Abstract
Oligonucleotide- and cDNA-based microarrays comprising a subset of Neisseria meningitidis genes were assessed for study of the meningococcal heat shock response and found to be highly suitable for transcriptional profiling of N. meningitidis. Employing oligonucleotide arrays encompassing the entire genome of N. meningitidis, we analyzed the meningococcal heat shock response on a global scale and identified 55 heat shock-deregulated open reading frames (34 induced and 21 repressed).
Sequencing of the genomes of Neisseria meningitidis serogroup A and serogroup B strains provided us with a tremendously broad range of information (15, 23). The next step is the elucidation of gene expression patterns and gene product function on a genome-wide scale. DNA microarrays offer an ideal tool for high-throughput investigation of gene regulation on the transcriptional level (for review, see references 4, 13, and 17). The two most commonly employed DNA microarray platforms are oligonucleotide and cDNA arrays. Here we performed a comparative analysis of the suitability of both technology platforms for transcriptional profiling of N. meningitidis.
The two main features of DNA microarray performance are sensitivity (signal intensity) and specificity (ratio of specific to nonspecific hybridization). Additional care must be taken to standardize experimental conditions and to avoid the detection of false-positive signals (12). In order to validate gene expression modulations of N. meningitidis observed using cDNA-based and oligonucleotide-based microarrays, we first performed parallel hybridizations of identical RNA samples to the same slide. Microarrays containing probes specific for 60 genes selected from the published genome sequence of N. meningitidis serogroup B strain MC58 (23) were produced (Table 1). For cDNA-arrays, internal fragments of each open reading frame (ORF) (300 to 560 bp) were PCR amplified. For oligonucleotide arrays, oligonucleotides (40-mers, three per gene) comprising gene-specific internal fragments (covering 5′, central, and 3′ parts) were designed. All oligonucleotides (manufactured by MWG-Biotech AG, Ebersberg, Germany) carried a C6 amino linker modification at the 5′ end for covalent attachment to the slide surface. Each probe was spotted 5 (oligonucleotides) or 10 times (PCR products) per array using the Affymetrix 417 Arrayer (MWG-Biotech AG). PCR products were spotted on CMT-GAPS-Coated Slides (Corning, Wiesbaden, Germany), oligonucleotides were spotted on Super Aldehyde Slides (TeleChem International, Sunnyvale, Calif.), and the slides were processed according to the manufacturers' instructions.
TABLE 1.
N. meningitidis serogroup B genes present on PCR product and oligonucleotide arrays comprising a subset of meningococcal ORFs
| Locia for: | Product (gene name) |
|---|---|
| Genes relevant under heat shock conditions | |
| NMB0027 | FkbP-type peptidyl-prolyl cis-trans isomerase (fbp) |
| NMB0059 | Heat shock protein (dnaJ) |
| NMB0550 | Thiol/disulfide interchange protein (dsbC) |
| NMB0554 | Heat shock protein 70 (dnaK) |
