thermoviolaceus OPC-520. Appl. Environ. Microbiol. 63:661-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Heijne, G. 1986. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 14:4683-4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaura, I., T. Matsumoto, M. Funatsu, and E. Mukai. 1990. Purification and some properties of endo-1,3-β-d-xylanase from Pseudomonas sp. PT-5. Agric. Biol. Chem. 54:921-926. [PubMed] [Google Scholar]