| NMB0791 | Peptidyl-prolyl cis-trans isomerase |
| NMB1027 | Truncation |
| NMB1131 | Chaperone protein HscA (hscA-1) |
| NMB1313 | Trigger factor (tig) |
| NMB1366 | Thioredoxin |
| NMB1519 | Thiol disulfide interchange protein (dsbD) |
| NMB1522 | Peptidyl-prolyl cis-trans isomerase, FkbP-type (slyD) |
| NMB1649 | Disulfide bond formation protein B (dsbB) |
| NMB1845 | Thioredoxin |
| NMB1972 | Heat shock protein (groEL) |
| NMB1973 | Chaperonin (groES) |
| Competence/transformation genes | |
| NMB0018 | Pilin, PilE (pilE) |
| NMB0049 | PilC2 protein, authentic frameshift (pilC2) |
| NMB0116 | DNA processing chain A (dprA) |
| NMB0118 | DNA topoisomerase (topA) |
| NMB0269 | Competence protein |
| NMB0329 | Type IV pilus assembly protein (pilF) |
| NMB0332 | Type IV prepilin peptidase (pilD) |
| NMB0405 | Competence protein (comM) |
| NMB0692 | Tpc protein (tpc) |
| NMB0702 | Competence protein A (comA) |
| NMB0703 | Competence protein L (comL) |
| NMB1445 | RecA protein (recA) |
| NMB1588 | CDP-diacylglycerol-glycerol-3-phosphate 3-phosphatidyltransferase (pgsA) |
| NMB1715 | Multiple transferable resistance system protein MtrD (mtrD) |
| NMB1808 | PilM protein (pilM) |
| NMB1809 | PilN protein (pilN) |
| NMB1810 | PilO protein (pilO) |
| NMB1811 | PilP protein (pilP) |
| NMB1812 | PilQ protein (pilQ) |
| NMB1847 | PilC1 protein, authentic frameshift (pilC1) |
| NMB1936 | ATP synthase F1 subunit (atpA) |
| NMB1940 | ATP synthase F0 subunit (atpB) |
| NMB2044 | Phosphoenolpyruvate protein phosphotransferase (ptsl) |
| Genes encoding restriction/modification systems | |
| NMB0829 | Type I restriction enzyme EcoR124II M protein (hsdM) |
| NMB0831 | Type I restriction enzyme S protein, degenerate |
| NMB0835 | Type I restriction enzyme EcoR124II R protein |
| NMB1289 | Type II restriction enzyme |
| NMB1375 | Modification methylase, putative, authentic frameshift |
| Housekeeping genes | |
| NMB0207 | Glyceraldehyde 3-phosphate dehydrogenase (gapA-1) |
| NMB0955 | 2-Oxoglutarate dehydrogenase, E1 component (sucA) |
| NMB1341 | Pyruvate-dehydrogenase pdhA |
| NMB2159 | Glyceraldehyde 3-phosphate dehydrogenase (gapA-2) |
| NMB0950 | Succinate dehydrogenase, flavoprotein subunit (sdhA) |
| NMB0951 | Succinate dehydrogenase, iron-sulfur protein (sdhB) |
| Sigma factors | |
| NMB1538 | RNA polymerase sigma-70 factor (rpoD) |
| NMB0712 | RNA polymerase sigma-32 factor (rpoH) |
| NMB2044 | Sigma factor |
| NMB2144 | Sigma factor |
| nqr operon | |
| NMB0564 | Na(+)-translocating NADH-quinone reductase, subunit F (nqrF) |
| NMB0565 | Na(+)-translocating NADH-quinone reductase, subunit E (nqrE) |
| NMB0566 | Na(+)-translocating NADH-quinone reductase, subunit D (nqrD) |
| NMB0567 | Na(+)-translocating NADH-quinone reductase, subunit C (nqrC) |
| NMB0568 | Na(+)-translocating NADH-quinone reductase, subunit B (nqrB) |
| NMB0569 | Na(+)-translocating NADH-quinone reductase, subunit A (nqrA) |
| NO reductase | |
| NMB1622 | Nitric oxide reductase (norB) |
ORF numbers in the published N. meningitidis serogroup B genome sequence (23) are given.
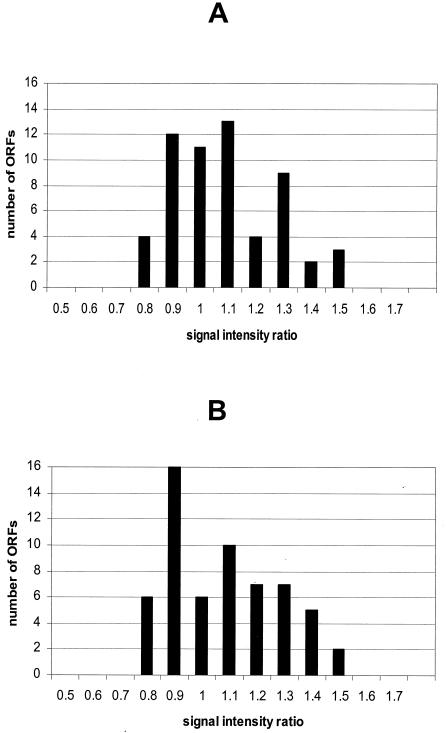
Cultures of N. meningitidis strain MC58 (24) were grown to mid-logarithmic growth phase (optical density at 600 nm [OD600] = 0.5/5 × 108 CFU/ml) at 37°C in supplemented proteose peptone medium and RNA isolated as previously described (5). The RNA was split into two aliquots, and one-half was labeled with Cy3-dCTP, the other with Cy5-dCTP (Amersham Pharmacia, Freiburg, Germany) during a first-strand reverse transcription (RT) reaction using Superscript II RNase H− reverse transcriptase (Life Technologies, Karlsruhe, Germany) and a balanced mixture (1 pmol each) of C-terminal primers specific for all genes present on the microarrays. Before performance of the RT reaction, four different Saccharomyces-specific in vitro-derived transcripts (CHS1, CHS2, GAS1, and FKS1) were added as internal standards to the two labeling reaction mixtures in defined ratios. The two differentially labeled cDNA samples were mixed and again split in halves for hybridization to a cDNA array and an oligonucleotide array under identical conditions (3× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 0.1% [wt/vol] sodium dodecyl sulfate [SDS], 50°C) for 16 h. Arrays were washed and scanned using the Affymetrix 418 Scanner (MWG-Biotech AG). Average signal intensity and local background measurements were obtained for each spot using ImaGene 4.0 software (Biodiscovery Inc., Los Angeles, Calif.). The two channels were normalized with respect to the mean values of all N. meningitidis DNA spots. The Cy3/Cy5 fluorescence ratios were calculated from the normalized values, and the average signal ratios of all replicates per gene were determined (Fig. 1). Under identical conditions, all probes present should exhibit signals at a 1:1 ratio. On the cDNA arrays, the majority of genes (91%) were present at ratios ranging from 0.8 to 1.3, and five ORFs were present at ratios of 1.4 or 1.5 (Fig. 1A). Utilizing oligonucleotide arrays, 88% of the ORFs were present at ratios of 0.8 to 1.3, and seven ORFs were present at a ratio of 1.4 to 1.5 (Fig. 1B). The standard deviation was 0.19 for the PCR product arrays, and for oligonucleotide arrays, 0.20 for the combined three oligonucleotides per gene. Standard deviation and fluorescence intensity were inversely correlated for both array types. Signal intensity ratios of Saccharomyces internal standards reflected their ratios in the RNA samples (data not shown).
FIG. 1.
Performance of cDNA-based versus oligonucleotide-based microarrays. RNA was isolated from a single N. meningitidis serogroup B culture, the RNA was split, and the halves were labeled independently. cDNAs were combined and split again for competitive hybridization with cDNA (A) and oligonucleotide arrays (B). After hybridization and washes, arrays were scanned and images were normalized. Histograms of average hybridization intensity ratios for individual ORFs are shown.
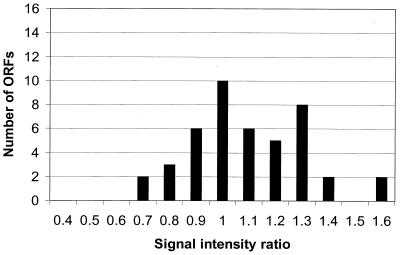
We next analyzed the standard deviation for RNAs isolated from different cultures of N. meningitidis. RNAs were isolated from cultures at mid-logarithmic growth phase (OD600 = 0.5) grown on different days. RNAs were labeled differentially and employed for competitive hybridization of cDNA microarrays (Fig. 2). The majority of ORFs exhibited signal ratios ranging from 0.8 to 1.2 (68%), some ORFs had a signal ratio of 0.7, and several had ratios of 1.3 to 1.6; the standard deviation was 0.21. The standard deviation of the microarray data is therefore mainly due to labeling and hybridization of RNAs; differences in culture conditions or RNA isolation have a minor impact. Based on these results, a 2-fold deregulation (equivalent to 3.29-fold log2 of the standard deviation of our experimental setting) has a level of confidence of above 99.9%, and a 1.7-fold deregulation (2.58-fold standard deviation) exhibits a confidence level of 99%. Usually, changes above 2-, 3-, and 4-fold variation are considered significant (25).
FIG. 2.
Reproducibility of N. meningitidis cultures. RNAs were isolated from two neisserial cultures at mid-logarithmic growth phase (OD600 = 0.5) grown on different days. RNAs were labeled differentially and employed for competitive hybridization of cDNA arrays. A histogram of hybridization intensities for individual ORFs is shown.
Sensitivity and specificity of PCR product and oligonucleotide arrays were compared for the analysis of the heat shock response of N. meningitidis. Meningococcal cultures were grown at 37°C to an OD600 of 0.5 and split into halves; one half was subjected to heat shock at 44°C for 5 min, and the other half remained at 37°C. RNA was isolated from both cultures 10 min post-heat shock. RNAs were labeled differentially, combined, and hybridized to cDNA and oligonucleotide microarrays. We found both array types to reproducibly detect ratios of transcript levels (Table 2). Eight of sixty ORFs were identified as being deregulated more than twofold by PCR product-based arrays; of these, six were also detected by oligonucleotide arrays. On the other hand, two ORFs were deregulated only according to the data analysis with oligonucleotide arrays. For the ORFs identified as deregulated by both array platforms, the levels of deregulation were in excellent concordance. The majority of ORFs were detected by both platforms as being not deregulated (83%). Hence, there is a very good agreement of the data for oligonucleotide and cDNA microarrays. Similarly, cDNA and oligonucleotide arrays were recently found to exhibit a similar sensitivity and specificity for Escherichia coli (9) and Saccharomyces (8) transcriptome analysis.
TABLE 2.
Analysis of N. meningitidis genes deregulated by heat shock at 44°C by PCR product and oligonucleotide arrays
| Locusa/gene name | cDNA array
|
Oligonucleotide array
|
||
|---|---|---|---|---|
| Transcript ratio | SD | Transcript ratio | SD | |
| NMB0059/dnaJ | 1.31 | 0.28 | 3.50 | 0.98 |
| NMB0554/dnaK | 3.77 | 0.19 | 1.79 | 1.12 |
| NMB0565/nqrE | 0.45 | 0.01 | 0.54 | 0.18 |
| NMB0791/peptidyl-prolyl cis-trans isomerase | 1.30 | 0.15 | 2.08 | 0.23 |
| NMB1366/thioredoxin | 3.80 | 0.12 | 4.18 | 1.03 |
| NMB1810/pilO | 0.39 | 0.02 | 0.51 | 0.05 |
| NMB1936/atpA | 0.56 | 0.14 | 0.49 | 0.00 |
| NMB1940/atpB | 0.50 | 0.02 | 0.89 | 0.08 |
| NMB1972/groEL | 2.76 | 0.13 | 3.91 | 1.39 |
| NMB1973/groES | 4.02 | 0.22 | 4.52 | 3.01 |
ORF numbers in N. meningitidis serogroup B genome sequence (23).
RT-PCR was employed as an independent method to confirm the data obtained by microarray analysis for heat-shocked (44°C) versus untreated (37°C) meningococci. For dnaK (NMB0554), the increased transcript level was confirmed by RT-PCR. The RT-PCR assay also corroborated the microarray-based observation that the transcription of pdhA (NMB1341) was not changed by a shift to 44°C.
When analyzing the temperature dependence of the N. meningitidis heat shock response, we interestingly found only two ORFs to be deregulated more than twofold at 42 and 43.5°C, but 24 ORFs were deregulated more than twofold at 45°C (data not shown). While 45°C is certainly a nonphysiological condition for meningococci, the limited response at 42 and 43.5°C is in contrast to observations for Neisseria gonorrhoeae, where heat shock induction was observed on the RNA and protein level at 43°C (10, 22).
After the suitability of oligonucleotide and cDNA microarrays had been demonstrated, the meningococcal heat shock was analyzed for all 2,160 ORFs present in the genome of strain MC58 using oligonucleotide arrays manufactured according to the specifications given above. Three heat shock experiments were performed, comprising independent RNA isolations, labeling reactions, and hybridizations. Heat shock was performed at 45°C, and neisserial RNAs were isolated immediately after heat shock. Reference RNAs were isolated from parallel 37°C cultures. RNAs were labeled differentially, combined, and hybridized to whole-genome oligonucleotide microarrays. These arrays allowed hybridization signals specific for 1,498 different ORFs to be detected above the background level, equivalent to 69.35% of all MC58 ORFs. Data from the three independent experiments were combined, and average transcript ratios were calculated. Fifty-five ORFs (equivalent to 3.7% of the ORFs detected as being transcriptionally active) were identified as being heat shock responsive (34 upregulated, 21 downregulated; Table 3). This immediate deregulation after a shift to 45°C demonstrates the rapid onset of the heat shock response in N. meningitidis. The excellent reliability of the whole-genome microarrays for the analysis of the meningococcal transcriptome is demonstrated by the similar degree of deregulation found for genes organized in operons (NMB0164-0165, NMB0787-0788, NMB0906-0907, NMB0946-0947,NMB1468-1469, NMB1563-1564, NMB1789-1790, NMB1808-1812, and NMB1972-1973). The majority of meningococcal genes (95.9%) are not altered significantly due to heat shock, similar to the heat shock response of E. coli (18).
TABLE 3.
N. meningitidis genes deregulated directly after heat shock at 45°C detected by whole genome arrays
| Locusa | Gene name/product | Transcript ratio | SD |
|---|---|---|---|
| Genes upregulated | |||
| NMB0059 | dnaJ | 2.90 | 1.10 |
| NMB0554 | dnaK | 5.33 | 0.79 |
| NMB0712 | rpoH | 1.63 | 0.48 |
| NMB0791 | Peptidyl-prolyl cis-trans isomerase | 2.59 | 0.89 |
| NMB1366 | Thioredoxin | 3.81 | 1.00 |
| NMB1538 | rpoD | 3.06 | 1.08 |
| NMB1972 | groEL | 3.70 | 0.59 |
| NMB1973 | groES | 5.33 | 0.78 |
| NMB0164 | 50S ribosomal protein L36 | 2.27 | 0.17 |
| NMB0165 | 30S ribosomal protein S13 | 2.24 | 0.24 |
| NMB0214 | Oligopeptidase A | 2.18 | 0.17 |
| NMB0294 | dsbA | 2.48 | 1.17 |
| NMB0370 | Hypothetical protein | 3.72 | 2.03 |
| NMB0557 | Conserved hypothetical protein | 3.18 | 0.97 |
| NMB0561 | grpE | 4.55 | 1.37 |
| NMB0604 | Alcohol dehydrogenase, zinc- containing | 4.55 | 1.22 |
| NMB0906 | Hypothetical | 2.50 | 0.43 |
| NMB0907 | Hypothetical | 2.63 | 0.63 |
| NMB0946 | Peroxiredoxin 2 family Protein/glutaredoxin | 4.18 | 1.38 |
| NMB0947 | Lipoamide dehydrogenase, putative | 2.54 | 0.61 |
| NMB1056 | Hypothetical | 4.60 | 2.10 |
| NMB1231 | ATP-dependent protease La | 4.19 | 1.26 |
| NMB1334 | Hypothetical | 2.41 | 0.32 |
| NMB1377 | l-lactate dehydrogenase | 2.22 | 0.06 |
| NMB1468 | Hypothetical | 2.68 | 0.51 |
| NMB1469 | Hypothetical | 2.06 | 0.04 |
| NMB1472 | clpB | 2.97 | 1.67 |
| NMB1563 | transcriptional regulator, GntR family | 2.27 | 0.36 |
| NMB1564 | Conserved hypothetical protein | 3.64 | 1.03 |
| NMB1789 | secB | 2.34 | 0.20 |
| NMB1790 | Glutaredoxin 3 | 2.76 | 0.28 |
| NMB1796 | Conserved hypothetical protein | 2.86 | 0.85 |
| NMB2000 | Conserved hypothetical protein | 2.63 | 1.52 |
| NMB2013 | Hypothetical | 2.37 | 0.56 |
| Genes downregulated | |||
| NMB0187 | Ribosome recycling factor | 0.62 | 0.25 |
| NMB0535 | Glucose/galactose transporter | 0.63 | 0.26 |
| NMB0565 | nqrE | 0.60 | 0.20 |
| NMB0567 | nqrC | 0.78 | 0.13 |
| NMB0568 | nqrB | 0.81 | 0.09 |
| NMB0623 | Spermidine/putrescine ABC Transporter periplasmic Spermidine/putrescine-binding protein | 0.56 | 0.09 |
| NMB0787 | Amino acid ABC transporter, periplasmic amino acid-binding protein | 0.38 | 0.26 |
| NMB0788 | Amino acid ABC transporter, permease protein | 0.50 | 0.19 |
| NMB0838 | Cold-shock domain family protein | 0.41 | 0.12 |
| NMB0960 | sucD | 0.54 | 0.05 |
| NMB1343 | Hypothetical | 0.56 | 0.04 |
| NMB1669 | Iron starvation | 0.54 | 0.01 |
| NMB1808 | pilM | 0.78 | 0.09 |
| NMB1809 | pilN | 0.58 | 0.10 |
| NMB1810 | pilO | 0.67 | 0.10 |
| NMB1811 | pilP | 0.75 | 0.17 |
| NMB1812 | pilQ | 0.52 | 0.06 |
| NMB1933 | atpH | 0.58 | 0.09 |
| NMB1936 | atpA | 0.78 | 0.08 |
| NMB1937 | atpD | 0.61 | 0.11 |
| NMB2051 | petC | 0.51 | 0.06 |
ORF numbers in N. meningitidis serogroup B genome sequence (23).
Most upregulated genes represent typical heat shock genes or are likely to be involved in the stress response, like those encoding chaperons and proteases, which protect stressed cells against protein misfolding. The heat shock sigma factor RpoH/σ32, the principal sigma factor RpoD/σ70, and a transcriptional regulator of the GntR family may be involved in the regulation of the heat shock response. Interestingly, two ORFs encoding ribosomal proteins were also identified as being upregulated more than twofold. For 12 upregulated ORFs, no function has been identified so far.
The 21 downregulated ORFs are involved in aerobic metabolism and pilus synthesis and encode components of four different transporters, a cold shock protein, an iron starvation protein, and one ORF of unknown function. The exact mode of repression after heat shock in N. meningitidis is unclear. While upregulation is normally due to increased transcriptional activity, either downregulated ORFs may be transcribed at lower rates or the specific mRNAs may undergo more rapid degradation. For E. coli, the downregulation is not due to an increased decay of non-heat shock mRNAs (7). Although the heat shock response has been studied for many years, little has been reported about repressed genes. Here, the high proportion of ORFs involved in aerobic metabolism may indicate that their downregulation is caused by a decreased pO2 of the culture medium at elevated temperatures. Similarly, in E. coli strains overexpressing recombinant proteins, a correlation of the induction of heat shock genes and the repression of genes involved in aerobic metabolism was demonstrated (14). The upregulation of the GntR family transcriptional regulator may play an important role in the neisserial heat shock response, since these regulators comprise repressors of genes involved in bacterial metabolism (19).
Our data demonstrate the physiological relevance of the microarray-determined transcriptional response of N. meningitidis to heat shock. The heat shock response is highly conserved among different organisms and allows cells to adapt rapidly to environmental and metabolic changes and to survive stress conditions. It is well studied among a wide range of microorganisms, with Escherichia coli being analyzed in the most detail (2). Whole-genome DNA arrays were recently applied to the analysis of the heat shock response for E. coli (18), group A Streptococcus (21), Bacillus subtilis (16), and S. cerevisiae (11). Among the Neisseriae, the heat shock response of N. gonorrhoeae has been elucidated on the protein (10, 26) and transcriptional levels (22). While increased levels of GroEL were found in cultures of N. meningitidis cultured under stationary growth conditions (1), this is the first analysis of gene regulation in N. meningitidis upon transient temperature increase.
Most microarray studies are performed with cDNA-based microarrays. However, oligonucleotide arrays offer an attractive alternative. The production of cDNA microarrays requires the PCR amplification of all genes to be included in the array. In addition, PCR products can have disadvantages, such as varying degrees of GC content and possible inclusion of sequence stretches with high homology to alternative ORFs, which causes nonspecific signal to interfere with the specific target sequence (3, 18). Regions sharing a homology above 75% of the length of oligonucleotide probes were recently shown to be sufficient for cross-hybridization (9). Oligonucleotides, in contrast, can be designed to the optimum, especially within a sequenced genome, enabling researchers to prevent nonspecific hybridization. Their specificity even allows the detection of single-nucleotide polymorphisms (6). Oligonucleotide arrays also allow the design of probes of minimal secondary structure and similar length and GC content. The 40-mer oligonucleotides chosen for this study were as sensitive and specific as PCR probes 300 to 560 bp in length. Recently microarrays using even 25-mer oligonucleotides were successfully employed for transcriptome analysis with E. coli (20).
In conclusion, our experimental data clearly demonstrate the suitability of oligonucleotide and cDNA microarrays for the study of gene regulation in N. meningitidis, and oligonucleotide-based whole-genome microarrays allowed the analysis of the N. meningitidis heat shock transcriptome.
Acknowledgments
M. Guckenberger and S. Kurz contributed equally to this work.
We are grateful to J. Reidl and S. Schlör for helpful discussions, to M. Dietrich and F. Dietrich, I. Gentschev, and J. F. Viret for critical reading of the manuscript, and to A. Glück for expert technical assistance.
We thank Creatogen AG and the Bayerische Forschungsstiftung, grant “Molecular biological analysis for the development of novel antiinfectives,” for financial support.
REFERENCES
- 1.Arakere, G., M. Kessel, N. Nguyen, and C. E. Frasch. 1993. Characterization of a stress protein from group B Neisseria meningitidis. J. Bacteriol. 175:3664-3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arsene, F., T. Tomoyasu, and B. Bukau. 2000. The heat shock response of Escherichia coli. Int. J. Food Microbiol. 55:3-9. [DOI] [PubMed] [Google Scholar]
- 3.Bartosiewicz, M., M. Trounstine, D. Barker, R. Johnston and A. Buckpitt. 2000. Development of a toxicological gene array and quantitative assessment of this technology. Arch. Biochem. Biophys. 376:66-73. [DOI] [PubMed] [Google Scholar]
- 4.Cummings, C. A., and D. A. Relman. 2000. Using DNA microarrays to study host-microbe interactions. Emerg. Infect. Dis. 6:513-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietrich, G., U. E. Schaible, K. D. Diehl, H. J. Mollenkopf, S. Wiek, J. Hess, K. Hagens, S. H. E. Kaufmann, and B. Knapp. 2000. Isolation of RNA from mycobacteria grown under in vitro and in vivo conditions. FEMS Microbiol. Lett. 186:177-180. [DOI] [PubMed] [Google Scholar]
- 6.Guo, Z., R. A. Guilfoyle, A. J. Thiel, R. Wang, and L. M. Smith. 1994. Direct fluorescence analysis of genetic polymorphisms by hybridization with oligonucleotide arrays on glass supports. Nucleic Acids Res. 22:5456-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry, M. D., S. D. Yancey and S. R. Kushner. 1992. Role of the heat shock response in stability of mRNA in Escherichia coli K-12. J. Bacteriol. 174:743-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes, T. R., M. Mao, A. R. Jones, J. Burchard, M. J. Marton, K. W. Shannon, S. M. Lefkowitz, M. Ziman, J. M. Schelter, M. R. Meyer, et al. 2001. Expression profiling using microarrays fabricated by an ink-jet oligonucleotide synthesizer. Nat. Biotechnol. 19:342-347. [DOI] [PubMed] [Google Scholar]
- 9.Kane, M. D., T. A. Jatkoe, C. R. Stumpf, J. Lu, J. D. Thomas, and S. J. Madore. 2000. Assessment of the sensitivity and specificity of oligonucleotide (50mer) microarrays. Nucleic Acids Res. 28:4552-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klimpel, K. W., and V. L. Clark. 1989. The heat shock response of type 1 and type 4 gonococci. Sex. Transm. Dis. 16:141-147. [DOI] [PubMed] [Google Scholar]
- 11.Lashkari, D. A., J. L. DeRisi, J. H. McCusker, A. F. Namath, C. Gentile, S. Y. Hwang, P. O. Brown, and R. W. Davis. 1997. Yeast microarrays for genome wide parallel genetic and gene expression analysis. Proc. Natl. Acad. Sci. USA 94:13057-13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee, M. L., F. C. Kuo, G. A. Whitmore, and J. Sklar. 2000. Importance of replication in microarray gene expression studies: statistical methods and evidence from repetitive cDNA hybridizations. Proc. Natl. Acad. Sci. USA 97:9834-9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lockhart, D. J., and E. A. Winzeler. 2000. Genomics, gene expression and DNA arrays. Nature 405:827-836. [DOI] [PubMed] [Google Scholar]
- 14.Oh, M. K., and J. C. Liao. 2000. DNA microarray detection of metabolic responses to protein overproduction in Escherichia coli. Metab. Eng. 2:201-209. [DOI] [PubMed] [Google Scholar]
- 15.Parkhill, J., M. Achtman, K. D. James, S. D. Bentley, C. Churcher, S. R. Klee, G. Morelli, D. Basham, D. Brown, T. Chillingworth, et al. 2000. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature 404:502-506. [DOI] [PubMed] [Google Scholar]
- 16.Peterson, A., M. Brigulla, S. Haas, J. D. Hoheisel, U. Völker, and M. Hecker. 2001. Global analysis of the general stress response of Bacillus subtilis. J. Bacteriol. 183:5617-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rappuoli, R. 2000. Pushing the limits of cellular microbiology: microarrays to study bacteria-host cell intimate contacts. Proc. Natl. Acad. Sci. USA 97:13467-13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richmond, C. S., J. D. Glasner, R. Mau, H. Jin, and F. R. Blattner. 1999. Genome-wide expression profiling in Escherichia coli K-12. Nucleic Acids Res. 27:3821-3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sa-Noguiera, I., and L. J. Mota. 1997. Negative regulation of l-arabinose metabolism in Bacillus subtilis: characterization of the araR (araC) gene. J. Bacteriol. 179:1598-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selinger, D. W., K. J. Cheung, R. Mei, E. M. Johansson, C. S. Richmond, F. R. Blattner, D. J. Lockhart, and G. M. Church. 2000. RNA expression analysis using a 30 base pair resolution Escherichia coli genome array. Nat. Biotechnol. 18:1262-1268. [DOI] [PubMed] [Google Scholar]
- 21.Smoot, L. M., J. C. Smoot, M. R. Graham, G. A. Somerville, D. E. Sturdevant, C. A. L. Migliaccio, G. L. Sylva, and J. M. Musser. 2001. Global differential gene expression in response to growth temperature alteration in group A Streptococcus. Proc. Natl. Acad. Sci. USA 98:10416-10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tauschek, M., C. W. Hamilton, L. A. Hall, C. Chomvarin, J. A. Fyfe, and J. K. Davies. 1997. Transcriptional analysis of the groESL operon of Neisseria gonorrhoeae. Gene 189:107-112. [DOI] [PubMed] [Google Scholar]
- 23.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. E. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, et al. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 24.Virji, M., H. Kayhty, D. J. P. Ferguson, J. E. Heckels, and E. R. Moxon. 1991. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Mol. Microbiol. 5:1831-1841. [DOI] [PubMed] [Google Scholar]
- 25.Wodicka, L., H. Dong, M. Mittmann, M. H. Ho, and D. J. Lockhart. 1997. Genome-wide expression monitoring in Saccharomyces cerevisiae. Nat. Biotechnol. 15:1359-1367. [DOI] [PubMed] [Google Scholar]
- 26.Woods, M. L., R. Bonfiglioli, Z. A. McGee, and C. Georgopoulos. 1990. Synthesis of a select group of proteins by Neisseria gonorrhoeae in response to thermal stress. Infect. Immun. 58:719-725. [DOI] [PMC free article] [PubMed] [Google Scholar]